Evans Review No. 3: Structure–function relationships of the plant cuticle and cuticular waxes — a smart material?
Hendrik Bargel A C , Kerstin Koch B , Zdenek Cerman B and Christoph Neinhuis A DA Institut für Botanik, Technische Universität Dresen, Zellescher Weg 22, 01062 Dresden, Germany.
B Nees-Institut für Biodiversität der Pflanzen, Universität Bonn, Meckenheimer Allee 170, 53115 Bonn, Germany.
C Present address: Biotechnical Center, Technische Universität Darmstadt, Petersenstraße 20, 64287 Darmstadt, Germany.
D Corresponding author. Email: christoph.neinhuis@tu-dresden.de
E This paper is part of The Evans Review Series, named for Dr Lloyd Evans. The series contains reviews that are critical, state-of-the-art evaluations that aim to advance our understanding, rather than being exhaustive compilations of information, and are written by invitation.
F This paper is dedicated to Prof. Wilhelm Barthlott on the occasion of his 60th birthday.
Functional Plant Biology 33(10) 893-910 https://doi.org/10.1071/FP06139
Submitted: 30 May 2006 Accepted: 18 August 2006 Published: 2 October 2006
Abstract
The cuticle is the main interface between plants and their environment. It covers the epidermis of all aerial primary parts of plant organs as a continuous extracellular matrix. This hydrophobic natural composite consists mainly of the biopolymer, cutin, and cuticular lipids collectively called waxes, with a high degree of variability in composition and structure. The cuticle and cuticular waxes exhibit a multitude of functions that enable plant life in many different terrestrial habitats and play important roles in interfacial interactions. This review highlights structure–function relationships that are the subjects of current research activities. The surface waxes often form complex crystalline microstructures that originate from self-assembly processes. The concepts and results of the analysis of model structures and the influence of template effects are critically discussed. Recent investigations of surface waxes by electron and X-ray diffraction revealed that these could be assigned to three crystal symmetry classes, while the background layer is not amorphous, but has an orthorhombic order. In addition, advantages of the characterisation of formation of model wax types on a molecular scale are presented. Epicuticular wax crystals may cause extreme water repellency and, in addition, a striking self-cleaning property. The principles of wetting and up-to-date concepts of the transfer of plant surface properties to biomimetic technical applications are reviewed. Finally, biomechanical studies have demonstrated that the cuticle is a mechanically important structure, whose properties are dynamically modified by the plant in response to internal and external stimuli. Thus, the cuticle combines many aspects attributed to smart materials.
Keywords: AFM, anti-adhesive surfaces, biomimetics, biopolymer, epicuticular waxes, Lotus-Effect®, mechanical properties, plant cuticle, self-assembly, structure–function relationships, template effect.
Introduction
Interfacial interactions at and physical properties of boundary layers are of crucial importance for plant life at various scaling levels. One of such an interface is the thin extracellular membrane called the cuticle, which covers primary aerial parts of vascular plants and many bryophytes (Kolattukudy 2001). Its lipid-derived components are synthesised by the epidermis and deposited on the outer periclinal walls, forming a continuous protective layer with striking properties. Since its appearance in early land plant evolution during the Silurian, the cuticle has proved to be of tremendous importance, enabling the survival of plants in many different terrestrial habitats. Indeed, macrofossil records interpreted as cuticles date back to the very earliest terrestrial plant species known (Edwards et al. 1996). The critical role of this crucial protective layer is stressed by the fact that it represents one of the largest interfaces between biosphere and atmosphere (Riederer and Schreiber 1995). It is thus no surprise that many researchers and papers have dealt with the multiple properties and facets of this delicate structure. Knowledge of the microstructural features, chemical composition, ontogeny, permeability, biosynthesis, biotic and abiotic interactions, and functions of the cuticle has increased significantly, while the molecular biology has gained much interest in the recent years. A detailed summary of up-to-date concepts and results is presented in the book by Riederer and Müller (2006).
After a general introduction on structure and composition, this review highlights three structure–function relationships of the cuticle that have not been treated in context so far and have been the subjects of recent studies with intriguing results. Special attention is paid to epicuticular wax crystals, in particular to the coherence between chemistry and specific morphology, as well as the self-assembly of individual molecules. Next, the functions of microstructured hydrophobic plant surfaces, in particular water-repellent and self-cleaning properties, are addressed. Up-to-date concepts and progress of the rapidly expanding field of micro- and nanoscale biomimetic technology are reported. Finally, current knowledge of the biomechanics of the membrane and its importance for structural stability and maintenance of the physiological integrity of the plant is reviewed. This topic has come into focus in recent years and represents a new functional aspect of the plant cuticle.
The continuous extracellular matrix: structure and composition
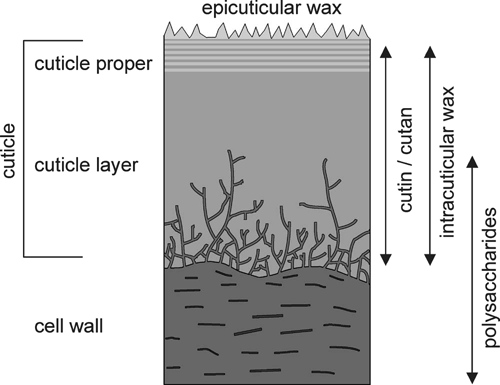
The cuticle represents a natural composite comprising two major hydrophobic components: the insoluble polymer, cutin, and soluble lipids of diverse chemistry, collectively called waxes (Kolattukudy 1980). Variable amounts of polysaccharide fibres and the frequently abundant pectin layer originate from the cell wall and link the biopolymer to the underlying tissue (Holloway 1982; Jeffree 1996). Ultrastructural studies have established the generalised view of a hypothetical bi-layered structure with a thin outer ‘cuticle proper’ (CP) and a more prominent inner ‘cuticle layer’ (CL) (Fig. 1). The delicate CP (usually <200 nm) is formed as the primary protective layer succeeding a procuticle during ontogeny. It shows a variable number of alternating layers supposed to contain wax and cutin — the so-called lamellate region (Holloway 1994; Jeffree 1996). This outer region makes up to 99% of the barrier efficiency (Riederer and Schreiber 1995). The CL is variable in thickness, up to 17 µm in some xerophytes, and has a mostly reticulate appearance, a result of impregnation of the epidermal cell wall during maturation (Holloway 1994; Jeffree 1996). The cuticle appears to be very inhomogeneous in structure and composition and shows a high degree of variability between different plant species and organs. Summaries of cuticle type classification can be found in papers by Holloway (1994) and Jeffree (2006).
|
The basic framework cutin is a biopolyester composed of saturated C16 ω-hydroxy and unsaturated C18 hydroxy-epoxy fatty acid monomers (Kolattukudy 1980). Cutin has a variable and dynamic composition: the ratio of C16 : C18 fatty acids may be organ- as well as species-specific, and changes during ontogeny (Espelie et al. 1979; Baker et al. 1982; Marga et al. 2001). Common structural constituents of suberin, which is abundant in roots and periderm tissue, were recently also identified to be an integral part of cutin. Among them are glycerol and glyceryl esters (Graca et al. 2002) or dicarboxylic acids proven to be important structural monomers in Arabidopsis thaliana L. stem and leaf cuticle (Bonaventure et al. 2004; Xiao et al. 2004; Franke et al. 2005). Whether or not this latter type of cutin is the exception to the rule is yet to be determined. The structure of the matrix polyester is commonly thought to be built up by esterified primary hydroxyl groups of the fatty acid monomers forming a linear polyester, while branching combined with cross-linking by ester-bound and non-ester-bound mid-chain groups and abundant constituents yields a three-dimensional network (Stark et al. 2000; Villena et al. 2000; Kolattukudy 2001). Modelling and calculation of the molecular structure of the matrix revealed an average pore size of 0.3–0.5 nm (Matas and Heredia 1999; Popp et al. 2005). However, the three-dimensional structure of cutin still remains puzzling. A summary of current knowledge is given by Stark and Tian (2006).
Cutan is a second, highly resistant biomacromolecule originally found in fossilised cuticles. It is composed of ether-linked long chain alkyl moieties with chain lengths mainly of even numbers of carbon ranging from C22 to C34 (Collinson et al. 1998; Schouten et al. 1998; van Bergen et al. 2004; Deshmukh et al. 2005). Cutan may be also abundant in cuticles of extant taxa, e.g. Agave americana L., Clivia miniata Regel, Gingko biloba L. or A. thaliana, although of a somewhat different chemical composition (Mösle et al. 1997; Villena et al. 1999; Suh et al. 2005). One of the open questions is how the three-dimensional matrix network is built in vivo. Croteau and Kolattukudy (1974) postulated the existence of an acyltransferase that transfers exogenously applied fatty acid monomers from their acyl-CoA form to the growing cutin polymer in Vicia faba cell-free preparations. Reina and Heredia (2001) reported the characterisation of a putative acyl-CoA : cutin transferase isolated from Agave americana. Recently, an A. thaliana bodyguard (bdg) mutant with altered cuticle formation was described (Kurdyukov et al. 2006). The authors proposed that BDG, a member of the α-,β-hydrolase fold protein superfamily, encodes an extracellular synthase responsible for the formation of cuticle. However, the loss-of-function bdg mutant resulted in an overall increase in the level of cutin and wax constituents, contrasting with the author’s main hypothesis.
Cuticular waxes are either embedded as filler into the cutin matrix to waterproof the membrane or deposited as epicuticular waxes onto the surface, where they often form complex three-dimensional crystalline microstructures. These are useful characters in plant systematics, since particular surface wax types are restricted to certain taxa (Barthlott 1981; Gülz 1994; Barthlott et al. 1998, 2003). Among them are platelets, rodlets, tubules, and several others. Most of these structures appear together with an underlying wax film (Conn and Tewari 1989; Ensikat et al. 2000; Koch et al. 2003a). The cuticular lipids are a complex mixture of aliphatic and aromatic components, most of them resembling derivatives of n-acyl alkanes with chain lengths of C20–C40 (von Wettstein-Knowles 1995; Jetter et al. 2006). Substitution by functional groups (-hydroxyl, -carboxyl, -ketoyl) broadens the spectrum to fatty acids, primary alcohols, aldehydes, β-diketones and secondary alcohols, respectively (Walton 1990; Bianchi 1995; Kunst and Samuels 2003). Exceptions are polymeric aldehydes present in the waxes of sugarcane (Saccharum officinarum Salisb.), rice (Oryza sativa L.) leaves and traps of the pitcher plant (Nepenthes alata Blanco) (Haas et al. 2001; Riedel et al. 2003), as well as aromatic compounds such as flavonoids (Wollenweber 1984; Wollenweber and Schneider 2000). Similar to cutin, cuticular wax composition is subject to great variation, either among plant species or during organ ontogeny, indicating a very customisable system (Riederer and Schreiber 2001). It has been shown that intracuticular wax is chemically distinct from the epicuticular form (Jetter et al. 2000; Guhling et al. 2005). The majority of existing studies on wax chemistry are based on solvent-extracted waxes, which also contain intracuticular waxes to a variable degree. New selective isolation methods for superficial wax projections have been developed, i.e. embedding the waxes in glycerol (Ensikat et al. 2000) or water-based glue (Jetter et al. 2000; Jetter and Schäffer 2001). After drying / freezing of the embedding medium, the surface lipids can be distinctively separated from the inner lipid fraction for further analysis.
The analysis of model species, e.g. A. thaliana, Hordeum vulgare L. and Zea mays L., has been widely established and has revealed new insights into the molecular biology and biosynthesis of the plant cuticle and cuticular waxes. One advantage of a model plant approach is the increase of data of the chosen species within a relatively short time. A further point is that available data, in the best case, provides a detailed knowledge of key regulative mechanisms. As a result, an increasing number of genes associated with cuticular lipid and cutin biosynthesis has now been cloned, encoding metabolic or regulatory loci, or proposed to be involved in the transport of compounds (Post-Beittenmiller 1996; Jenks and Ashworth 1999; Yephremov and Schreiber 2005; Kunst et al. 2006). However, knowledge and function of other important gene candidates is only slowly emerging and, thus, little remains known about the molecular biology of the cuticle. Moreover, it is established that chemical composition and structure of the cuticle and cuticular waxes is highly variable, either among plant species, among the organs of one species and even individual cells, or during organ ontogeny. Based on this, the validity of molecular mechanisms of wax and cutin biosynthesis of model species is limited. This also holds true for other aspects, such as micromorphology of epicuticular waxes, demonstrated by the example of Brassica oleracea L., a model species for epicuticular waxes. Baker (1974) studied Brassica oleracea var. gemmifera (Brussels sprout) plants grown at low air humidities and reported changes in wax micromorphology. However, these effects could not be detected for β-diketone tubules of Eucalyptus gunnii L. or nonacosanol wax tubules of Tropaeolum majus L. (Koch et al. 2006b). Thus, it is highly desirable that studies are extended to non-model species in order to obtain a more representative picture of the biology of the plant cuticle and its regulatory cues.
