Flooding tolerance: suites of plant traits in variable environments
T. D. Colmer A C and L. A. C. J. Voesenek BA School of Plant Biology, Faculty of Natural and Agricultural Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
B Plant Ecophysiology, Department of Biology, Utrecht University, Padualaan 8, 3584 CH, Utrecht, The Netherlands.
C Corresponding author. Email: tdcolmer@cyllene.uwa.edu.au
This paper is part of an ongoing series: ‘The Evolution of Plant Functions’.
Functional Plant Biology 36(8) 665-681 https://doi.org/10.1071/FP09144
Submitted: 5 June 2009 Accepted: 15 June 2009 Published: 23 July 2009
Abstract
Flooding regimes of different depths and durations impose selection pressures for various traits in terrestrial wetland plants. Suites of adaptive traits for different flooding stresses, such as soil waterlogging (short or long duration) and full submergence (short or long duration – shallow or deep), are reviewed. Synergies occur amongst traits for improved internal aeration, and those for anoxia tolerance and recovery, both for roots during soil waterlogging and shoots during submergence. Submergence tolerance of terrestrial species has recently been classified as either the Low Oxygen Quiescence Syndrome (LOQS) or the Low Oxygen Escape Syndrome (LOES), with advantages, respectively, in short duration or long duration (shallow) flood-prone environments. A major feature of species with the LOQS is that shoots do not elongate upon submergence, whereas those with the LOES show rapid shoot extension. In addition, plants faced with long duration deep submergence can demonstrate aspects of both syndromes; shoots do not elongate, but these are not quiescent, as new aquatic-type leaves are formed. Enhanced entries of O2 and CO2 from floodwaters into acclimated leaves, minimises O2 deprivation and improves underwater photosynthesis, respectively. Evolution of ‘suites of traits’ are evident in wild wetland species and in rice, adapted to particular flooding regimes.
Additional keywords: abiotic stress, adventitious roots, aerenchyma, anoxia tolerance, emergent properties, fermentation, hyponasty, reactive oxygen species, rice, shoot elongation, submergence, synergistic traits, oxidative stress, underwater photosynthesis, waterlogging, wetland plants.
Introduction
Flooding is an environmental stress in many natural and man-made ecosystems worldwide. Anthropogenically-induced global climate change is expected to increase the frequency and severity of flooding events (Arnell and Liu 2001). Most crops and wild plants will not tolerate these floods as the poor gas exchange under water disrupts their energy and carbohydrate economies (Voesenek et al. 2006; Bailey-Serres and Voesenek 2008). Many plants suffer severe growth reduction, or even death, when only the root system is surrounded by excess water (i.e. soil waterlogging) (Armstrong 1979; Jackson and Drew 1984). When overland floods occur, and shoots are also either partially or even fully submerged, plants must also cope with the additional adverse effects directly on shoots (Voesenek et al. 2006). Knowledge of mechanisms of flooding tolerance in plants will underpin both increases in crop production (Xu et al. 2006; Singh et al. 2009) and understanding of the distribution of wild species (Blom and Voesenek 1996; Silvertown et al. 1999) in flood-prone environments.
Recent research has improved understanding of mechanisms of flooding tolerance in plants, as dependent upon contrasting flooding regimes in various habitats. Temporary floods differ in seasonal timing, and with much variation in durations, depths and frequencies (Vervuren et al. 2003). Disparity in these factors results in a multi-dimensional continuum of flooding regimes in environments inhabited by terrestrial plants. This spectrum of environmental conditions determines species distributions and abundances in flood-prone areas (Armstrong et al. 1985; Silvertown et al. 1999; Voesenek et al. 2004). Such diversity in environments would, as hypothesised by Darwin (1859), impose specific selection pressures for various traits associated with flooding tolerance, based on the assumption that trait benefits outweigh costs (Voesenek et al. 2004).
Contrasting flooding regimes are numerous, so only selected examples can be given here. In rice (Oryza sativa L.)-growing areas of Asia, hydrological regimes can be broadly classified as deepwater, paddy, rain-fed lowland, and rain-fed upland; these differ in depths (several metres above ground to absence) and durations of overland flooding and soil waterlogging (Grist 1986). In river flood-plains, water levels are highly dynamic, which together with geomorphological characteristics results in deep, short-term floods at river shores, up to shallow floods that last for several months at some locations on the plains (e.g. Rhine river, Blom and Voesenek 1996). In coastal marshes, tides flood some areas daily and others only monthly or seasonally, with depths varying depending on elevation in the marsh (Armstrong et al. 1985; Silvestri et al. 2005). A final example is the spectacular seasonal flooding in the Amazon Floodplain in which floodwaters show a mean amplitude of 10 m (range 6–14 m) (Parolin 2009). These floodwaters are also very turbid so that light penetration is poor (Piedade et al. 1991), yet several species of trees can survive prolonged submergence (Parolin 2009) and some herbaceous species with leaves that remain in contact with air can be very productive (e.g. 8 t ha−1 month−1 for the emergent C4 grass Echinochloa polystachya (Kunth) A.S.Hitchc., Piedade et al. 1991).
In this review, we first summarise the adverse conditions resulting from soil waterlogging and from floods causing submergence, and discuss the various adaptations in plants to these conditions. The main purpose of our review is to consider the prevailing plant traits associated with flooding tolerance in contrasting types of flood-prone environments, as defined by depths and durations of waterlogging and submergence. Moreover, we highlight that these traits co-occur and act synergistically to enhance fitness. Such suites of traits can be considered as an ‘emergent property’ (Bhalla and Iyangar 1999) at the organismal level. Our focus is mainly on terrestrial and semi-aquatic plants that occasionally are exposed to higher water levels.
The problem: stresses caused by waterlogging and submergence
During periods of excess rainfall, soil waterlogging can occur so that roots in soil become surrounded by water. During flooding events, the shoots can also be partially or completely submerged. A primary effect of this altered condition is the 104-fold slower diffusion of gases dissolved in water, as compared with in air. Gas diffusion is important for plants; CO2 influx to chloroplasts is required for photosynthesis and rapid diffusion of O2 to mitochondria enables respiration. Furthermore, regulation of endogenous concentrations of the volatile hormone ethylene depends strongly on rates of outward diffusion. Fast diffusion of these critical gases is severely hampered when the gas-filled soil pores (waterlogging), or complete shoot space (submergence) become water-filled. The slow outward diffusion in water-saturated soils also results in accumulation of CO2 and methane (Ponnamperuma 1984). As a consequence, levels of O2 typically decline, whereas ethylene increases, in submerged tissues (i.e. roots in waterlogged soil; shoots and roots when fully submerged). In the case of CO2, levels can increase in roots in waterlogged soils (Greenway et al. 2006), whereas when the shoot is also fully submerged, slow entry of CO2 into leaves typically limits photosynthesis (Mommer and Visser 2005).
In addition to the greatly altered availability of gases, electrochemical changes also occur in water-saturated soils, induced by microorganisms that use oxidised chemicals as electron acceptors (Ponnamperuma 1984; Laanbroek 1990). The soil concentrations of certain potentially-toxic compounds, such as the reduced forms of manganese (Mn2+), iron (Fe2+) and H2S and S2–, often increase. These compounds can enter roots, and move to shoots, with adverse effects on both organs (Baba et al. 1965; Armstrong and Armstrong 2005). Finally, when completely submerged, an additional stress can be the very low light levels, owing to the often turbid nature of floodwaters. Low light, together with low CO2 availability, greatly hampers photosynthesis under water (Mommer and Visser 2005).
The flooding-induced changes of the plant environment from air to water, results in several major problems inside the plant body:
-
‘Energy crisis’. The majority of ATP for cellular metabolism in plants is generated by oxidative phosphorylation in respiration. This process requires O2 as the terminal acceptor of electrons. Although the Km of cytochrome oxidase is low (0.013% O2), indicating a very high affinity to scavenge O2, respiration is inhibited in cells, tissues and organs at much higher external O2 concentrations, owing to diffusion limitations through liquid-phase boundary layers and through the tissues themselves (Berry and Norris 1949; Armstrong et al. 2009). When cells become anoxic, oxidative phosphorylation ceases, but some ATP can be produced in glycolysis, provided that NAD+ is regenerated, for example via conversion of pyruvate to ethanol. Despite this ATP generation, the ‘energy crisis’ ensues since the production of ATP by glycolysis is much less than compared with oxidative phosphorylation (Gibbs and Greenway 2003). Low ATP availability leads to cellular damage, owing to a deterioration of cellular components, such as membranes (Gibbs and Greenway 2003), and/or cytoplasmic acidosis in sensitive species (Xia and Roberts 1996). Such damage can, however, be avoided (or substantially delayed) in anoxia-tolerant plants (e.g. rice coleoptiles, Kulichikhin et al. 2009).