Epicuticular waxes: morphology, crystallinity and self-assembly
How do wax crystals form in vivo, and what influence do abundant structures have on crystallisation and predetermined pattern formation at the molecular level? Some answers have been proposed only recently for model wax types.
Coherence between chemical composition and wax morphology
Despite the great variability in appearance and chemistry, epicuticular waxes often consist of a single predominating constituent or compound class that determine a characteristic morphology. This coherence between chemistry and morphology is well documented for wax tubules and some wax platelets.
Two types of tubules can be distinguished chemically as well as morphologically. The first type contains large amounts of secondary alcohols, predominantly 10-nonacosanol and its homologues (Baker 1982; Jetter and Riederer 1994, 1995). These tubules are common in gymnosperms and many basal dicotyledonous families (e.g. Ranunculaceae, Magnoliaceae) (Jeffree et al. 1975, 1976; Barthlott et al. 2003) and some mosses (Neinhuis and Jetter 1995). Holloway et al. (1976) showed that tubules dominated by secondary alcohols usually did not contain a single compound, but homologous series of secondary alcohols and also asymmetrical diols. The second type of tubule contains large amounts of β-diketones as hentriacontan-14,16-dione (Tulloch and Hoffman 1974; Baum et al. 1989; Meusel et al. 2000b). This particular type of wax tubule is characteristic for several Poaceae but also occurs in various groups scattered among angiosperms (Barthlott et al. 2003). Morphologically, tubules based on secondary alcohols are usually shorter (0.3–1.1 µm long by 0.1–0.2 µm wide) than the β-diketone tubules, which typically are 2–3 µm long and 0.2–0.3 µm wide (Jeffree et al. 1975).
Wax platelets are found in all major groups of plants and vary considerably in shape, chemical composition and spatial pattern (Barthlott et al. 2003). In some species wax platelets are dominated by triterpenoids, e.g. in Sedum rupestre (Crassulaceae) (Stevens et al. 1994), whereas platelets found in the Poaceae, Fabaceae and the within the genus Eucalyptus are dominated by primary alcohols and probably represent the most common type (Hallam and Chambers 1970; Bianchi et al. 1979; Tulloch et al. 1980; Koch et al. 2006a). Other wax platelets are chemically characterised by large amounts of alkanes, aldehydes, esters, secondary alcohols or flavonoids (Hallam and Chambers 1970; Jeffree et al. 1975; Baker 1982), but there is still only limited information on the connection between morphology and chemical composition.
The morphology of three-dimensional wax structures is not necessarily determined by the dominating chemical compound or compound class. One example of wax crystals determined by a minor component of a complex mixture is transversely ridged rodlets (Meusel et al. 1999), while the origin of longitudinally ridged rodlets is still puzzling (Meusel et al. 1994). An example of a genus with very complex wax crystal morphology is B. oleracea, in which several cultivars form several different wax types (Barthlott et al. 1998; Gomez-Campo et al. 1999). This polymorphism most likely arises from the presence of several dominant wax compounds (Baker 1982; Shepherd et al. 1995). In this context it was shown that environmental factors such as temperature and relative humidity can affect total wax mass and crystal density, but rarely chemical composition (Whitecross and Armstrong 1972; Richardson et al. 2005; Koch et al. 2006b).
Crystallinity
The high similarity of individual wax structures, such as polygonal platelets and rodlets and the physical properties of the waxes, e.g. a defined melting point between 60 and 95°C, suggest a crystalline order. De Bary (1871) introduced the term ‘Krystalloid’ for plant waxes, but the crystalline nature of various wax types was verified nearly one century later by X-ray powder diffraction, electron diffraction (Reynhardt and Riederer 1994; Meusel et al. 2000a; Matas et al. 2003) and nuclear mass resonance (NMR) spectroscopy (Schreiber et al. 1997). Whereas intracuticular waxes may be either amorphous or crystalline, epicuticular waxes are assumed always to be crystalline (Bianchi 1995; Riederer and Schreiber 1995). Crystal structure data of pure epicuticular waxes are rarely published, e.g. Meusel et al. (2000b). Very recently, Ensikat et al. (2006) investigated the waxes of 35 species, including nine different wax morphologies. Electron diffraction and X-ray diffraction analysis of native and re-crystalised structures showed that epicuticular waxes can be divided into three of seven existing crystal symmetry classes. The orthorhombic class is characteristic for the majority of wax morphologies. In this, the long aliphatic carbon chains are aligned in parallel lines and with a zig-zag formation of the backbone (Kreger and Schamhart 1956). Wax tubules with large amounts of β-diketones had hexagonal symmetries of the methylene sub cells. The triclinic order has been reported for even-chain alkanes and branched fatty acids (Larsson 1994; Dorset 2002), but has now also been found for nonacosan-ol tubules (Ensikat et al. 2006). Jeffree et al. (1975) described the background epicuticular wax layer to form a continuous amorphous wax coating of the plant cuticle. By using a modified ‘cryo-adhesion’ preparation method developed by Ensikat et al. (2000), it was possible to preserve the natural crystal structure of the epicuticular waxes and to separate the three-dimensional crystals from the underlying thin wax film for crystallographic analysis (Ensikat et al. 2006). It was shown that the crystal structure of the associated wax films has an orthorhombic order, whereas wax tubules showed a triclinic symmetry, respectively hexagonal crystalline symmetry. It was also found that re-crystallised waxes, not always, but in several cases, showed a remarkably lower degree of order compared with mechanically isolated waxes. According to these new results the common view of wax crystal structure probably requires refinement. However, the traditional method of re-crystallisation of plant waxes led to the proven perceptions of coherence between wax morphology and chemistry, and thus remains a suitable method for studying the self-assembly processes of plant waxes.
Self-assembly driven structure formation
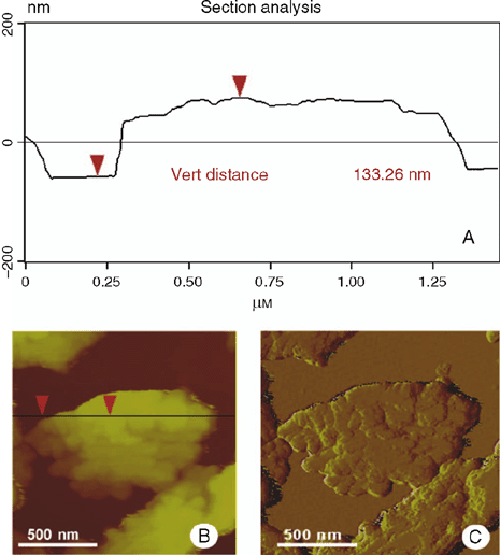
A wide range of biological and non-biological nano-structures originates from self-assembly (Benyus 1997; Whitesides et al. 1991; Zhang 2003). It is generally established that the various epicuticular wax crystal types also result from self-assembly processes, mainly based on the chemical composition of the waxes. Self-assembly implies that if a particular system or structure is disassembled in appropriate subunits, these are able to reform into the original structure under favourable conditions. When waxes are dissolved in an organic solvent and subsequently re-crystallised, morphologies similar to the plant surface emerge (von Wettstein-Knowles 1974; Jeffree et al. 1975, 1976; Chambers et al. 1976; Jetter and Riederer 1994, 1995; Meusel et al. 2000b; Neinhuis et al. 2001; Koch et al. 2006a). Re-crystallisation of waxes is characterised by several principles of molecular self-assembly, e.g. interaction, mobility, and reversibility of the subunits. The wax molecules interact with one another, whereby the molecules may be the same, as found for the wax platelets composed of primary alcohols (Hallam and Chambers 1970; Jeffree et al. 1975; Koch et al. 2006a) or different, as found for wax tubules dominated by nonacosan-10-ol (Jeffree et al. 1975, 1976; Jetter and Riederer 1994) and alkanediols (Jetter and Riederer 1995). Self-assembly of wax crystals requires mobility of the subunits and segregation of different components or component classes of the wax mixtures. During the crystallisation process of an extracted wax in or from a solution, temperature, the concentration and the chemical nature of the solvent determine the mobility of the molecules. It is still a matter of debate how wax components stay mobile in situ, as they have to move from inside the cell through the cuticle and finally onto the ridges and edges of the growing crystals. In addition to the concept of two possible paths of cuticular transport, i.e. a lipophilic pathway for non-ionic, lipophilic molecules and a polar pathway for polar and charged molecules (Schreiber 2005; Riederer and Müller 2006), Neinhuis et al. (2001) proposed a co-transport of wax components with evaporating water that potentially keeps the wax molecules in a mobile category. Both of these models can explain the transport of wax molecules across the cuticle, but as far as the size or chemical nature of the postulated polar paths is concerned, no evidence of trans-cuticular structures that could serve as pathways for wax molecules such as pores have yet been found in the plant cuticle by SEM, TEM or AFM investigations (Canet et al. 1996; Koch et al. 2004). Schreiber (2006) speculated that the chemical nature of the polar pores originate in functional groups abundant in the cuticular membrane, for example non-esterified carboxyl and / or hydroxyl groups of cutin monomers or polar carbohydrates. So far, few data about the dynamic process of self-assembly exist. The most suitable microscopy techniques for studying such dynamic processes at the molecular scale are scanning probe microscopes (SPM). SPMs represent a group of different techniques, such as atomic force microscopy (AFM), scanning tunnelling microscopy (STM) and magnetic force microscopy. SPMs can be operated in a variety of environmental conditions, in liquid, air, or other gases, and have been used for studying self-assembly processes of organic monolayer formation (Buchholz and Rabe 1992; Cyr et al. 1996; Le Poulennec et al. 2000; De Feyter and De Schryver 2005). Molecular steps on the surface of single wax crystals can be visualised by AFM (Fig. 2). Such steps are virtually undetectable by SEM (Ensikat et al. 2000). Another advantage of AFM is the analysis of self-assembly of plant surface wax under environmental conditions in vivo (Koch et al. 2003b, 2004). These investigations visualised the very early stages of wax crystal formation on leaves of various species at the molecular level for the first time. After partial removal of the epicuticular waxes, AFM time-series images of regenerating wax layers showed extension of mono- and bimolecular wax films and the growth of three-dimensional platelets, either directly on the cuticle or on already existing wax layers. Whereas von Wettstein-Knowles (1974) assumed that newly extruded wax accumulates at the base of existing structures, AFM studies of the surface of an attached Galanthus nivalis L. leaf clearly showed that the extension is occurring at the distal end of the growing crystal (Koch et al. 2004). The same principle of distal growth was also observed for re-crystallising wax platelets from Triticum aestivum L. cv. Naturastar (Koch et al. 2006a) (Fig. 3).
|
|
Wax tubule formation is assumed to be based on a rolling-in process of plate-like wax structures (Jeffree 2006). The observation of morphological mixtures of tubules and plates, found on neighbouring cells or even on the surface of one cell (Hallam and Chambers 1970; Tulloch 1973; Barthlott et al. 1998), and spiral lines on the surfaces of some nonacosanol tubules (Thair and Lister 1975) led to the thesis that tubules might arise from spontaneous folding of wax platelets. Jetter and Riederer (1994) demonstrated that the pure (S)-nonacosan-10-ol forms tubules under kinetic control, while planar crystals result from thermodynamic control during crystallisation. Particular convolute and curved platelets were interpreted as transitions between platelets and tubules (Hallam and Chambers 1970) and helically twisted plates as precursor forms of tubules (Jetter and Riederer 1994). However, the proposed platelet folding has never been observed experimentally. Recently, in-vitro re-crystallisation of waxes forming tubules extracted from Leymus arenarius Hochst., Eucalyptus gunii Hook.f., Tropaeolum majus L., Thalictrum flavum L., Nelumbo nucifera Adan. and other species was studied by AFM (K Koch unpubl. data). Both β-diketone and nonacosanol tubules grew by a continuous supplementation of wax, either on top of the tubules if growth took place perpendicular to the substrate, or at one preferred end of the tubules if growth occurred parallel to the substrate. Thus, the thesis of morphological deduction of tubules from platelet structures can no longer be supported.
Although SPM techniques allow visualisation of the molecular ordering in monolayers and the dynamics within these monolayers, real time studies of self-assembly may be limited by the scan speed of the device, which is generally slow compared with the time scale of single molecule dynamics (Gesquière et al. 2000). Additionally, AFM of biological surfaces might be limited by the surface roughness and softness of the specimen (Hoh and Hansma 1992; Morris et al. 1999).
Several comparative studies showed that certain wax types are able to crystallise in different morphological forms, depending on the growth conditions (Jeffree et al. 1975; Jetter and Riederer 1994; Meusel et al. 2000a). One important crystallisation condition is the crystallisation speed. In the natural system, the speed of the crystal growth is determined by wax biosynthesis and transport processes (Koch et al. 2004), whereas the re-crystallisation from or in a solution occurs relatively fast and, thus, time for separation of molecules is limited. In addition, substrate characters (e.g. polarity, order) can also have a direct influence on self-assembly processes and the formation of wax crystals, leading to a template effect at the molecular level (Koch et al. 2006a).
Template-mediated self-assembly
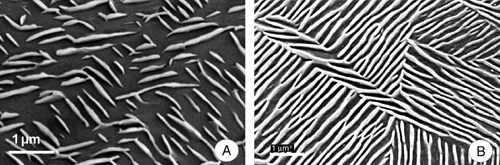
Applied molecules do interact with substrates on the molecular level. These interactions might lead to a template effect, i.e. the substrate organises the assembly of the molecules in a specific spatial arrangement (Cyr et al. 1996; De Feyter and De Schryver 2005). Such a template effect of the substrate on re-crystallising waxes was only recently reported for wax platelets formed by primary alcohols (Koch et al. 2006a). It was demonstrated by STM and AFM that epitaxial growth of primary alcohol platelets occurs on highly ordered pyrolytic graphite (HOPG) substrate. On this particular substrate, the spatial pattern of the wax molecules strictly followed the hexagonal symmetry of the crystalline substrate, resulting in a highly ordered pattern of wax microstructures (Fig. 4) with a uniform and parallel alignment of the growing platelets (Koch et al. 2006a). Thus, growth of wax platelets dominated by primary alcohols on HOPG is not a pure self-assembly process driven by the chemical constituents of the wax molecules, but guided by the structural template effect of the underlying substrate.
|
Concerning wax pattern in situ, it is conceivable that the cuticular matrix might account for a naturally occurring template effect. Highly ordered spatial distribution of wax crystals have been described for several plant species (Barthlott et al. 1998; Carr et al. 1985). Based on several studies of wax re-crystallisation, however, it was postulated that neither the cuticle nor an artificial substrate has an influence on wax self-assembly (Jeffree et al. 1975; Chambers et al. 1976; Baker 1982). Recently, isolated and de-waxed cuticles of mature Convallaria majalis leaves were used for re-crystallisation of waxes (Koch et al. 2006a). The results clearly showed that fixed positions within the cutin network of this particular cuticle are essentially responsible for the spatial distribution of the re-crystallised platelets. The influence of the cuticle is not yet totally understood, but a first evidence of cuticle involvement in pattern formation is available. AFM studies of wax regeneration on living plant surfaces showed that three-dimensional wax crystals grew directly on the cuticle as well as on existing wax layers (Koch et al. 2004). These findings indicate that the cuticle resembles a template for the molecular arrangement of the first wax layers to grow, which then itself provides a template for the consecutive formation of three-dimensional waxes. Due to the morphological variability of plant cuticles and chemical diversity of cuticular waxes; however, these results cannot be canonically generalised. Wax tubules dominated by secondary alcohols and diols are able to self-assemble as tubules on various substrates without any influence of the substrate on crystal morphology (Jeffree et al. 1976; Jetter and Riederer 1994). SEM was used for the visualisation of waxes in these and other studies of wax self-assembly and therefore the masses applied to the substrates must have been higher than necessary for SPM techniques used in more recent work. It is important for the interpretation of the results to note that the influence of a substrate could be lost after two or more layers of wax molecules have been formed on the substrate (Mo et al. 2004). Another important point is that during in vitro crystallisation the concentration of wax molecules in the solvent increases when the solvent evaporates, and nucleation of the wax molecules might start within the solution without any influence of the substrate.
Biomimetic surfaces
Template-affected self-assembly of waxes on artificial substrates could create well-defined biomimetic systems, with wax crystal morphologies similar to those on the plant surfaces. Such biomimetic systems facilitate high-resolution study of interactions at the micro- and nanometer scale, and processes occurring at the plant boundary layer, e.g. in spray application of pesticides or nutrients in agricultural systems, could be studied under defined conditions. Furthermore, biomimetic systems could be used to study the adhesion of particles, insects and microorganisms on waxy surfaces. Templating may be important to induce order, but also gives rise to self-assembled three-dimensional aggregates on the molecular level. However, this strategy for self-assembly is just beginning to be explored and will provide stimulus for molecular self-assembly studies for years to come. The wide range of morphological and chemical variation in plant epicuticular waxes reveals a great pool of organic material for such studies of self-assembly. The rapid development of nano-technology will probably provide new features of biomimetic structures within a short time.
Wettability and self-cleaning property of plant surfaces
The evolution of the cuticle is strongly linked to plant life on land. The development of a hydrophobic outer coverage was one of the key innovations that enabled plants to leave their primarily aquatic habitat and to overcome the physical and physiological problems associated with an atmospheric environment, such as desiccation, gravitation or UV-radiation (Edwards et al. 1996; Bateman et al. 1998). Thus, the cuticle represents a multifunctional protective interface between plants and the environment, and the waxes, particularly, play an important role in the interfacial interactions. The plethora of processes in which the cuticle is involved ranges from transport phenomena and control, to interaction with the biotic and abiotic environment, developmental cues, signalling, and mechanical containment (Bird and Gray 2003; Bargel et al. 2004a; Riederer and Müller 2006). Figure 5 provides a schematic overview of the most prominent functions of the plant surface. One important and far-reaching aspect is the ability of many plant surfaces to repel water and, in contiguity, to stay virtually clean owing to a striking self-cleaning property. Water-repellency is advantageous for the plant owing to reduction of both leaching from interior and water availability, which creates unfavourable conditions for the successful colonisation of pathogens and parasitic fungi. These aspects are reviewed by other authors, e.g. Juniper (1991) and Leveau (2006).
|
Wetting of surfaces
Wetting of a surface is determined by the three interfacial tensions between solid / gas, solid / liquid and liquid / gas, and can be calculated by Young’s equation (Barthlott and Neinhuis 1997). Surfaces with contact angles <90° are usually called wettable (hydrophilic), while surfaces with contact angles >90° are usually considered non-wettable (hydrophobic), although a clear distinction between both states is not possible. The basic principles of surface wetting were published in the 19th century and several books and reviews on surface wetting were published in the last century. The last comprehensive summary of the physical and chemical basics of surface wetting was published by de Gennes et al. (2004).
The introduction of scanning electron microscopy into botany allowed plant surfaces to be studied in relation to wetting (Holloway 1969, 1970, 1971). It became clear that plant micro- and nanostructures, i.e. cellular protrusions, cuticular folding and the epicuticular waxes, minimise the contact area between water (liquid) and the plant surface (solid) by the combination of hydrophobic chemistry and micro-roughness, and form an enlarged water / air interface, thus constituting a composite surface with air enclosures between the epicuticular wax crystals. On such ‘low energy’ surfaces water forms spherical droplets owing to surface tension, which rest on the outermost tips of the wax crystals with minimised contact area, a phenomenon called water repellency (Barthlott and Neinhuis 1997). Neinhuis and Barthlott (1997) determined the contact angles of ~200 water repellent plant species showing contact angles from 153° to 165°. This publication was also the first to point to the significance of combined structures on different length scales resulting in a high degree of water repellency: The combination of papillose epidermal cells coated with micro- and nanostructures formed by epicuticular waxes creates a hierarchically structured surface that is less prone to erosion and mechanical damage while, at the same time, the state of the droplet resting on the papillae is stabilised. Wagner et al. (2003) provided further corroboration of this thesis. The authors examined various water repellent surfaces in relation to the critical surface tension of the wetting liquid at which droplets stick to the leaves. It was shown that droplets of water / methanol solutions containing up to 75% methanol roll off from hierarchically structured plant surfaces (e.g. sacred lotus) at low inclination angles, while those with only one level of structuring are readily wetted by solutions containing 5% methanol. Concurring AFM and CLM (confocal white light microscopy) analysis revealed that those plant surfaces with optimised water-repellency have an aspect ratio of ~0.6. Such surfaces exhibit a special form of hydrophobicity, the so-called superhydrophobicity, which is defined by a very high water contact angle and a very low roll-off angle at inclination (Marmur 2003). Two possible wetting states for super-hydrophobic surfaces dependent on surface structure have been described, the so-called ‘Wenzel’ and ‘Cassie’ states (Lafuma and Quéré 2003; Marmur 2003). In the first, large contact angles are obtained, but the droplet rests between the structures of the surface, while in the latter, a droplet is elevated onto the tips of the structures. Only in this case, droplets roll off the surfaces at the lowest angles of inclination. Surfaces of plants with a double-structured hierarchy seem to stabilise this ‘Cassie state’ (Lafuma and Quéré 2003). Nosonovsky and Bhushan (2005) proposed a comprehensive analytical model to analyse the relationship between various local roughness distributions and contact angle.
An alternative and, at first glance, astonishing mechanism by which plants achieve super-hydrophobicity is described by Otten and Herminghaus (2004). Plants such as Alchemilla vulgaris L. are characterised by a hydrophobic surface in combination with hydrophilic hairs. The hydrophobic cuticle is non-wettable and causes the droplets to stick to the individual elastic hairs. The droplets then move up the hairs and remain at their tips far above the epidermal cell surface.
Epicuticular waxes and self-cleaning surfaces
One of the, presumably, most important functions of structured plant surfaces remained undiscovered for a long time. This function is closely linked to water-repellency of micro- and nano-structured surfaces. Droplets of water roll off superhydrophobic surfaces at the slightest inclination angle. During their course they pick up contaminating particles from the surface. Dust, soot, spores, algae or bacteria are readily removed from the plant surface by rain (Fig. 6). This self-cleaning property of certain plant surfaces was known at the end of the 19th century and was mentioned anecdotally by German botanists (Engel 1939; Günther and Wortmann 1966; Rentschler 1973). The correlation between the ultrastructure of waxes and self-cleaning was recognised for the first time by Barthlott and Ehler (1977), who also gave a functional explanation. Barthlott (1990) suggested that self-cleaning may be one of the most important functions of epicuticular waxes. The high efficiency of self-cleaning was quantitatively verified by Barthlott and Neinhuis (1997). As for water droplets, the contact area between the particles and plant surface is minimised. Similarly, particles rest only on the tips of the epicuticular waxes and, consequently, adhesion forces, particularly van-der-Waals forces, are present only at these few contact points (Fürstner et al. 2000). If a droplet rolls over the particle the capillary forces between droplet and particle are predominant, resulting in adhesion between droplet and particle, which is then removed from the leaf surface. The basic principle for this purely physico-chemical self-cleaning process is merely the structure of the surface combined with a hydrophobic chemistry such as that of epicuticular waxes. This phenomenon is not restricted to plants; some insects also evolved self-cleaning surfaces (Wagner et al. 1996). The purpose of the efficient self-cleaning property of plants seems to be the protection against pathogens. Experiments on leaf surfaces with disrupted wax microstructure caused by detergents (Noga et al. 1987; Lownds 1988; Wolter et al. 1988) or environmental pollutants (Turunen and Huttunen 1990) showed an increase in contamination and a significant increase of fungal spores compared with the controls. Moreover, self-cleaning plant surfaces have an extremely low water-capacity and hence, are virtually dry, a condition not beneficial for the majority of plant pathogens (Beattie and Lindow 1995).