-
‘Carbohydrate crisis’. Soluble sugars and the mobilisation of starch in plants are of importance to sustain glycolysis and thus ATP generation, via either respiration or fermentation, depending on available O2. Due to light and CO2 limitations during submergence, the sugar and starch reserves are not replenished. Ultimately, exhaustion of sugars will result in cell and organ death (Bailey-Serres and Voesenek 2008).
-
Toxicities. Reduced soil components such as Mn2+, Fe2+ and S2– can accumulate to toxic levels in root tissues (Jackson and Drew 1984). In addition, volatile lower organic acids (e.g. propionic and butyric acids) can accumulate in waterlogged soils and damage roots (Armstrong and Armstrong 1999). These organic acids, as well as high CO2, can impose ‘acid loads’ on cells of roots in waterlogged soils (Greenway et al. 2006). Upon re-aeration after a period of O2 deprivation, ethanol remaining in tissues will be converted into acetaldehyde that can induce post anoxic cell injuries (Bailey-Serres and Voesenek 2008).
-
Reactive Oxygen Species (ROS). Excessive formation of ROS (e.g. superoxide radicals, hydroxyl radicals, hydrogen peroxide, singlet oxygen) during low O2 conditions and upon re-aeration is common amongst plants (Blokhina et al. 2003). An important source of superoxide are the mitochondria in which accumulated electrons at Complex III (ubiquinone : cytochrome c reductase) of the electron transport chain are donated to O2. The production of superoxide radicals is stimulated at low catalysing rates of cytochrome c oxidase during low O2 conditions. Superoxide can quickly dismutate to hydrogen peroxide (Blokhina et al. 2003; Bailey-Serres and Chang 2005).
-
Water deficits. Waterlogging can cause wilting of shoot organs in a range of plant species. This response is mediated by a decrease in the hydraulic conductivity of roots (Holbrook and Zwieniecki 2003). Tournaire-Roux et al. (2003) demonstrated that upon O2 deficiency, cytoplasmic acidification in root cells of Arabidopsis signals for a permeability decrease of aquaporins in the plasma membrane of these cells.
Although the submerged environment poses many challenges for plant functioning, an aquatic environment also has some advantages over terrestrial life with shoots in air. Water has a much higher density than air and thus provides plants with support and buoyancy. As a consequence, submerged plants can invest less in support structures without loosing their upright position to reach for light (Bowes 1987). Furthermore, as hypothesised by Bowes (1987), submerged plant shoots can take up nutrients from the surrounding water (e.g. rice coleoptile, Huang et al. 2003; aquatic leaves, Adamec and Kovarova 2006; seagrasses, Lee et al. 2007), providing access to nutrients in addition to those in sediments/soils. Uptake of nutrients by shoots of completely submerged plants might be necessary to overcome constraints on root-to-shoot transport imposed by a lack of transpiration, although xylem guttation driven by root-pressure can provide some flow in aquatic species when completely submerged (Pedersen 1993). Floodwater in a deepwater rice region of Thailand contained higher concentrations of N, P and K than found in oligotrophic lakes, but these concentrations are still considered low compared with those found in most soil solutions (Setter et al. 1987).
Plant adaptations to flooding stress: an overview
Terrestrial plants originated from aquatic predecessors. Flood tolerant plants regained their aquatic competence independently over 200 times (Jackson et al. 2009), suggesting that a restricted number of mutations can alter flooding tolerance (Voesenek and Pierik 2008). Internal O2 movement from shoots to roots is crucial for plant tolerance to soil waterlogging and submergence (Armstrong 1979; Jackson and Drew 1984; Colmer 2003a; Voesenek et al. 2006). This O2 movement occurs within aerenchyma, an interconnected series of large gas-filled spaces providing a low-resistance pathway from the shoot to root extremities (Fig. 1). Traits in addition to internal transport of O2, however, are also essential. Plant adaptations to complete submergence have recently been classified into two main strategies (Bailey-Serres and Voesenek 2008): the Low Oxygen Quiescence Syndrome (LOQS) and the Low Oxygen Escape Syndrome (LOES).
|
Plants with the LOQS are characterised by traits that enable them to: (i) use ATP economically, (ii) increase the abundance of enzymes necessary to make some ATP without molecular O2, and (iii) to increase the production of components that counteract harmful cellular changes associated with flooding. A major feature of this syndrome is that when submerged, shoots do not elongate and in extreme cases cease all growth, thus conserving substrates and prolonging survival until waters recede. This ‘wait out the stress’-type response was the basis for naming this a ‘quiescence syndrome’ (Bailey-Serres and Voesenek 2008). The LOQS has been studied in detail in lowland rain-fed rice (described in the section ‘Submergence – short duration’).
Plants with the LOES are characterised by traits that enable them to: (i) re-orientate the growth direction and increase the rate of growth of shoot organs, such as stems and petioles, so as to emerge above floodwaters, (ii) preserve or develop anatomical structures that facilitate internal gas diffusion or pressurised through-flow, and (iii) retain or develop structures that facilitate gas exchange between plants and their submerged environment (Bailey-Serres and Voesenek 2008). The LOES has been studied in detail in Rumex palustris Sm. and deepwater rice (described in Shallow submergence – long duration section).
These two syndromes and the traits involved as related to waterlogging and submergence stress are discussed in detail below. The focus of our discussion is on the development of ‘suites of complementary traits’ that act synergistically to enhance fitness of plants in the various flooding environments (Table 1; Fig. 2).
|
Flooding regimes exert selection pressures leading to distinct suites of adaptive traits
The wide spectrum of environmental conditions in flood-prone environments determines species distributions and abundances (Armstrong et al. 1985; Silvertown et al. 1999; Voesenek et al. 2004). In this section, we discuss the prevailing ‘suites of complementary traits’ that act synergistically to enhance fitness of plants in some of the main types of flooding environments. Table 1 provides a matrix of the environments and traits considered.
The environments and plant traits will be discussed under the two main headings of waterlogging and submergence, and in the order: soil waterlogging, short duration; soil waterlogging, long duration; submergence, short duration; shallow submergence, long duration; deep submergence, long duration. Root traits will be discussed first in the sections on waterlogging. As submergence impacts on shoots as well as roots, shoot traits will be the focus of the sections on submergence, but any consequences for roots in addition to those described for waterlogging will be considered.
Waterlogging
Waterlogging is the situation of excess water in the root zone. Soil pores that normally would be gas-filled become water-filled. Waterlogged soils are usually O2-deficient and often also contain potentially-toxic reduced soil constituents (Ponnamperuma 1984). Water levels can be dynamic with space and time (e.g. Setter and Waters 2003) and waterlogging can damage dryland species even when below the surface of the soil (Malik et al. 2001) or when transient (e.g. days, Malik et al. 2002). By contrast, wetland species grow well in waterlogged soil (Justin and Armstrong 1987), owing to several traits discussed below. Examples of species that inhabit environments with short-duration or transient waterlogging events, and have been confirmed to display waterlogging tolerance, are Lolium multiflorum Lam. and Hordeum marinum Huds. (McDonald et al. 2002; Garthwaite et al. 2003). Examples of species that inhabit areas with long-duration waterlogging are paddy rice, Phragmites australis (Cav.) Steud, various Typha spp., Juncus effuses L.; with many more examples listed also in Justin and Armstrong (1987).
Soil waterlogging – short duration
Oxygen deficiency in waterlogged soils results from the consumption of O2 by plant roots and soil organisms, while the excess water largely prevents O2 entry into the soil (Armstrong and Drew 2002). During short-term waterlogging, the main stress is likely to be O2 shortage (hypoxia or anoxia) resulting in an energy crisis in roots.