|
Technology transfer: biomimetic applications
The self-cleaning property of plant and animal surfaces has a high potential for technological applications and materials, as this feature depends only on the physico-chemical properties of the surface (Barthlott and Neinhuis 1997; Neinhuis and Barthlott 1997). Numerous methods have now been developed to reproduce such surfaces by industrial processes. A comparison between the self-cleaning efficiency of plant surfaces and technical counterparts is drawn by Fürstner et al. (2005). The authors also demonstrated for the first time the generation of micro- and nanostructured metal surfaces with highly efficient self-cleaning properties, comparable to their natural model (Fig. 6). Other researchers work on lotus-like surfaces with superhydrophobic or even amphiphilic properties (Jiang et al. 2004; Patankar 2004; Xie et al. 2004; Burton and Bhushan 2005; Guo et al. 2005; Sun et al. 2005). Essentially, all surfaces exposed to contamination can be cleaned easily by water based on biomimetic self-cleaning surfaces. Façade paints, varnish, glass, metals, textiles, plastics and many other applications are possible. For example, ready-for-market façade paints and textiles have been developed with an efficiency strikingly similar to the biological model (Barthlott and Neinhuis 2001). Great possibilities are predicted for plastics with self-cleaning surfaces, and Erbil et al. (2003) described a simple method to generate structured superhydrophobic plastics. Important properties of patterned surfaces are isotropy and anisotropy of wetting / dewetting (Gleiche et al. 2000). Whereas the lotus leaf is isotropic in wetting owing to homogenous distribution of the papillae, the panicles of rice (Oryza sativa) and some other species have an anisotropic dewetting tendency owing to its microstructures being oriented parallel to the leaf margin (Feng et al. 2002). This patterning can be used for controllable wettability, adhesion or capillary transport on a solid surface. Thus, for more than 2000 years the sacred lotus has been a symbol of purity in Asian cultures; today it is also regarded as a symbol of high water-repellency and self-cleaning for technical applications and has almost revolutionised surface technology.
Mechanical containment and structural stability: biomechanics of the cuticular membrane
The mechanical and rheological properties of the outer plant envelope are of considerable importance for both the structural stability and maintenance of the physiological integrity of the plant. The view that the cuticle exhibits a mechanical function is not new, but quantitative analysis of cuticle mechanics is available only from the past decade onward, and a much clearer picture is now emerging of the mechanical performance of the biopolymer and its importance for mechanical containment.
The role of the cuticle as a structural stabilisation component
The plant surface membrane has to withstand mechanical stresses exerted either from inside or outside. The cuticle was described as the primary mechanical barrier against microorganisms and herbivores (Juniper 1991), while it has to be sufficiently stable not to suffer mechanical failure, yet flexible enough to accommodate externally applied forces on the foliage or shoot exerted by wind (Edelmann et al. 2005). Studies on life form, growth, and biomechanics of early terrestrial plants proposed that these depended mainly on a turgor-driven ‘hydroskeleton’ with the outer cuticular envelope and little internal stabilising turgor-driven, thin-walled cortical tissue (Edwards et al. 1996). Knowledge of the rheological and mechanical properties of the membrane could provide insights into adaptations of growth processes and the potential impact on plant organ integrity, as well as failure symptoms such as fruit cracking, e.g. in ripe tomato fruit (Matas et al. 2004a; Bargel and Neinhuis 2005; Edelmann et al. 2005). Moreover, transport properties of the biopolymer may be influenced by its mechanical properties, being affected by factors such as polymer density and the amount and type of fillers (Heredia 2003). Hence, the mechanical properties of the cuticle are of particular interest.
Only a few reports have addressed the biomechanical properties solely of the plant cuticle with an experimental approach so far. Lees (1984) reported that the mechanical damage to whole leaves of forage legumes was mainly determined by the strength of the cuticle and epidermal cells, and correlated mean thickness of epidermal cell wall plus cuticle with resistance to mechanical damage by sonication. Pesacreta et al. (1991) and Hasenstein et al. (1993) reported on the thigmonasticity of the staminal filaments of a thistle (Cirsium horridulum Michx.) and the involvement of a pre-stressed cuticle providing the force for the contraction of the filaments. The authors also pointed out that the cuticle is visco-elastic, i.e. shows a time-dependent elastic behaviour. The same material behaviour was reported for the surface of tomato fruit (Petracek and Bukovac 1995; Matas et al. 2004a) and leaves of several species (Edelmann et al. 2005). The biological function of a visco-elastic cuticle might be a reduced risk of mechanical failure owing to energy dissipation and reduction of stress peaks upon naturally occurring dynamic loads. Wiedemann and Neinhuis (1998) characterised the mechanical properties of isolated cuticular membranes and reported a modulus of elasticity — a measure of resistance against deformation — ranging between 0.6 and 1.3 GPa for leaf cuticles of four species with different leaf shapes. Hydration of the membranes caused a decrease in the modulus of ~35–50% and generally increased the total extensibility. These results indicate that the mechanical properties of the biopolymer are considerably influenced by hydration and, as a consequence, water acts as a plasticiser, increasing the extensibility at a given stress as well as the viscous component (Petracek and Bukovac 1995; Wiedemann and Neinhuis 1998; Edelmann et al. 2005). This was supported by nanomechanical studies that revealed a decrease of the surface elastic modulus of de-waxed tomato fruit cutin at saturation (Round et al. 2000). However, neither AFM indentation testing nor NMR relaxation measurements could resolve a plasticising effect of water in the native cuticle on a molecular scale (Stark and Tian 2006). A possible reason for this is that nanoindentation mainly measures surface properties at a high spatial resolution with only local impacts of the AFM tip, i.e. in the native tomato fruit cuticle the properties of the superficial wax layer are characterised. The cuticular wax itself is not strongly affected by hydration, as demonstrated by NMR relaxation measurements of cuticular wax in native cuticles (Stark and Tian 2006). Second, the intracuticular wax may restrict segmental motion of the methylene chains due to acyl-chain interactions and reduced free volume, leading to less efficient segmental dynamics for the chain methylenes of the native cuticle than the dewaxed cutin. The authors proposed that this might attenuate the plasticising effect of water in the native cuticle on a molecular level compared with the dewaxed samples. However, hydration clearly promotes softening of the cuticular membrane on the macroscopic scale (Wiedemann and Neinhuis 1998; Bargel and Neinhuis 2004; Bargel 2005). Temperature is an abiotic factor that also has a considerable effect on cuticular mechanics. Increased temperature regimes resulted in increased instantaneous extensibility and overall reduced stiffness and strength (Edelmann et al. 2005; Matas et al. 2005). These temperature-dependent effects can be as small as 1°C and are likely to be related to secondary phase transitions of the semicrystalline cutin polymer matrix.
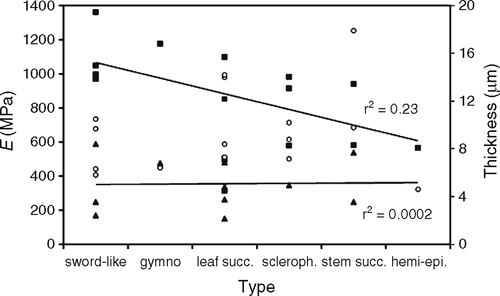
The mechanical properties of the biopolymer are remarkably high, e.g. in comparison with technical polymers such as polyethylene. Thus, the cuticle could be of importance for leaves as a reinforcing element. According to biophysical principles, the location at the outer perimeter of plant organs, a favourable secondary moment of area, and its relatively rigid appearance indeed indicate that the cuticle, depending on the species, could function as an external stiffening component that adds mechanical support for tissue integrity and impacts on morphogenetic processes. A further study of enzymatically isolated cuticular membranes (CMs) of leaves and stems of various species, different life forms and from different ecological habitats confirmed this view (Bargel 2005). The results also revealed considerable heterogeneity in the mechanical properties of the CM (Fig. 7). The highest stiffness was measured for cuticles of elongated sword-like leaves including Yucca and Clivia species, while the lowest stiffness was measured for the hemi-epiphyte V. planifolia. Data available indicate that smaller leaves from more mesic habitats generally show a trend to have a less stiff cuticle, although no significant correlation between tensile resistance and a particular leaf type or life form could be determined. Hydration led to strain-softening with a higher breaking strain for virtually all tested CMs, i.e. the cuticle was more elastic and capable of larger deformation. This behaviour is typical for ductile semi-crystalline polymers (Nielsen and Landel 1994), and is also a characteristic feature of visco-elastic or plastic polymers. Biologically, these features are prerequisites for the cuticle to remain intact and fully functional when subjected to mechanical stress.
|
Dynamics of the cuticle mechanics during fruit ripening
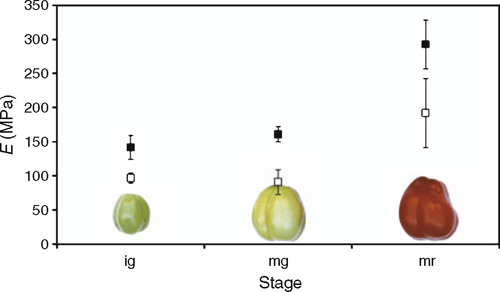
The mechanical properties of the cuticle, in particular that of soft fruits, are of commercial interest. The mechanical performance of a fruit surface affects fruit appearance, handling and storage, but also plays a prominent role in fruit cracking. It is therefore not surprising that several studies were carried out to analyse fruit cracking symptoms of important crops such as tomato or sweet cherry (Prunus avium L.) fruit (Bargel et al. 2004a, 2004b and references therein; Knoche et al. 2004). Only recently, Matas et al. (2004b) reported a correlation between CM thickness, mechanical properties and resistance to cracking of cherry tomato fruit. Regarding fruit growth regulation, it was proposed that the epidermis plays a major role in resistance to turgor-driven tomato fruit growth, since both stiffness and stress at rupture are high when growth ceases (Thompson 2001; Andrews et al. 2002a). Moreover, it was shown that the modulus of elasticity of both the exocarp and the isolated CM increased from immature green stage towards fully ripe stage, with the highest values for the epidermal strips, while the cuticle mirrored the changing mechanical properties of the epidermis to a large extent (Bargel and Neinhuis 2005) (Fig. 8). It follows that the cuticle becomes increasingly important for the structural integrity of the tomato fruit during ripening, and even seems to affect morphogenetic processes in addition to the epidermal cell wall. Stiffening of the epidermal cell walls was attributed to the increased peroxidase activity pattern in the epidermis starting at the mature green stage (Thompson et al. 1998; Brownleader et al. 1999; Andrews et al. 2002b). Whether extracellular peroxidase activity also affects stiffening of the cuticle, for example owing to cutin deposition (Ferrer et al. 1991), remains to be established. There is, however, evidence that the chemical composition of the tomato fruit cuticle changes dynamically during fruit maturation, and these changes are likely to account for the different mechanical properties of the CM from immature to fully ripe fruits. This issue is addressed further below.