Metabolic anoxia tolerance is presumably of importance as an adaptive trait for roots of plants during short-term waterlogging, as aerenchyma can take time to develop (e.g. Zea mays L., Konings 1982; Triticum aestivum L., Thomson et al. 1990). Aerenchyma provides a gas-filled pathway for low-resistance movement of O2, and other gases, through plant organs. This internal pathway is inter-connected from shoot tissues (stems or sheaths or petioles, and in many cases from leaves – e.g. mid-rib in leaves of rice) to root extremities. Constitutive aerenchyma would be of immediate benefit to roots faced with waterlogging, as O2 will diffuse into and along the roots. Many wetland species possess roots with constitutive aerenchyma (Justin and Armstrong 1987). Different parts of root systems will likely experience different O2 status; surface roots may, for example, have access to O2, whereas deeper roots might be without O2. So, even in species with constitutive aerenchyma, some tissues will still need to cope with O2 deficiency.
Central to anoxia tolerance is dealing with the imposed energy crisis; anaerobic catabolism can produce at least some ATP, but apportionment of the scarce energy to essential processes is also needed (Gibbs and Greenway 2003; Greenway and Gibbs 2003). Consistent with the above, glycolytic flux and ethanolic fermentation were stimulated in roots of grey poplar when waterlogged. No changes were observed in leaves, as the shoot remained in contact with air. Various genes involved in biosynthetic pathways such as secondary cell wall formation and other energy-demanding processes, such as transport of nutrients, were downregulated in roots, but again not in leaves (Kreuzwieser et al. 2009). Whether roots of wetland species possess greater anoxia tolerance than those of dryland species, however, is still uncertain (Gibbs and Greenway 2003).
Hypoxic tissues and tissues experiencing re-entry of O2 following anoxia are subject to potential oxidative stress (Blokhina et al. 2003). Oxidative damage can result from increased production of reactive oxygen species (ROS) and/or a reduced capacity to detoxify ROS. In a waterlogging-sensitive species, like wheat, cycles of anoxia and re-entry of O2 can increase levels of oxidative stress (Goggin and Colmer 2005). Oxidative stress can occur in relatively short time frames following changes in tissue O2 status, and so likely impacts on roots of some species during transient waterlogging. Whether roots of tolerant and sensitive species differ in oxidative damage during waterlogging is uncertain (Blokhina et al. 2003), and only some components of the oxidative defence system have been measured in the few studies available. Upon transfer of intact plants from N2-flushed to aerobic nutrient solution, the ratio of reduced-to-oxidised glutathione decreased in roots of waterlogging-intolerant species, but not in tolerant species, indicating potentially more oxidative stress in the roots of the less tolerant species (Biemelt et al. 1996). However, whether these contrasting responses reflect inherent differences in biochemical root traits, or instead are a consequence of the roots of the more tolerant wetland species possibly containing higher amounts of aerenchyma and so not suffering the same degree of O2 deprivation, remains unknown. Experiments avoiding or controlling internal O2 transport to roots are needed so that data on ROS and defence systems in roots can be obtained under well controlled root O2 status; such as use of excised roots submerged in solutions of known O2 concentrations or manipulations of shoot O2 as well as root-medium O2 so that the supply via aerenchyma is also controlled. Nevertheless, even intact, aerenchymatous roots of some wetland species can suffer oxidative damage upon re-entry of O2 into the root zone (Chen and Qualls 2003).
Several waterlogging-sensitive species, such as sunflower (Helianthus annuus L.), tobacco (Nicotiana tabacum L.) and tomato (Solanum lycopersicum L.), show epinastic leaf growth (downward leaf movement) in response to waterlogging. This epinastic growth has been suggested to ameliorate the dehydrating effect of a drop in hydraulic conductance of roots in waterlogged soil (Jackson 2002). Waterlogging causes a decrease in root hydraulic conductance (Holbrook and Zwieniecki 2003), which in Arabidopsis has been associated with O2-deficit-induced acidification of root cell cytoplasm causing gating of aquaporins (Tournaire-Roux et al. 2003). Such gating of an Arabidopsis aquaporin by changes in pH was recently confirmed in studies of proteoliposomes (Verdoucq et al. 2008). Vandeleur et al. (2005) hypothesised that gating of aquaporins in roots experiencing O2-deficit might be a mechanism by which water uptake is re-directed to other roots in more favourable regions of the soil (e.g. from O2-deficient deeper roots, to those in surface layers with access to some O2).
In summary (Table 1), tolerance of short-duration waterlogging is enhanced by the presence of constitutive aerenchyma, a trait in many wetland species. In addition, tolerance of anoxia in root tissues is also hypothesised to be of importance, especially in roots lacking constitutive aerenchyma. Even when constitutive aerenchyma is present, anoxia tolerance is presumed also to be of importance for some tissues, for example, those most distant from the O2 source (e.g. root tips) would likely still experience O2 deprivation. Hypoxic tissue conditions and the re-entry of O2 following drainage can lead to formation of ROS in plant cells, so an efficient oxidative defence system is another trait likely to be of importance for plants in environments with transient waterlogging.
Soil waterlogging – long duration
Soil O2 deficiency occurs soon after waterlogging commences, depending on biological activity. With time, waterlogging also changes other soil factors; CO2, ethylene (C2H4), and reduced compounds, such as Mn2+, Fe2+, S2– and carboxylic acids can increase (Ponnamperuma 1984). Internal O2 transport from air, via the shoots, to roots is essential to survival and functioning of roots (Armstrong 1979).
Waterlogging tolerant species form a large adventitious root system when in saturated soils. The newly-formed adventitious roots usually contain aerenchyma (Figs 1 and 2c) and these roots can replace the stress-damaged roots that formed before waterlogging (Jackson and Drew 1984). Waterlogging tolerant species tend to develop larger adventitious root systems than intolerant species, and these newly-formed roots contain more aerenchyma (e.g. Rumex species, Laan et al. 1989). The initiation and outgrowth of adventitious roots has been studied in deepwater rice and Rumex species, respectively. Accumulation of ethylene signals this response, and auxin and H2O2 are also involved (Visser et al. 1996; Steffens and Sauter 2009).
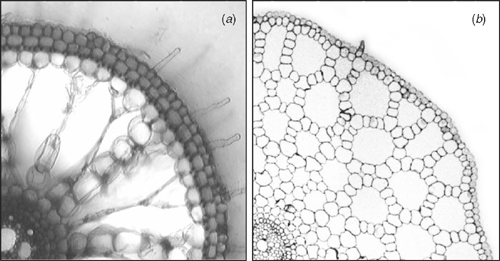
Aerenchyma in roots either forms constitutively in drained soil conditions and/or is induced upon soil waterlogging. It develops in existing plant organs or concomitant with the outgrowth of new adventitious roots. Induction of lysigenous aerenchyma (Fig. 1a) in roots is promoted by the accumulation of ethylene and subsequently a cascade leading to programmed cell death (Drew et al. 2000; Shiono et al. 2008). In roots, genes associated with cell wall breakdown show increased expression in response to waterlogging or hypoxia (Saab and Sachs 1996; Liu et al. 2005; Lasanthi-Kudahettige et al. 2007). Even in situations where only the lower parts of roots are in waterlogged soil, functional aerenchyma can form both in the portion under water and in that above the water-saturated zone (Malik et al. 2003).
In wetland species with constitutive aerenchyma, the volume is often further enhanced during soil waterlogging (Justin and Armstrong 1987). Porosity in roots (% gas volume per unit root volume) of some wetland species can increase to as high as 53% (Justin and Armstrong 1987). High porosity greatly reduces resistance to gas-phase diffusion through plant organs and tissues (Armstrong 1979). In addition, many wetland species form a barrier to radial O2 loss (ROL) in basal zones of roots, a feature that further promotes longitudinal O2 diffusion down roots, by preventing losses to the surrounding anoxic soil (Fig. 3; Armstrong 1979; Visser et al. 2000; Colmer 2003a; Garthwaite et al. 2003). In rice, growth in stagnant conditions increased aerenchyma formation and induced a barrier to ROL in basal root zones (Colmer 2003b). An inducible barrier to ROL also occurs in several other species that inhabit wet areas (e.g. L. multiflorum Lam., McDonald et al. 2002; H. marinum Huds., Garthwaite et al. 2003), whereas in other species the barrier appears to be constitutive (e.g. J. effuses L., Visser et al. 2000). Ethylene enhances the development of the additional aerenchyma but not formation of the barrier to ROL, in roots of rice (Colmer et al. 2006). The barrier to ROL, together with rhizosphere re-oxidation around root tips and laterals, can also diminish entry of potentially toxic compounds in highly-reduced soils (Armstrong 1979; Armstrong et al. 1996; Armstrong and Armstrong 2005).
|
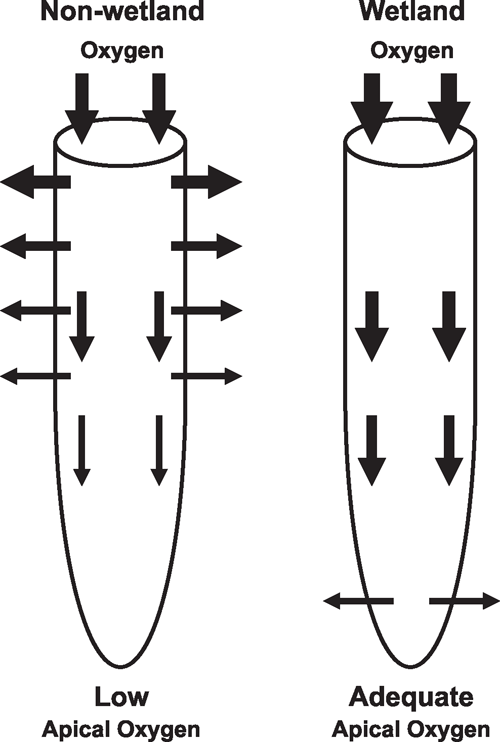
Modelling predicts that the amount of O2 available within organs is inversely related to diffusion-path lengths (Armstrong 1979). Plants have developed traits to promote diffusion to the most distant destination, the root meristems. Traits that bring the O2 source closer to roots are formations of lenticels at stem bases and development of vertically-growing roots that reach air, such as pneumatophores (Kozlowski 1984). A specialised trait that also brings an O2 source closer to roots is convective flow of gases through shoot organs and along rhizomes of some wetland species (Dacey 1981; Armstrong et al. 1992). Such flows elevate the O2 concentrations in rhizomes, thus enhancing O2 diffusion into the subtending roots (e.g. P. australis, Armstrong et al. 1992). Another shoot trait of relevance during soil waterlogging, possessed by several species with rosette-type shoots that inhabit flood-prone environments (Grimoldi et al. 1999), is a capacity to change leaf orientation from rather prostrate to almost vertical (hyponastic growth). This acclimation prevents partial submergence when some water ponds on waterlogged soils.
The situation for possible oxidative damage in roots experiencing changes in O2 regimes was discussed above under short duration waterlogging. Oxidative stress during long-term anoxia has not been studied in roots, as root tissues do not tolerate long-term anoxia (Gibbs and Greenway 2003). Other organs, such as rhizomes, can experience long-term anoxia associated with long duration waterlogging. The classical paper by Monk et al. (1987) of oxidative stress during re-aeration of rhizomes of Iris species differing in flooding tolerance, showed less lipid peroxidation in the tolerant Iris pseudacorus L. compared with intolerant Iris germanica L., owing to differences in the oxidative defence systems of these two species.
Roots experience the direct effects of waterlogging, whereas the shoots of plants in waterlogged soils suffer any consequences of root dysfunction. For example, shoots can be damaged by deficiencies of mineral nutrients, particularly nitrogen, and by an influx of reduced soil toxins (e.g. Fe2+ and Mn2+ toxicities) (Jackson and Drew 1984). Soil element toxicities associated with waterlogging might, in combination with the O2-deprivation stress, determine relative performances of different wheat genotypes on different soil types (Setter et al. 2009). Even wetland species can suffer from entry of reduced compounds from the soil, depending on soil type and waterlogging duration. Rice grown on some high organic-content soils, for example, can show leaf bronzing and poor growth caused by ingress of Fe2+ and S2–, and cultivars differ in susceptibility to development of these symptoms (Baba et al. 1965; Tanaka et al. 1968). Formation of root barriers to restrict entry of these compounds, as well as having some ROL from key sites (root tips and laterals) to re-oxidise these substances in the rhizosphere, are regarded as important traits for minimising these toxicities (Armstrong 1979; Armstrong and Armstrong 2005). Future research should also assess whether species differ in cellular tolerance of these soil-derived compounds when entry does occur into roots and shoots.
Roots in waterlogged soils might also need to cope with exposure to high CO2 (Greenway et al. 2006). Carbon dioxide accumulated to 30–35 kPa in soil of a waterlogged soybean field (Boru et al. 2003). In pots of soil from rice paddy fields, CO2 ranged 8–40 kPa, depending amongst other factors on initial soil pH (IRRI 2005; Greenway et al. 2006). High CO2 could interfere with root metabolism via an imposed acid load, by general disruption of metabolism (e.g. from high HCO3− in cellular fluids), or by direct inhibition of respiratory activity (reviewed in Greenway et al. 2006). There is some evidence that species differ in tolerance of high CO2 in the root zone, with wetland rice reported as more tolerant than dryland soybean (Boru et al. 2003), but further work is needed to confirm the reported differences (Greenway et al. 2006), and to determine how other wetland species cope with high CO2 in the root zone.
In summary (Table 1), tolerance of long duration waterlogging requires an efficient system for internal O2 transport to roots, as well as the continued development of new roots. Traits such as aerenchyma and a barrier against ROL in roots, aerenchyma in shoots, lenticels at stem bases, and in some species pressure-driven flows along rhizomes, are found in various combinations, and these act synergistically to enhance internal aeration. Hyponastic growth of petioles of some rosette-type plants avoids leaves also experiencing submergence from shallow ponding of water. In addition to the O2-deprivation stress, reduced soil toxins can accumulate during long duration waterlogging, and these can damage root, rhizome and shoot tissues. Formation of root barriers to restrict entry of these compounds, as well as having some ROL from key sites (root tips and laterals) to re-oxidise these substances in the rhizosphere, are important traits.
Submergence
Submergence refers to the situation when floodwaters rise to levels that shoots are completely under water. This prevents direct exchange of gases between the entire plant body and the atmosphere resulting in reduced O2 and CO2 levels. Exchange of gases, however, does occur between plant organs and floodwaters, but at slow rates. Furthermore, complete submergence frequently reduces the available light level. Thus, low CO2 and/or low light hampers rates of underwater photosynthesis.
Three contrasting submergence regimes, based on temporal and depth criteria, have been studied in relation to natural and agricultural systems: short duration floods; long duration, but shallow floods; and long duration, but deep floods (Piedade et al. 1991; Jackson and Ram 2003; Voesenek et al. 2004). Short duration submergence occurs during flash-flooding events, with variable depths, such as occur frequently in lowland rice areas, and also at various times in most low-lying land areas of the world. Long-lasting submergence can either be shallow or deep. In river systems, the deep floods typically occur on positions that are in direct contact with the river, such as river foregrounds in Europe, and delta areas in Asia supporting deepwater rice cultivation, or in the Amazon. Long-lasting, shallow floods, by contrast, occur in more distal areas of the plains when water flows over embankments that subsequently impede reverse-runoff. In many cases, the flooding durations are further prolonged owing to poor drainage of soils in these areas.