|
The analysis of mutants, e.g. those with affected maturation, composition or permeability, might provide further insights in cuticle mechanics, structure and biosynthesis and might provide additional hints about its role in growth regulation of tomato fruit. One of the few examples existing so far is the analysis of the non-ripening tomato mutant, nor (Bargel and Neinhuis 2004). NOR was proposed to encode a higher regulatory developmental transcription factor (Giovannoni 2004), and nor fruits remain yellowish-orange and show reduced flesh softening at mature-ripe stage owing to failure of elevated ethylene biosynthesis at the onset of ripening. Compared with the CM of the wild type fruits, the membrane of the nor mutant had altered mechanical properties and was significantly less stiff and weaker at full maturity (Bargel and Neinhuis 2004). Since virtually no morphological changes were detectable, the altered mechanical performance of the mutant cuticle is most likely aroused by ethylene-mediated and ripening-related metabolic mechanisms. This may affect the cuticle and its composition directly, e.g. by chalcone synthase activity and flavonoid biosynthesis (Bargel et al. 2006), or indirectly, e.g. by signals deriving from cell wall metabolism that may trigger the biosynthesis of cuticular components. Several enzymatic systems fundamentally influence tomato fruit ripening. Among them are cell-wall-modifying enzymes (Fischer and Bennett 1991), reactive oxidative substances such as hydrogen peroxide (H2O2), and radicals and their counterparts, the antioxidant system, including peroxidases and superoxide dismutase (Jimenez et al. 2002; Mondal et al. 2004). Either these enzymes or their metabolic products might be part of such a signalling pathway. Since nor fruits remain firmer at maturity than the corresponding wild type fruits, cell-wall disassembly and probably the level of signalling molecules putatively involved in the biosynthesis of cuticular components seem to be reduced, resulting in the observed altered mechanical properties of the nor cuticle. Further studies in the area of signalling related to cell-wall disassembly or stress and biosynthesis of cuticular components may help to create a clearer picture of key regulatory factors of plant physiology and cuticle formation.
Relationship between chemical composition and mechanical properties of the cuticle
The non-homogeneity of the cuticle with respect to its mechanical properties and the dynamic changes during fruit growth indicate that the specific chemical composition determines the mechanics of the biomembrane. Particularly, cutin matrix composition and molecular architecture as well as composition and content of polysaccharide, waxes or phenolic compounds are of interest. More rigid cuticles could be classified by C16 cutin monomers, while more elastic cuticles correspond to mixed C16 / C18 cutin monomers (Holloway 1982; Marga et al. 2001). As an example, the compliant leaf cuticle of Hedera helix L. was classified as C18 cutin (Holloway and Brown 1981; Graca et al. 2002) and displayed a relatively low stiffness and high extensibility (Wiedemann and Neinhuis 1998; Edelmann et al. 2005). Baker et al. (1982) analysed the composition of the tomato fruit cuticle in relation to fruit maturation and found that the relative amount of tri-hydroxy C18 fatty acids decreased towards the fully ripe stage, while the amount of C16 monomers increased. Since stiffness of the tomato fruit cuticle increased and the extensibility decreased from immature to mature ripe stage, it seems that a large amount of hydroxyl groups enhances the hydrophilic character and the hydration state of the cutin matrix of immature fruit, while the opposite could be anticipated for the mature ripe fruit (Bargel and Neinhuis 2004, 2005). In addition to the biopolyester cutin, the second highly resistant residue, cutan, might be a target candidate to account for the rigid appearance of some cuticles. Whereas cutan was reported to be absent in the cuticular membrane of tomato fruit (Ramírez et al. 1992), leaves of A. americana, C. miniata, and Prunus laurocerasus have mixed cutin / cutan cuticles of up to 56% cutan (Riederer and Schönherr 1988; Villena et al. 1999). However, a direct comparison of the mechanical properties of the CM of these species did not reveal a clear picture, and more data on chemical composition with respect to cutan content is needed in order to relate the matrix composition to the mechanics of the cuticle (Bargel 2005).
The number and type of cross-linkages in the cutin matrix also affect the mechanical behaviour of the membrane. A common view is that 50% of the secondary hydroxyl groups in the matrix are esterified. Other linkages exist via hydrogen bonds at functional groups (Kolattukudy 2001; Heredia 2003). The actual number of cross-linkages may not only determine the rigidity of the membrane, but also the effect of water on the mechanics of the cuticle. On the molecular scale, water molecules bind to polar groups, e.g. hydroxyl groups of the cutin monomers, are incorporated in very narrow macromolecular holes in the cutin matrix, or embedded between carboxyl groups of the polyester and hydroxyl groups of polysaccharides (Marechal and Chamel 1996). The abundant polysaccharide fibres may contribute significantly to the water sorption in the plant cuticle, depending on the polysaccharide content (Chamel et al. 1991). Dominguez and Heredia (1999) even proposed that water could disrupt hydrogen-bond cross-links in the cutin matrix, and thus alter its rheological properties. Since the chemical composition appears to vary in a species-specific manner, the contribution of each component to the hydration state of the cuticle may be also subject of variation. Moreover, the biopolymer may have a so-far unknown complex cross-sectional hydration profile, and the increase of permeability upon cuticular hydration and the evidence of polar domains also imply a lateral heterogeneity of the membrane at least in some species (Edelmann et al. 2005; Schreiber 2005). Thus, data on the natural state of cuticular hydration are crucial to fully interpret the mechanical behaviour of the plant cuticle, but these are currently not available, and further studies and new approaches are necessary.
Cell-wall polysaccharides incorporated into the cutin matrix may not only affect hydration, but are likely to be responsible for the linear elastic behaviour and the high modulus of stiff cuticular membranes at dry condition. Dry crystalline cellulose is very stiff, and cutinised cellulose fibres could stiffen the membrane according to the theory of fibre-reinforced composite materials (Courtney 1990). Cellulose, xyloglucans and hemicellulose polysaccharides originating in the cell wall are integral constituents of virtually all cuticles studied so far (Jeffree 1996). However, data on the volume fraction, fibre length and fibre orientation of the polysaccharides in the cuticle are virtually unavailable. It is nevertheless very likely that the polysaccharide volume fraction is species-specific and hence, the contribution of the cellulosic material to the mechanical properties will vary from species to species. Marga et al. (2001) reported that the percentage of hemicellulose monosaccharides in the cutin of the stiff leaf cuticle of a thistle was ~70%, while it was ~14% in the more elastic cuticles of the filament. Different polysaccharide contents were proposed to explain even cultivar-specific mechanical properties of tomato fruit cuticle (Matas et al. 2004a). The quantitative analysis of the mechanical contribution of the cell wall fibres in the surface membrane provides an interesting issue for future studies. One step in this direction could be wide- and small-angle X-ray scattering (WAXS / SAXS) analysis of various cuticles currently under way (H Bargel unpubl. data).
In the mechanical context, the intracuticular waxes can be described as filler, i.e. reduction of free volume and segmental mobility within the cutin matrix (Wiedemann and Neinhuis 1998). The modulus of elasticity of de-waxed cuticles from Yucca leaves and tomato fruit in uni-axial testing decreased by ~30% compared with the control (H Bargel unpubl. data). However, the surface waxes may not have such a significant contribution to bulk mechanical properties of the cuticle. Round et al. (2000) reported a surface elastic modulus of the native cuticle five times smaller than the dewaxed cutin matrix. The authors used AFM nanoindentation tests that have a high spatial resolution, i.e. that in the case of the native cuticle, the much softer epicuticular wax layer seemed to shield the underlying matrix properties. The influence of cuticular waxes and their composition on the mechanics of the cuticle has not yet been thoroughly analysed and provides an interesting area for future research. Phenolic compounds, in particular the class of cinnamic acids (p-coumaric acid, ferulic acid) and flavonoids (naringenin, chalconaringenin), are cuticular constituents of several species, if not ubiquitous, providing important protection against damage from UV irradiation (Holton and Cornish 1995; Krauss et al. 1997; Fernandez et al. 1999). They are covalently bound to the cutin matrix, associated in molecular clusters, or part of the cuticular waxes (Hunt and Baker 1980; Laguna et al. 1999). An effect of the phenolics, in particular the flavonoids, on the mechanical properties of the plant cuticle has been proposed for the tomato fruit cuticle related to fruit ripening either based on chemical / structural and mechanical studies (Bargel and Neinhuis 2004; Bargel et al. 2006) or thermophysical studies (Matas et al. 2004c). The authors proposed that the amount of phenolic compounds is correlated with a more rigid cutin matrix at full maturity, either by restricted segmental mobility of cross-linked moieties, or by a reduced free volume in the network increasing the overall matrix rigidity. From an ecological point of view, this should also hold true in high-radiation environments, which would be consistent with the mechanical properties of cuticles, for example, from sclerophyllous species (Bargel 2005).
In conclusion, the analysis of the correlations between cuticular chemical composition, molecular structure, and the mechanical properties and performance of the cuticle provides fundamental insights into how plants maintain their structural and physiological integrity. It also identifies drawbacks of the outer protective envelope under the influence of biotic and abiotic environmental factors. These aspects provide a fruitful area for future studies, also having implications for applied horticulture and crop protection strategies.
Acknowledgments
Funding of the author’s research by several grants from the Deutsche Forschungsgemeinschaft (DFG), Bundesministerium für Bildung und Forschung (BMBF) and Deutsche Bundesstiftung Umwelt (DBU) is gratefully acknowledged. The authors thank Peter Wagner, Rhenotherm AG, for the picture of the metal foil with honey droplets.
Andrews J,
Adams SR,
Burton KS, Edmondson RN
(2002a) Partial purification of tomato fruit peroxidase and its effect on the mechanical properties of tomato fruit skin. Journal of Experimental Botany 53, 2393–2399.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Andrews J,
Adams SR,
Burton KS, Evered CE
(2002b) Subcellular localization of peroxidase in tomato fruit skin and the possible implications for the regulation of fruit growth. Journal of Experimental Botany 53, 2185–2191.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Baker EA
(1974) The influence of environment on leaf wax development in Brassica oleracea var. gemmifera. New Phytologist 73, 955–966.
| Crossref | GoogleScholarGoogle Scholar |