Traits for tolerance of soil waterlogging, discussed in the preceding section, are also present in many plants when completely submerged (Table 1). These traits were, in the main, in roots. During floods, soils are also waterlogged, therefore many of these root traits remain of relevance. The functionality of aerenchyma and a barrier against ROL in adventitious (sediment) roots is dependent upon there being an O2 source via the shoots (Armstrong 1979). During short-term submergence, if these traits are present in existing roots, then the resultant O2 movement would enhance root survival. With shoots emergent above long-lasting, shallow floods, these root aeration traits would enhance root growth and functioning, as described previously for waterlogged plants. When floods are prolonged and too deep for shoots to reach the surface, existing roots would still benefit from these traits enabling some O2 supply, depending on entry from the water column and/or underwater photosynthesis (Pedersen et al. 2006). Availability of O2 in completely submerged plants shows marked diurnal cycles, as dependent on underwater photosynthesis (Pedersen et al. 2006; Colmer and Pedersen 2008a). Interestingly, when some species remain completely submerged, hardly any new adventitious roots emerge into the sediment (Van der Sman et al. 1993). By contrast, new adventitious roots emerge into the water column, these have been termed ‘aquatic roots’ (rice, Lorbiecke and Sauter 1999; Rumex, Van der Sman et al. 1993). In aquatic roots of some species, chloroplasts develop in cortical cells, thereby enabling photosynthesis in the roots and providing an internal source of O2 (and sugars) when under water (Rich et al. 2008).
In addition, many of the traits described in the preceding section for roots also occur in shoots, such as aerenchyma formation, anaerobic energy production, and protection against ROS (Voesenek et al. 2006; Bailey-Serres and Voesenek 2008). Submergence-specific traits of shoots are also evident and their contributions to tolerance in relation to the three contrasting flooding regimes (Table 1) are discussed below.
Submergence – short duration
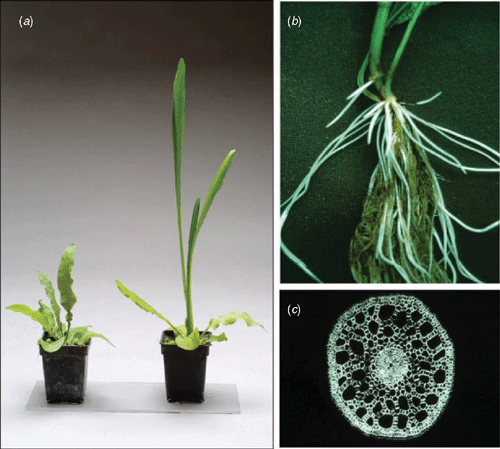
Plants with the LOQS, characterised by a lack of a shoot extension so as to conserve substrates for enhancement of survival until waters recede, have fitness advantages in environments with short-duration floods (Bailey-Serres and Voesenek 2008). An association of the LOQS with environments prone to short-duration floods, has been demonstrated for rice (Jackson and Ram 2003; Xu et al. 2006) and also several species in natural plant communities (Voesenek et al. 2004). Non-elongating cultivars of rice are more tolerant of flash-floods, even when these short-duration floods are deep, as carbohydrates are conserved for processes essential for survival (reviewed by Bailey-Serres and Voesenek 2008). The elongation response in lowland rain-fed rice cultivars is controlled by one polygenic locus (Sub1) on chromosome 9. A transcription factor within this Sub1 locus (Sub1A-1), belonging to the multi-gene family of AP2/ERF transcription factors, influences submergence tolerance in rice (Fukao et al. 2006). The expression of this gene is regulated by ethylene and constrains underwater shoot elongation and carbohydrate catabolism (Xu et al. 2006; Perata and Voesenek 2007). Most probably Sub1A resulted from gene duplication events that occurred before, and after, rice domestication (Fukao et al. 2009). When Sub1A-1 was introgressed into rain-fed lowland cultivars lacking this gene at the Sub1 locus, tolerance of submergence lasting 12–17 days was improved (e.g. Fig. 4); the yield advantage under field conditions was up to 3.8 t ha−1 (Singh et al. 2009).

|
In addition to the traits associated with the LOQS as described in Bailey-Serres and Voesenek (2008), another plant trait that improves submergence tolerance of rice is the so-called ‘plant plastron’ (Raven 2008) or leaf gas films. A thin layer of gas is retained on submerged leaves of some species, depending on leaf surface traits (Colmer and Pedersen 2008b). The gas films enlarge the water-gas interface, thus improving gas exchange between submerged shoots and the surrounding water (Colmer and Pedersen 2008b). Gas films on leaves of rice can enhance underwater photosynthesis to provide additional sugars and O2. Even in darkness, O2 entry from floodwaters was improved, and O2 that entered shoots moved via the aerenchyma to the roots (Pedersen et al. 2009). During short duration floods, O2 entry from the water column might be adequate to sustain submerged shoots (e.g. in rice), but distal portions of roots would be expected to become O2 deficient during nights (Waters et al. 1989; Colmer and Pedersen 2008a), so that anaerobic energy production would be of relevance for survival of roots (see sections above on waterlogging and roots).
In addition to cessation of growth during complete submergence, other processes may also be downregulated so as to conserve energy. This information is largely gleaned from molecular studies using model systems. For example, Arabidopsis protoplasts that are not well aerated demonstrate large reductions in accumulation of many gene transcripts, especially those coding for energy consuming processes such as ribosome biogenesis and anabolism (Baena-González et al. 2007). Furthermore, in Arabidopsis seedlings a large portion (70%) of cellular mRNA’s become poorly translated upon low O2 stress (Branco-Price et al. 2005, 2008). Even in highly-tolerant species with relatively high rates of ethanolic fermentation supporting anoxic shoot elongation (e.g. rice coleoptile and shoot of Potamogeton pectinatus L.), selective protein synthesis conserves energy (Huang et al. 2005; Dixon et al. 2006). This restriction of the energetically costly protein synthesis significantly reduces the consumption of ATP. A major energy saving is associated with the selective repression of translation of mRNAs encoding the highly abundant ribosomal protein (Branco-Price et al. 2008). The downregulation of energy consuming processes is rapidly reversed upon reoxygenation, indicating that cellular sensing of energy status controls the modulation of these processes (Branco-Price et al. 2008). Thus, tolerance to anoxia involves both ATP production via glycolysis linked to ethanolic fermentation and apportionment of the limited ATP supply to processes essential for survival (Greenway and Gibbs 2003).
In conditions of hypoxia, an active downregulation of the rate of respiration has been suggested to prolong survival as cells would maintain higher O2 levels under these conditions (Geigenberger 2003). Further work on this concept is needed, so as to separate effects of physical diffusion limitations (see Armstrong et al. 2009) and the proposed downregulation. Recently, Zabalza and co-workers (2009) showed that the pyruvate concentration in pea roots is positively correlated with the respiration rate. This led them to suggest that in hypoxic tissues fermentation not only functions to regenerate NAD+ for continuation of glycolysis but that it may help to reduce the levels of pyruvate and thus restrict respiration and so reduce consumption of available O2 during hypoxia. The likely spatial separation of these processes, however, needs also to be considered, as fermentation could mainly occur in an inner ‘anoxic core’ and respiration in the outer cell layers (reviewed by Gibbs and Greenway 2003).
If severe hypoxia or anoxia occurs in some situations or plant parts, a subset of upregulated genes are associated with regulation of scarce energy resources. Examples are the use of pyrophosphate (PPi) over ATP as a high-energy donor (Huang et al. 2008) and the preferred use of sucrose synthase (SuSy) and UDPglucose pyrophosphorylase for the degradation of sucrose (Geigenberger 2003). More generally, certain mRNAs circumvent translational repression and/or are transcriptionally upregulated thus selectively ensuring production of a specific set of proteins (Branco-Price et al. 2005, 2008). These ‘anaerobic proteins’ are involved in (i) glycolysis, (ii) conversion of pyruvate to fermentation end products, (iii) sucrose degradation, and (iv) controlling reactive oxygen species (ROS) (Sachs et al. 1996; Klok et al. 2002; Branco-Price et al. 2005; Huang et al. 2005; Liu et al. 2005; Loreti et al. 2005; Lasanthi-Kudahettige et al. 2007; Igamberdiev and Hill 2009; Kreuzwieser et al. 2009; van Dongen et al. 2009). There is evidence that protein kinases belonging to the SnRK1 group, such as Arabidopsis KIN10 and KIN11, orchestrate transcriptional networks to globally promote catabolism (cell wall, starch, sucrose, amino acid, lipid and protein degradation) to provide alternative sources of energy and metabolites and suppress anabolism during hypoxia-induced carbohydrate starvation and/or energy deprivation (Baena-González et al. 2007).
When floodwaters recede, post-submergence damage can also occur. Lodging occurs if shoots have elongated (e.g. rice, Jackson and Ram 2003), and tissues can also be challenged by oxidative stress (Blokhina et al. 2003). Interestingly, oxidative damage in leaves following de-submergence of young rice plants that were under water for 6 days, was greater in an intolerant cultivar (IR42) than in submergence-tolerant FR13A (Ella et al. 2003). Oxidative damage was measured as lipid peroxidation products, and in addition to the genotypic differences, oxidative damage was greater under conditions of high light than at lower light (PAR, 1000 and 300 μmol m−2 s−1, respectively), indicating that photoreduction of O2 to O2− in Photosystem I might have been greater in chloroplasts altered by the submergence treatments (Ella et al. 2003). Leaves of the more tolerant FR13A contained higher concentrations of ascorbate and had higher glutathione reductase activity, than those of the intolerant IR42. The lower endogenous ascorbate concentration in the flooding intolerant IR42 was found also in a second study, and when exogenous ascorbate was supplied to discs taken from submerged leaves of IR42, oxidative damage was reduced (Kawano et al. 2002). In addition to oxidative damage in leaves of rice upon de-submergence, injury can also result from acetaldehyde toxicity, when ethanol remaining in tissues is converted into acetaldehyde (Mustroph et al. 2006). Studies of oxidative stress do not seem to have been conducted for leaves of ‘wild’ wetland species.
In summary (Table 1), tolerance of short-duration submergence is characterised by the LOQS (Bailey-Serres and Voesenek 2008). Major features of the LOQS are lack of shoot extension to conserve substrates, downregulation of other non-essential processes to further conserve energy, induction of anaerobic energy production in tissues that become anoxic, and upregulation of the ROS defence system to avoid damage upon O2 re-entry. In addition, leaf gas films on some species (e.g. rice) enhance underwater photosynthesis to provide additional sugars and O2 (Pedersen et al. 2009).
Shallow submergence – long duration
Species from a wide range of families share the capacity to initiate fast extension growth of shoot organs upon submergence (Ridge 1987; Voesenek and Blom 1999). In the river Rhine floodplains, this escape response is prevalent in plants inhabiting environments characterised by long, shallow floods (several weeks and less than 1 m). By contrast, this trait was generally absent in species from sites with deep floods and sites with short duration floods (Voesenek et al. 2004). Shoot extension is only of major benefit for submerged terrestrial species if it leads to emergence (Bailey-Serres and Voesenek 2008; Pierik et al. 2009). This shoot extension response is central to plants that respond to flooding events with the LOES, but these plants also possess other traits.
Hyponastic growth of shoot organs upon submergence has been described for several species, such as R. palustris (Cox et al. 2003), Ranunculus repens L., Caltha palustris L., (Ridge 1987), Leontodon taraxacoides Lam. (Grimoldi et al. 1999), Paspalum dilatatum Poir. (Insausti et al. 2001), and even Arabidopsis (Millenaar et al. 2005). Hyponastic growth in R. palustris occurs mainly in petioles of younger leaves, and results from differential cell elongation across the petiole base (Cox et al. 2004). Hyponastic growth alone, is a trait of relevance as vertical re-orientation by itself can bring leaves above the water; an example is L. taraxacoides that does not display a shoot elongation response (Grimoldi et al. 1999). Even if hyponastic growth occurs and leaves do not reach the water surface, potential benefits might still be improved access to light, shortening of the submergence period, and prevention of sediment/debris covering leaves. Furthermore, hyponastic growth is a prerequisite for submergence-induced petiole elongation in some species (e.g. R. palustris, Cox et al. 2003).
Both hyponastic growth and petiole/internode elongation bring leaf tips closer to the water surface or ultimately above floodwaters that are not too deep. Shoot emergence is highly beneficial for plants in flooded environments, due to the improved exchange of gases and the re-start of aerial photosynthesis (He et al. 1999; Mommer et al. 2005; Pierik et al. 2009). These adaptive elongation responses are initiated by the accumulation of the volatile hormone ethylene inside submerged plant tissues (Jackson 2008). Subsequently, the interplay of various other plant hormones such as gibberellic acid (GA) and abscisic acid (ABA) and more downstream targets at the cell wall level, such as cell wall acidification and expansins (Vreeburg et al. 2005) result in the elongation of shoot organs (reviewed by Bailey-Serres and Voesenek 2008; Jackson 2008).
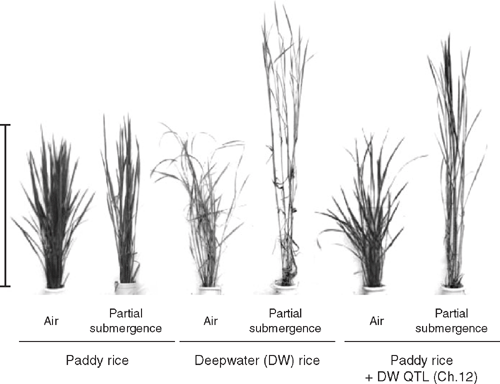
Deepwater rice also displays a remarkable capacity for stem elongation as floodwaters rise. Deepwater rice grows in areas with water depths of 0.5 m (or more) for at least 1 month, although in some regions water levels can reach several metres (Grist 1986; Catling 1992). Deepwater rice tends to avoid complete submergence as stem elongation proceeds as floodwaters rise, typically keeping a portion of the shoot above water (Catling 1992). When floods recede, the stems show ‘kneeing’; the lower nodes root into the sediments whereas the uppermost nodes grow upwards so that floral structures and grains are held in air, rather than being spoilt by damp soil conditions (Catling 1992). As waters rise, internode elongation is stimulated via ethylene-induced reductions in ABA and increased tissue sensitivity to GA (Kende et al. 1998). This stem elongation response in deepwater rice, and in a wild relative (Oryza rufipogon Griffiths), is determined by three major QTLs common to both species (Hattori et al. 2007). When a major QTL on chromosome 12 of deepwater types was introduced into a non-elongating rice genotype (i.e. a paddy type O. sativa ssp. japonica), the plant demonstrated a deepwater elongation response upon partial submergence with rising water levels (Fig. 5). Hattori et al. (2007) proposed that the gene associated with the QTL might encode a product that senses and switches on the deepwater elongation response, with the downstream machinery already being present in the non-elongating type. Thus, like the Sub1 locus (chromosome 9) for shoot elongation during complete submergence of lowland rain-fed rice (Xu et al. 2006), alteration in a major locus (QTL on chromosome 12) can also have a major effect on the response of deepwater rice to rising partial submergence (Hattori et al. 2007).
|
In many cases, shoots of plants with a LOES not only elongate, but also produce new leaves when submerged (e.g. R. palustris) (Mommer and Visser 2005). These leaves have a higher specific leaf area (SLA), thinner epidermal cell walls, and cuticles and chloroplasts oriented close to the epidermis (Mommer et al. 2005). These traits reduce the diffusion resistance for gases and as a consequence increase the rates of CO2 entry for underwater photosynthesis (Mommer et al. 2006a) and inward O2 diffusion for respiration (Mommer et al. 2004). Leaf gas films are another plant trait that improves gas exchange between submerged shoots and the surrounding water. The gas films enlarge the water–gas interface, thus facilitating entry of CO2 for photosynthesis when in light, and of O2 during darkness (Colmer and Pedersen 2008b). Gas films contribute to the LOES by increased rates of net underwater photosynthesis and improved internal aeration of roots (Pedersen et al. 2009). In both cases, leaves produced and then positioned further towards the water surface during elongation growth, would benefit from the higher light in surface layers promoting net photosynthesis.
Thus, the shoot elongation response acts synergistically with other leaf traits enhancing underwater gas exchange. Rapid shoot elongation, however, depletes carbohydrates, ultimately hampering further growth (Groeneveld and Voesenek 2003) and can compromise survival if leaves do not reach air (Setter and Laureles 1996). As shoot elongation occurs, however, these leaves grow into better illuminated water layers. This higher light availability, together with the leaf traits enhancing underwater photosynthesis, results in higher carbon gain to sustain further elongation growth. Moreover, the improved O2 status, derived from photosynthesis and/or enhanced entry of O2 from the water column, due to these leaf traits, would also prolong survival during submergence. Maintenance of healthy tissues for as long as possible is advantageous as this enables elongation to be sustained for longer periods, and tissues in a healthy condition would presumably also survive better after recession of floodwaters.