Bargel H, Neinhuis C
(2004) Altered tomato (Lycopersicon esculentum Mill.) fruit cuticle biomechanics of a pleiotropic non-ripening mutant. Journal of Plant Growth Regulation 23, 61–75.
| Crossref | GoogleScholarGoogle Scholar |

Bargel H, Neinhuis C
(2005) Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. Journal of Experimental Botany 56, 1049–1060.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bargel H,
Spatz HC,
Speck T, Neinhuis C
(2004b) Two-dimensional tension testing in plant biomechanics — sweet cherry fruit skin as a model system. Plant Biology 6, 432–439.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Barthlott W
(1981) Epidermal and seed surface characters of plants: systematic applicability and some evolutionary aspects. Nordic Journal of Botany 3, 345–355.

Barthlott W, Ehler N
(1977) Raster-Elektronenmikroskopie der Epidermisoberflächen von Spermatophyten. Tropische und Subtropische Pflanzenwelt 19, 367–467.

Barthlott W, Neinhuis C
(1997) Purity of the sacred lotus or escape from contamination in biological surfaces. Planta 202, 1–7.
| Crossref | GoogleScholarGoogle Scholar |

Barthlott W, Neinhuis C
(2001) The lotus effect: a self-cleaning surface based on a model taken from nature. Tekstil 50, 461–465.

Barthlott W,
Neinhuis C,
Cutler D,
Ditsch F,
Meusel I,
Theisen I, Wilhelmi H
(1998) Classification and terminology of plant epicuticular waxes. Botanical Journal of the Linnean Society 126, 237–260.
| Crossref | GoogleScholarGoogle Scholar |

Bateman RM,
Crane R,
DiMichele WA,
Kenrick PR,
Rowe NP,
Speck T, Stein WE
(1998) Early evolution of land plants: phylogeny, physiology, and ecology of the primary terrestrial radiation. Annual Reviews of Ecology and Systematics 29, 263–292.
| Crossref | GoogleScholarGoogle Scholar |

Baum BR,
Tulloch AP, Bailey LG
(1989) Epicuticular waxes of the genus Hordeum: a survey of their chemical composition and ultrastructure. Canadian Journal of Botany 67, 3219–3226.

Beattie GA, Lindow SE
(1995) The secret life of foliar bacterial pathogens on leaves. Annual Review of Phytopathology 33, 145–172.
| Crossref | GoogleScholarGoogle Scholar |

Bianchi G,
Lupotto E, Russo S
(1979) Composition of epicuticular wax of rice, Oryza sativa. Experientia 35, 1417.
| Crossref | GoogleScholarGoogle Scholar |

Bird SM, Gray JE
(2003) Signals from the cuticle affect epidermal cell differentiation. New Phytologist 157, 9–23.
| Crossref | GoogleScholarGoogle Scholar |

Bonaventure G,
Beisson F,
Ohlrogge J, Pollard M
(2004) Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. The Plant Journal 40, 920–930.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brownleader MD,
Jackson P,
Mobasheri A,
Pantelides AT,
Sumar S,
Trevan M, Dey PM
(1999) Molecular aspects of cell wall modifications during fruit ripening. Critical Reviews in Food Science and Nutrition 39, 149–164.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Buchholz S, Rabe JM
(1992) Molecular imaging of alkanol monolayers on graphite. Angewandte Chemie International Edition 31, 189–191.
| Crossref | GoogleScholarGoogle Scholar |

Burton Z, Bhushan B
(2005) Hydrophobicity, adhesion, and friction properties of nanopatterned polymers and scale dependence for micro- and nanoelectromechanical systems. Nano Letters 5, 1607–1613.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Canet D,
Rohr R,
Chamel A, Guillain F
(1996) Atomic force microscopy study of isolated ivy leaf cuticles observed directly and after embedding in Epon®. New Phytologist 134, 571–577.
| Crossref | GoogleScholarGoogle Scholar |

Carr DJ,
Carr SGM, Lenz JR
(1985) Oriented arrays of epicuticular wax plates in Eucalyptus species. Protoplasma 124, 205–212.
| Crossref | GoogleScholarGoogle Scholar |

Chambers TC,
Ritchie IM, Booth MA
(1976) Chemical models for plant wax morphogenesis. New Phytologist 77, 43–49.
| Crossref | GoogleScholarGoogle Scholar |

Chamel A,
Pineri M, Escoubes M
(1991) Quantitative determination of water sorption by plant cuticles. Plant, Cell & Environment 14, 87–95.
| Crossref | GoogleScholarGoogle Scholar |

Collinson ME,
Mösle B,
Finch P,
Scott AC, Wilson R
(1998) The preservation of plant cuticles in the fossil record: a chemical and microscopical investigation. Ancient Biomolecules 2, 251–265.

Conn KL, Tewari JP
(1989) Ultrastructure of epicuticular wax in canola. Zeitschrift für Naturforschung C 44, 705–711.

Croteau R, Kolattukudy PE
(1974) Biosynthesis of hydroxy fatty acid polymers. Enzymatic synthesis of cutin from monomers acids by cell-free preparations from the epidermis of Vicia faba leaves. Biochemistry 13, 3193–3202.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cyr DM,
Venkataraman B, Flynn GW
(1996) STM investigations of organic molecules physisorbed at the liquid–solid interface. Chemistry of Materials 8, 1600–1615.
| Crossref | GoogleScholarGoogle Scholar |

De Feyter S, De Schryver FC
(2005) Self-assembly at the liquid / solid interface: STM reveals. Journal of Physical Chemistry B. Materials, Surfaces, Interfaces, & Biophysical 109, 4290–4302.

Deshmukh AP,
Simpson AJ,
Hadad CM, Hatcher PG
(2005) Insights into the structure of cutin and cutan from Agave americana leaf cuticle using HRMAS NMR spectroscopy. Organic Geochemistry 36, 1072–1085.
| Crossref | GoogleScholarGoogle Scholar |

Dominguez E, Heredia A
(1999) Water hydration in cutinized cell walls: a physico-chemical analysis. Biochimica et Biophysica Acta 1426, 168–176.
| PubMed |

Dorset DL
(2002) From waxes to polymers — crystallography of polydisperse chain assemblies. Structural Chemistry 13, 329–337.
| Crossref | GoogleScholarGoogle Scholar |

Edelmann HG,
Neinhuis C, Bargel H
(2005) Influence of hydration and temperature on the rheological properties of plant cuticles and their impact on plant organ integrity. Journal of Plant Growth Regulation 24, 116–126.
| Crossref | GoogleScholarGoogle Scholar |

Engel H
(1939) Das Verhalten der Blätter bei Benetzung mit Wasser. Jahrbücher für wissenschaftliche Botanik 48, 816–861.

Ensikat HJ,
Neinhuis C, Barthlott W
(2000) Direct access to plant epicuticular wax crystals by a new mechanical isolation method. International Journal of Plant Sciences 161, 143–148.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ensikat HJ,
Boese M,
Mader W,
Barthlott W, Koch K
(2006) Crystallinity of plant epicuticular waxes: electron and X-ray diffraction studies. Chemistry and Physics of Lipids in press ,
| PubMed |

Erbil HY,
Demirel AL,
Avci Y, Mert O
(2003) Transformation of a simple plastic into a superhydrophobic surface. Science 299, 1377–1380.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Espelie KE,
Dean BB, Kolattukudy PE
(1979) Composition of lipid-derived polymers from different anatomical regions of several plant species. Plant Physiology 64, 1089–1093.
| PubMed |

Feng L,
Li S,
Li Y,
Li H,
Zhang L,
Zhai J,
Song Y,
Liu B,
Jiang L, Zhu D
(2002) Super-hydrophobic surfaces: from natural to artificial. Advanced Materials 14, 1857–1860.
| Crossref | GoogleScholarGoogle Scholar |

Fernandez S,
Osorio S, Heredia A
(1999) Monitoring and visualising plant cuticles by confocal laser scanning microscopy. Plant Physiology and Biochemistry 37, 789–794.
| Crossref | GoogleScholarGoogle Scholar |

Ferrer MA,
Munoz R, Barcelo AR
(1991) A biochemical and cytochemical study of the cuticle-associated peroxidases in Lupinus. Annals of Botany 67, 561–568.