Restoration of leaf-to-air contact, owing to shoot extension upon submergence, is only functional if shoots contain pathways for low resistance gas diffusion (Mommer et al. 2006b; Pierik et al. 2009). To this end, the bodies (i.e. shoots and roots) of many wetland plants are highly aerenchymatous. Upon emergence from floodwaters, internal O2 status is improved as O2 enters the aerenchyma. In many species (e.g. R. palustris, O. sativa) the O2 diffuses throughout the plant body, whereas in some (e.g. P. australis, Armstrong et al. 1992; waterlily (Nuphar lutea (L.) Sm), Dacey 1981) gases are transported through shoots and rhizomes via through-flows generated by pressure gradients. Through-flows can increase by two orders of magnitude the effective aeration distance in plants, as compared with diffusion alone (Armstrong et al. 1991). This enhanced O2 supply enables rhizomes to grow deeper into waters and/or anoxic sediments (Strand 2002). Moreover, higher O2 within rhizomes enhances the O2 status in attached roots; ROL was ~4-fold higher from adventitious roots of P. australis with through-flows active (Armstrong et al. 1992).
In summary (Table 1), tolerance to long duration, shallow, submergence is characterised by the LOES (Bailey-Serres and Voesenek 2008). Major features of the LOES are rapid re-orientation and extension growth of shoots towards the water surface, development of new ‘aquatic’-leaves (or possession of leaf gas films) to promote carbon acquisition during the period of extension growth, aerenchyma and other traits for enhanced internal aeration when leaves emerge, as well as defence against ROS. As submergence is prolonged, the capacity to form new roots (sediment and aquatic), and to deal with reduced soil constituents, would also be of importance.
Deep submergence – long duration
In some environments, floods are of such depths that plant organs will never reach the water surface or may take weeks of enhanced shoot elongation to emerge. A shoot elongation response was generally absent in species from sites with deep, long-lasting floods (Voesenek et al. 2004). Consistent with the lack of the elongation trait in many species in environments with deep floods, is that woody species in the Amazonian basin also generally do not elongate when deeply submerged (e.g. 10 m) (Parolin 2009). Interestingly, the emergent C4 grass E. polystachya can manage to keep a portion of shoot above deep Amazonian floods, owing to the formation of additional stem nodes; seven new internodes were formed when water levels were rising at ~1.5 m per month (Piedade et al. 1991).
Some plants in these environments with long-duration floods develop leaves with traits that significantly improve the exchange of gases with floodwaters (e.g. R. repens, Lynn and Waldren 2001). Thus, while the lack of shoot extension in many species that inhabit areas with prolonged and deep floods (Lynn and Waldren 2003; Voesenek et al. 2004) is similar to the LOQS, the leaf adaptations (Lynn and Waldren 2003) and acclimations (Mommer et al. 2006b; Mommer et al. 2007) promote avoidance, rather than escape, of the low O2 stress. Moreover, new leaves are produced underwater (e.g. Mommer et al. 2007), so these plants are not ‘quiescent’. Aquatic leaf acclimations (biophysical) upon submergence, such as dissected leaves, increased specific leaf area (i.e. larger surface area relative to mass), thinner epidermal cell walls and cuticles, and chloroplast re-orientation towards the epidermis, reduce the diffusion resistances for both CO2 and O2 and thus improve underwater photosynthesis (Mommer and Visser 2005; Mommer et al. 2006a) and respiration (Mommer et al. 2004). Such acclimations are relevant during flooding regimes in which light is available to submerged vegetation, such as during deeper floods of low turbidity. Nevertheless, these traits might also be beneficial for plants in deep and/or turbid waters, as the decreased resistance also enhances O2 entry for respiration (Mommer et al. 2004).
Improved O2 status in shoots of submerged plants, whether from aquatic leaf traits or from gas films, would also benefit the roots, owing to internal O2 diffusion to roots via the aerenchyma (Waters et al. 1989; Pedersen et al. 2009). Further evidence for the importance of this shoot-to-root O2 movement during submergence comes from strong correlations between aerenchyma content in petioles and survival when submerged, both in light and darkness, for diverse species (Mommer et al. 2006b).
Submerged plants experience diurnal fluctuations in tissue O2 status, as dependent on light and thus photosynthetic O2 production in shoots (Sorrell and Dromgoole 1987; Pedersen et al. 2006). O2 available at a specific location in the plant body is dependent upon resistances (and distance) and O2 consumption rates along the diffusion pathway (Armstrong 1979). As examples, the O2 concentration in the root cortex is generally higher than in the adjacent stele (Armstrong et al. 1994).These spatial and temporal differences in O2 supply result in tissue-specific (e.g. stele, Thomson and Greenway 1991) and time-dependent (e.g. rice roots, Waters et al. 1989) inductions of anaerobic energy production via glycolysis linked to fermentation. Similarly, submerged rhizomes of waterlily show large fermentation activity when O2 supply from aerenchyma is restricted, such as when no emergent leaves are present during long, deep winter submergence (Bucher et al. 1996). Thus, anaerobic energy production is likely to be of importance during submergence by long and deep floods. Furthermore, the variable O2 supply might increase ROS, so defence against ROS is also likely to be of importance in tissues with marked dynamics in O2 status.
Rhizomes and storage organs of marsh species can survive prolonged submergence when buried in anoxic mud over winter. Rhizomes of the marsh species Acorus calamus L. survived anoxia for more than 90 days (Crawford and Brändle 1996). Turions of Potamogeton species also survive winter in anoxic muds, and the shoot that emerges in spring is very anoxia tolerant; this tolerance is associated with the availability of large reserves of carbohydrates to fuel ethanolic fermentation, as well as regulation of the proteins synthesised during anoxia, so as to re-direct the flow of energy to essential processes (Sato et al. 2002; Dixon et al. 2006). In spring, shoots grow out of the anoxic sediments and into the water column, and O2 becomes available from photosynthesis in illuminated water and eventually from air when the shoots reach the atmosphere (Crawford and Brändle 1996). Anaerobic energy production will be a vital trait to energise this escape from anoxic sediments, such as demonstrated also for germinating seeds of rice (e.g. coleoptile elongation, Ismail et al. 2009; Magneschi and Perata 2009) and shoot elongation from turions (e.g. P. pectinatus L., Summers et al. 2000). Ethanol production rates in these specialist, highly anoxia-tolerant, shoot organs are amongst the fastest reported in plants (e.g. P. pectinatus, Summers et al. 2000). Yet, even in the coleoptiles of rice (Colmer et al. 2001; Huang et al. 2005) and in the elongating shoot of P. pectinatus (Dixon et al. 2006) several processes (e.g. protein synthesis) are downregulated so as to conserve energy, indicating that both energy production and regulation of consumption contribute to anoxia tolerance.
In summary (Table 1), tolerance of long duration, deep, submergence is characterised by some traits from the LOQS, and some from the LOES (Table 1). Plants cannot escape by reaching air, but instead acclimate so as to minimise the detrimental conditions. Thus, although species lack a strong shoot extension response, similar to the LOQS, many do not go into quiescence as new, aquatic-adapted leaves are produced. ‘Aquatic’ leaf acclimations reduce diffusion limitations against CO2 and O2 entry, thus enhancing underwater photosynthesis and respiration, so that the most adverse effects are somewhat ‘avoided’. Such plants must also possess aerenchyma so that roots can also receive O2, although many would be expected to also produce new aquatic roots. Significant diurnal fluctuations in tissue O2 status, as dependent on light and photosynthetic O2 production in shoots, mean that anaerobic metabolism for anoxia tolerance, and defence against ROS produced in this variable environment are of importance.