Fischer RL, Bennett AB
(1991) Role of cell wall hydrolases in fruit ripening. Annual Review of Plant Physiology and Plant Molecular Biology 42, 675–703.
| Crossref | GoogleScholarGoogle Scholar |

Franke R,
Briesen I,
Wojciechowski T,
Faust A,
Yephremov A,
Nawrath C, Schreiber L
(2005) Apoplastic polyesters in Arabidopsis surface tissues — a typical suberin and a particular cutin. Phytochemistry 66, 2643–2658.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fürstner R,
Neinhuis C, Barthlott W
(2000) The lotus effect: self-purification of microstructured surfaces. Nachrichten aus der Chemie 48, 24–28.

Fürstner R,
Barthlott W,
Neinhuis C, Walzel P
(2005) Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 21, 956–961.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gesquière A,
Abdel-Mottaleb MMS,
De Feyter S,
de Schryver FC,
Sieffert M,
Müllen K,
Calderone A,
Lazzaroni R, Brédas J-L
(2000) Dynamics in physisorbed monolayers of 5-alkoxy-isophrhalic derivatives at the liquid/solid interface investigated by scanning tunneling microscopy. Chemistry–A European Journal 6, 3739–3746.
| Crossref | GoogleScholarGoogle Scholar |

Giovannoni JJ
(2004) Genetic regulation of fruit development and ripening. The Plant Cell 16, S170–S180.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gleiche M,
Chi LF, Fuchs H
(2000) Nanoscopic channel lattices with controlled anisotropic wetting. Nature 403, 173–175.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gomez-Campo C,
Tortosa ME,
Tewari I, Tewari JP
(1999) Epicuticular wax columns in cultivated Brassica species and in their close wild relatives. Annals of Botany 83, 515–519.
| Crossref | GoogleScholarGoogle Scholar |

Graca J,
Schreiber L,
Rodrigues J, Pereira H
(2002) Glycerol and glyceryl esters of omega-hydroxyacids in cutins. Phytochemistry 61, 205–215.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Guhling O,
Kinzler C,
Dreyer M,
Bringmann G, Jetter R
(2005) Surface composition of myrmecophilic plants: cuticular wax and glandular trichomes on leaves of Macaranga tanarius. Journal of Chemical Ecology 31, 2323–2341.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gülz PG
(1994) Epicuticular leaf waxes in the evolution of the plant kingdom. Journal of Plant Physiology 143, 453–464.

Günther I, Wortmann GB
(1966) Dust on the surface of leaves. Journal of Ultrastructure Research 15, 522–527.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Guo Z,
Zhou F,
Hao J, Liu W
(2005) Stable biomimetic super-hydrophobic engineering materials. Journal of the American Chemical Society 127, 15 670–15 671.

Haas K,
Brune T, Rucker E
(2001) Epicuticular wax crystalloids in rice and sugar cane leaves are reinforced by polymeric aldehydes. Journal of Applied Botany–Angewandte Botanik 75, 178–187.

Hallam ND, Chambers TC
(1970) The leaf waxes of the genus Eucalyptus L’Heritier. Australian Journal of Botany 18, 335–386.
| Crossref | GoogleScholarGoogle Scholar |

Hasenstein KH,
Pescareta TC, Sullivan VI
(1993) Thigmonasticity of thistle staminal filaments II. Mechano-elastic properties. Planta 190, 58–64.
| Crossref | GoogleScholarGoogle Scholar |

Heredia A
(2003) Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochimica et Biophysica Acta 1620, 1–7.
| PubMed |

Hoh JH, Hansma PK
(1992) Atomic force microscopy for high-resolution imaging in cell biology. Trends in Cell Biology 2, 208–213.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Holloway PJ
(1969) The effects of superficial wax on leaf wettability. The Annals of Applied Biology 63, 145–153.

Holloway PJ
(1970) Surface factors affecting the wetting of leaves. Pesticide Science 1, 156–163.

Holloway PJ, Brown GA
(1981) Ultrahistochemical detection of epoxides in plant cuticular membranes. Journal of Experimental Botany 32, 1051–1066.

Holloway PJ,
Jeffree CE, Baker EA
(1976) Structural determination of secondary alcohols from plant epicuticular waxes. Phytochemistry 15, 1768–1770.
| Crossref | GoogleScholarGoogle Scholar |

Holton TA, Cornish EC
(1995) Genetics and biochemistry of anthocyanin biosynthesis. The Plant Cell 7, 1071–1083.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hunt GM, Baker EA
(1980) Phenolic constituents of tomato fruit cuticles. Phytochemistry 19, 1415–1419.

Jeffree CE,
Baker EA, Holloway PJ
(1975) Ultrastructure and recrystallization of plant epicuticular waxes. New Phytologist 75, 539–549.
| Crossref | GoogleScholarGoogle Scholar |

Jenks MA, Ashworth EN
(1999) Plant epicuticular waxes: function, production and genetics. Horticultural Reviews 23, 1–68.

Jetter R, Riederer M
(1994) Epicuticular crystals of nonacosan-10-ol: in-vitro reconstitution and factors influencing crystal habits. Planta 195, 257–270.
| Crossref | GoogleScholarGoogle Scholar |

Jetter R, Riederer M
(1995) In vitro reconstitution of epicuticular wax crystals: formation of tubular aggregates by long chain secondary alkanediols. Botanica Acta 108, 111–120.

Jetter R, Schäffer S
(2001) Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiology 126, 1725–1737.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jetter R,
Schäffer S, Riederer M
(2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant, Cell & Environment 23, 619–628.
| Crossref | GoogleScholarGoogle Scholar |

Jiang L,
Zhao Y, Zhai J
(2004) A lotus-leaf-like superhydrophobic surface: a porous microsphere / nanofiber composite film prepared by electrohydrodynamics. Angewandte Chemie International Edition 43, 4438–4441.

Jimenez A,
Creissen G,
Kular B,
Firmin J,
Robinson S,
Verhoeyen M, Mullineaux P
(2002) Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 214, 751–758.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Knoche M,
Beyer M,
Peschel S,
Oparlakov B, Bukovac MJ
(2004) Changes in strain and deposition of cuticle in developing sweet cherry fruit. Physiologia Plantarum 120, 667–677.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Koch K,
Neinhuis C,
Ensikat HJ, Barthlott W
(2004) Self assembly of epicuticular waxes on living plant surfaces imaged by atomic force microscopy (AFM). Journal of Experimental Botany 55, 711–718.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Koch K,
Barthlott W,
Koch S,
Hommes A,
Wandelt K,
Mamdouh W,
De-Feyter S, Broekmann P
(2006a) Structural analysis of wheat wax (Triticum aestivum cv ‘Naturastar’ L.): from the molecular level to three dimensional crystals. Planta 223, 258–270.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Koch K,
Hartmann KD,
Schreiber L,
Barthlott W, Neinhuis C
(2006b) Influence of air humidity on epicuticular wax chemical composition, morphology and wettability of leaf surfaces. Environmental and Experimental Botany 56, 1–9.
| Crossref | GoogleScholarGoogle Scholar |

Kolattukudy PE
(1980) Biopolyester membranes of plants: cutin and suberin. Science 208, 990–1000.

Krauss P,
Markstädter C, Riederer M
(1997) Attenuation of UV radiation by plant cuticles from woody species. Plant, Cell & Environment 20, 1079–1085.
| Crossref | GoogleScholarGoogle Scholar |

Kreger DR, Schamhart C
(1956) On the long crystal spacings in wax esters and their value in micro-analysis of plant cuticle waxes. Biochimica et Biophysica Acta 19, 22–44.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kunst L, Samuels AL
(2003) Biosynthesis and secretion of plant cuticular wax. Progress in Lipid Research 42, 51–80.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kurdyukov S,
Faust A,
Nawrath C,
Bär S, Voisin D , et al.
(2006) The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. The Plant Cell , 321–339.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lafuma A, Quéré D
(2003) Superhydrophobic states. Nature Materials 2, 457–460.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Laguna L,
Casado CG, Heredia A
(1999) Flavonoid biosynthesis in tomato fruit cuticles after in vivo incorporation of H-3-phenylalanine precursor. Physiologia Plantarum 105, 491–498.
| Crossref | GoogleScholarGoogle Scholar |

Le Poulennec C,
Cousty J,
Xie ZX, Mioskowski C
(2000) Self-organisation of physisorbed secondary alcohol molecules on a graphite surface. Surface Science 448, 93–100.
| Crossref | GoogleScholarGoogle Scholar |

Lees GL
(1984) Cuticle and cell wall thickness: relation to mechanical strength of whole leaves and isolated cells from some forage legumes. Crop Science 24, 1077–1081.

Lownds NK, Bukovac MJ
(1988) Studies on octylphenoxy surfactants: V. toxicity to cowpea leaves and effects or spray application parameters. Journal of the American Society for Horticultural Science 113, 205–210.

Marechal Y, Chamel A
(1996) Water in a biomembrane by infrared spectrometry. Journal of Physical Chemistry 100, 8551–8555.
| Crossref | GoogleScholarGoogle Scholar |

Marga F,
Pesacreta TC, Hasenstein KH
(2001) Biochemical analysis of elastic and rigid cuticles of Cirsium horridulum Michx. Planta 213, 841–848.
| PubMed |

Marmur A
(2003) Wetting on hydrophobic rough surfaces: to be heterogeneous or not to be? Langmuir 19, 8343–8348.
| Crossref | GoogleScholarGoogle Scholar |

Matas A, Heredia A
(1999) Molecular dynamics modellization and simulation of water diffusion through plant cutin. Zeitschrift für Naturforschung C 54, 896–902.

Matas AJ,
Sanz MJ, Heredia A
(2003) Studies on the structure of the plant wax nonacosan-10-ol, the main component of epicuticular wax conifers. International Journal of Biological Macromolecules 33, 31–35.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Matas AJ,
Cobb ED,
Bartsch JA,
Paolillo DJ, Niklas KJ
(2004a) Biomechanics and anatomy of Lycopersicon esculentum fruit peels and enzyme-treated samples. American Journal of Botany 91, 352–360.

Matas AJ,
Cobb ED,
Paolillo DJ, Niklas KJ
(2004b) Crack resistance in cherry tomato fruit correlates with cuticular membrane thickness. HortScience 36, 1354–1358.

Matas AJ,
Cuartero J, Heredia A
(2004c) Phase transitions in the biopolyester cutin isolated from tomato fruit cuticles. Thermochimica Acta 409, 165–168.
| Crossref | GoogleScholarGoogle Scholar |

Matas AJ,
Lópes-Casado G,
Cuartero J, Heredia A
(2005) Relative humidity and temperature modify the mechanical properties of isolated tomato fruit cuticles. American Journal of Botany 92, 462–468.

Meusel I,
Leistner E, Barthlott W
(1994) Chemistry and micromorphology of compound epicuticular wax crystalloids (Strelitzia type). Plant Systematics and Evolution 193, 115–123.
| Crossref | GoogleScholarGoogle Scholar |

Meusel I,
Neinhuis C,
Markstädter C, Barthlott W
(1999) Ultrastructure, chemical composition and recrystallisation of epicuticular waxes: transversely ridged rodlets. Canadian Journal of Botany 77, 706–720.
| Crossref | GoogleScholarGoogle Scholar |

Meusel I,
Barthlott W,
Kutzke H, Barbier B
(2000a) Crystallographic studies of plant waxes. Powder Diffraction 15, 123–129.

Meusel I,
Neinhuis C,
Markstadter C, Barthlott W
(2000b) Chemical composition and recrystallization of epicuticular waxes: coiled rodlets and tubules. Plant Biology 2, 462–470.
| Crossref | GoogleScholarGoogle Scholar |

Mo H,
Trogisch S,
Taub H,
Ehrlich SN,
Volkmann UG,
Hansen FY, Pino M
(2004) Studies on the structure and growth mode of dotriacontane films by synchrotron x-ray scattering and molecular dynamics simulations. Journal of Physics Condensed Matter 16, S2905–S2910.
| Crossref | GoogleScholarGoogle Scholar |

Mondal K,
Sharma NS,
Malhotra SP,
Dhawan K, Singh R
(2004) Antioxidant systems in ripening tomato fruits. Biologia Plantarum 48, 49–53.
| Crossref | GoogleScholarGoogle Scholar |

Mösle B,
Finch P,
Collinson ME, Scott AC
(1997) Comparison of modern and fossil plant cuticles by selective chemical extraction monitored by flash pyrolysis–gas chromatography–mass spectroscopy and electron microscopy. Journal of Analytical and Applied Pyrolysis 40–41, 585–597.
| Crossref | GoogleScholarGoogle Scholar |

Neinhuis C, Barthlott W
(1997) Characterisation and distribution of water-repellent, self-cleaning plant surfaces. Annals of Botany 79, 667–677.
| Crossref | GoogleScholarGoogle Scholar |

Neinhuis C, Jetter R
(1995) Ultrastructure and chemistry of epicuticular wax crystals in Polytrichales sporophytes. Journal of Bryology 18, 399–406.

Neinhuis C,
Koch K, Barthlott W
(2001) Movement and regeneration of epicuticular waxes through plant cuticles. Planta 213, 427–434.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Noga G,
Knoche M,
Wolter M, Barthlott W
(1987) Changes in leaf micromorphology induced by surfactant application. Angewandte Botanik 61, 521–528.

Nosonovsky M, Bhushan B
(2005) Roughness optimization for biomimetic superhydrophobic surfaces. Microsystem Technologies 11, 535–549.
| Crossref | GoogleScholarGoogle Scholar |

Otten A, Herminghaus S
(2004) How plants keep dry: a physicist’s point of view. Langmuir 20, 2405–2408.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Patankar NA
(2004) Mimicking the lotus-effect: influence of double roughness structures and slender pillars. Langmuir 20, 8209–8213.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pesacreta TC,
Sullivan VI,
Hasenstein KH, Durand JM
(1991) Thigmonasticity of thistle staminal filaments I. Involvement of a contractile cuticle. Protoplasma 163, 174–180.
| Crossref | GoogleScholarGoogle Scholar |

Petracek PD, Bukovac MJ
(1995) Rheological properties of enzymatically isolated tomato fruit cuticle. Plant Physiology 109, 675–679.
| PubMed |

Popp C,
Burghardt M,
Friedmann A, Riederer M
(2005) Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticular membranes: permeation of water and uncharged organic compounds. Journal of Experimental Botany 56, 2797–2806.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Post-Beittenmiller D
(1996) Biochemistry and molecular biology of wax production in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 405–430.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ramírez FJ,
Luque P,
Heredia A, Bukovac MJ
(1992) Fourier transform IR study of enzymatically isolated tomato fruit cuticular membrane. Biopolymers 32, 1425–1429.
| Crossref | GoogleScholarGoogle Scholar |

Reina JJ, Heredia A
(2001) Plant cutin biosynthesis: the involvement of a new acyltransferase. Trends in Plant Science 6, 296.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Reynhardt EC, Riederer M
(1994) Structures and molecular dynamics of plant waxes. II. Cuticular waxes from leaves of Fagus sylvatica L. and Hordeum vulgare L. European Biophysics Journal 23, 59–70.
| Crossref | GoogleScholarGoogle Scholar |

Richardson A,
Franke R,
Kerstiens G,
Jarvis M,
Schreiber L, Fricke W
(2005) Cuticular wax deposition in growing barley (Hordeum vulgare) leaves commences in relation to the point of emergence of epidermal cells from the sheaths of older leaves. Planta 222, 472–483.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Riedel M,
Eichner A, Jetter R
(2003) Slippery surfaces of carnivorous plants: composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta 218, 87–97.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Riederer M, Schönherr J
(1988) Development of plant cuticles: fine structure and cutin composition of Clivia miniata Reg. leaves. Planta 174, 127–138.
| Crossref | GoogleScholarGoogle Scholar |

Riederer M, Schreiber L
(2001) Protecting against water loss: analysis of the barrier properties of plant cuticles. Journal of Experimental Botany 52, 2023–2032.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Round AN,
Yan B,
Dang S,
Estephan R,
Stark RE, Batteas JD
(2000) The influence of water on the nanomechanical behavior of the plant biopolyester cutin studies by AFM and solid-state NMR. Biophysical Journal 79, 2761–2767.
| PubMed |

Schouten S,
Moerkerken P,
Gelin F,
Baas M,
de Leeuw JW, Damste JSS
(1998) Structural characterization of aliphatic, non-hydrolyzable biopolymers in freshwater algae and a leaf cuticle using ruthenium tetroxide degradation. Phytochemistry 49, 987–993.
| Crossref | GoogleScholarGoogle Scholar |

Schreiber L
(2005) Polar paths of diffusion across plant cuticles: new evidence for an old hypothesis. Annals of Botany 95, 1069–1073.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schreiber L,
Schorn K, Heimburg T
(1997) H-2 NMR study of cuticular wax isolated from Hordeum vulgare L. leaves: identification of amorphous and crystalline wax phases. European Biophysics Journal with Biophysics Letters 26, 371–380.

Shepherd T,
Robertson GW,
Griffiths DW,
Birch ANE, Duncan G
(1995) Effects of environment on the composition of epicuticular wax from kale and swede. Phytochemistry 40, 407–417.
| Crossref | GoogleScholarGoogle Scholar |

Stark RE,
Yan B,
Ray AK,
Chen Z,
Fang X, Garbow JR
(2000) NMR studies of structure and dynamics in fruit cuticle polyesters. Solid State Nuclear Magnetic Resonance 16, 37–45.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Stevens JF,
Hart HT,
Pouw AJA,
Bolck A, Zwaving JH
(1994) Epicuticular waxes of Sedum series Rupestria. Phytochemistry 36, 341–348.
| Crossref | GoogleScholarGoogle Scholar |

Suh MC,
Samuels AL,
Jetter R,
Kunst L,
Pollard M,
Ohlrogge J, Beisson F
(2005) Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiology 139, 1649–1665.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sun M,
Luo C,
Xu L,
Ji H,
Ouyang Q,
Yu D, Chen Y
(2005) Artificial lotus leaf by nanocasting. Langmuir 21, 8978–8981.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Thair BW, Lister GR
(1975) The distribution and fine structure of the epicuticular leaf wax of Pseudotsuga menziezii. Canadian Journal of Botany 53, 1063–1071.

Thompson DS
(2001) Extensiometric determination of the rheological properties of the epidermis of growing tomato fruit. Journal of Experimental Botany 52, 1291–1301.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Thompson DS,
Davies WJ, Ho LC
(1998) Regulation of tomato fruit growth by epidermal cell wall enzymes. Plant, Cell & Environment 21, 589–599.
| Crossref | GoogleScholarGoogle Scholar |

Tulloch AP
(1973) Composition of leaf surface waxes of Triticum species: variation with age and tissue. Phytochemistry 12, 2225–2232.
| Crossref | GoogleScholarGoogle Scholar |

Tulloch AP, Hoffman LL
(1974) Epicuticular waxes of Secale cereale and Triticale hexaploid leaves. Phytochemistry 13, 2535–2540.
| Crossref | GoogleScholarGoogle Scholar |

Tulloch AP,
Baum BR, Hoffman LL
(1980) A survey of epicuticular waxes among genera of Triticeae. 2. Chemistry. Canadian Journal of Botany 58, 2602–2615.

Turunen M, Huttunen S
(1990) A review of the response of epicuticular waxes of conifer needles to air pollution. Journal of Environmental Quality 19, 35–45.

Villena JF,
Dominguez E,
Stewart D, Heredia A
(1999) Characterization and biosynthesis of non-degradable polymers in plant cuticles. Planta 208, 181–187.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Villena JF,
Dominguez E, Heredia A
(2000) Monitoring biopolymers present in plant cuticles by FT–IR spectroscopy. Journal of Plant Physiology 156, 419–422.

von Wettstein-Knowles P
(1974) Ultrastructure and origin of epicuticular wax tubes. Journal of Ultrastructure Research 46, 483–498.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wagner T,
Neinhuis C, Barthlott W
(1996) Wettability and contaminability of insect wings as a function of their surface sculpture. Acta Zoologica 77, 213–225.

Wagner P,
Fürstner R,
Barthlott W, Neinhuis C
(2003) Quantitative assessment to the structural basis of water repellency in natural and technical surfaces. Journal of Experimental Botany 54, 1295–1303.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Whitecross MI, Armstrong DJ
(1972) Environmental effects on epicuticular waxes of Brassica napus L. Australian Journal of Botany 20, 87–95.
| Crossref | GoogleScholarGoogle Scholar |

Whitesides GM,
Mathias JP, Seto CT
(1991) Molecular self-assembly and nanochemistry — a chemical strategy for the synthesis of nanostructures. Science 254, 1312–1319.
| PubMed |

Wiedemann P, Neinhuis C
(1998) Biomechanics of isolated plant cuticles. Botanica Acta 111, 28–34.

Wollenweber E, Schneider H
(2000) Lipophilic exudates of Pteridaceae — chemistry and chemotaxonomy. Biochemical Systematics and Ecology 28, 751–777.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wolter M,
Barthlott W,
Knoche M, Noga GJ
(1988) Concentration effects and regeneration of epicuticular waxes after treatment with Triton X-100 surfactant. Angewandte Botanik 62, 53–62.

Xiao FM,
Goodwin SM,
Xiao YM,
Sun ZY,
Baker D,
Tang XY,
Jenks MA, Zhou JM
(2004) Arabidopsis CYP86A2 represses Pseudomonas syringae type III genes and is required for cuticle development. EMBO Journal 23, 2903–2913.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Xie Q,
Xu J,
Feng L,
Jiang L,
Tang WX,
Luo X, Han CC
(2004) Facile creation of a super-amphiphobic coating surface with bionic microstructure. Advanced Materials 16, 302–305.
| Crossref | GoogleScholarGoogle Scholar |

Yephremov A, Schreiber L
(2005) The dark side of the cell wall: molecular genetics of plant cuticle. Plant Biosystems 139, 74–79.

Zhang S
(2003) Fabrication of novel biomaterials through molecular self-assembly. Nature Biotechnology 21, 1171–1178.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