Conclusions and future prospects
Evidence has been presented for ‘suites of complementary traits’ occurring in specific combinations for plants inhabiting various types of flooding environments (Table 1). Traits contributing to tolerance of short-duration soil waterlogging are anoxia tolerance in root tissues, defence against ROS, and constitutive aerenchyma; and for longer-duration waterlogging the additional traits of induced aerenchyma, a barrier against ROL, and tolerance of reduced soil conditions. When overland floods submerge shoots, plant tolerance is determined by several shoot traits in addition to root traits described for waterlogging. The Low Oxygen Quiescence Syndrome (LOQS) improves survival in environments prone to flash-floods (short duration submergence), whereas the Low Oxygen Escape Syndrome (LOES) improves survival in environments with prolonged, shallow floods (Bailey-Serres and Voesenek 2008). Many species that inhabit areas with prolonged and deep floods also lack a shoot extension response (Voesenek et al. 2004), being a major component of the LOQS, but many of these plants produce new leaves adapted to the underwater environment (similarly to some plants displaying the LOES); this ‘mixed response’ conserves carbohydrates and also promotes avoidance of, rather than escape from, low O2 stress.
Several examples of beneficial interactions between these wetland plant traits were described. As examples:
-
the combination of shoot elongation and large volumes of aerenchyma, to access and enable O2 movement into the body of plants in areas with prolonged shallow floods. Although such O2 movement can occur solely by diffusion, some species also possess convective through-flows. Waterlily is one example, but again the effectiveness of this trait is determined by sub-traits; petiole elongation is a prerequisite for through-flows, as these can only occur when leaves are above water, and high aerenchyma in organs enhances through-flows, as the pathway resistance is less. The through-flows occur along rhizomes, so traits (e.g. large aerenchyma) that enhance O2 diffusion into the adjoining roots are also required.
-
the positive interaction between enhanced shoot elongation, leaf traits for improved underwater photosynthesis, and aerenchyma. While growing fast under water, leaves grow into better illuminated water layers. These better light conditions together with leaf traits that facilitate gas exchange can greatly enhance underwater photosynthesis. This not only improves the carbohydrate status of the plant thus sustaining elongation towards the water surface, but also improves internal aeration of the entire plant body, thus prolonging survival while still submerged.
-
temporal and spatial differences for the plant body in reliance on suites of traits, such as those enhancing underwater photosynthesis and internal aeration during light periods, but traits for anoxia tolerance during dark periods. Diurnal fluctuations in tissue O2 status, as dependent on light and photosynthetic O2 production in shoots, as well as spatial differences depending on location in the plant body, means that both these sets of traits are essential; their contributions to survival depend on temporal and spatial differences in O2 supply.
Future research on waterlogging and submergence tolerance should evaluate plants from various flooding regimes so as to reveal the synergies and trade-offs amongst traits possessed by plants in variable flooding environments.
Acknowledgements
We thank Rana Munns for suggestions on the final draft of this paper, and for inviting our review as part of the series in Functional Plant Biology to honour the 150th anniversary of the publication of Charles Darwin’s ‘On the Origin of Species by Means of Natural Selection’ (Munns 2009). We thank Julia Bailey-Serres and Ronald Pierik for constructive comments on a draft of this paper, and Eric Visser, Julia Bailey-Serres, Motoyuki Ashikari, and Abdel Ismail for advice/help with the Figures used. The ARC–NZ Research Network for Vegetation Function (WG39 – Wetland Plant Traits) provided us with an opportunity to discuss aspects of this review with colleagues. Waterlogging and flooding research in Colmer’s laboratory is supported by GRDC (FFI00004) and the Australian Research Council (LP0882350), and in Voesenek’s laboratory by NWO and CBSG2012 grants.
Adamec L, Kovarova M
(2006) Field growth characteristics of two aquatic carnivorous plants, Aldrovanda vesiculosa and Utricularia australis. Folia Geobotanica 41, 395–406.
| Crossref | GoogleScholarGoogle Scholar |

Armstrong W
(1979) Aeration in higher plants. Advances in Botanical Research 7, 225–332.
| Crossref | GoogleScholarGoogle Scholar |

Armstrong J, Armstrong W
(1999)
Phragmites die-back: effects of propionic, butyric and caproic acids in relation to pH. New Phytologist 142, 201–217.
| Crossref | GoogleScholarGoogle Scholar |

Armstrong J, Armstrong W
(2005) Rice: sulphide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Annals of Botany 96, 625–638.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Armstrong W,
Wright EJ,
Lythe S, Gaynard TJ
(1985) Plant zonation and the effects of the spring-neap tidal cycle on soil aeration in a Humber salt-marsh. Journal of Ecology 73, 323–339.
| Crossref | GoogleScholarGoogle Scholar |

Armstrong J,
Armstrong W, Beckett PM
(1992)
Phragmites australis: venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytologist 120, 197–207.
| Crossref | GoogleScholarGoogle Scholar |

Armstrong W,
Strange ME,
Cringle S, Beckett PM
(1994) Microelectrode and modelling study of oxygen distribution in roots. Annals of Botany 74, 287–299.
| Crossref | GoogleScholarGoogle Scholar |

Armstrong J,
Afreen-Zobayed F, Armstrong W
(1996)
Phragmites die-back: sulphide- and acetic acid-induced bud and root death, lignifications, and blockages within aeration and vascular systems. New Phytologist 134, 601–614.
| Crossref | GoogleScholarGoogle Scholar |

Armstrong W,
Webb T,
Darwent M, Beckett PM
(2009) Measuring and interpreting respiratory critical oxygen pressures in roots. Annals of Botany 103, 281–293.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Baena-González E,
Rolland F,
Thevelein JM, Sheen J
(2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bailey-Serres J, Chang R
(2005) Sensing and signalling in response to oxygen deprivation in plants and other organisms. Annals of Botany 96, 507–518.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bailey-Serres J, Voesenek LACJ
(2008) Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology 59, 313–339.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Berry LJ, Norris WE
(1949) Studies on onion root respiration. I. Velocity of oxygen consumption in different segments of roots at different temperatures as a function of partial pressure of oxygen. Biochimica et Biophysica Acta 3, 593–606.
| Crossref | GoogleScholarGoogle Scholar |

Bhalla US, Iyangar R
(1999) Emergent properties of networks of biological signalling pathways. Science 283, 381–387.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Biemelt S,
Albrecht G, Wiedenroth EM
(1996) The effect of post-hypoxia on roots in Senecio and Myosotis species related to the glutathione system. Folia Geobotanica et Phytotaxonomica 31, 65–72.
| Crossref | GoogleScholarGoogle Scholar |

Blokhina O,
Virolainen E, Fagerstedt KV
(2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany 91, 179–194.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Blom CWPM, Voesenek LACJ
(1996) Flooding: the survival strategies of plants. Trends in Ecology & Evolution 11, 290–295.
| Crossref | GoogleScholarGoogle Scholar |

Boru G,
VanToai T,
Alves J,
Hua D, Knee M
(2003) Responses of soybean to oxygen deficiency and elevated root-zone carbon dioxide concentration. Annals of Botany 91, 447–453.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Branco-Price C,
Kawaguchi R,
Ferreira RB, Bailey-Serres J
(2005) Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Annals of Botany 96, 647–660.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Branco-Price C,
Kaiser KA,
Jang CJH,
Larive CK, Bailey-Serres J
(2008) Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. The Plant Journal 56, 743–755.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bucher M,
Brändle R, Kuhlemeier C
(1996) Glycolytic gene expression in amphibious Acorus calamus L. under natural conditions. Plant and Soil 178, 75–82.
| Crossref | GoogleScholarGoogle Scholar |

Chen HJ, Qualls RG
(2003) Anaerobic metabolism in the roots of seedlings of the invasive exotic Lepidium latifolium. Environmental and Experimental Botany 50, 29–40.

Colmer TD
(2003a) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26, 17–36.
| Crossref | GoogleScholarGoogle Scholar |

Colmer TD
(2003b) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deepwater rice (Oryza sativa L.). Annals of Botany 91, 301–309.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Colmer TD, Pedersen O
(2008a) Oxygen dynamics in submerged rice (Oryza sativa). New Phytologist 178, 326–334.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Colmer TD, Pedersen O
(2008b) Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist 177, 918–926.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Colmer TD,
Huang S, Greenway H
(2001) Evidence for down regulation of ethanolic fermentation and K+ effluxes in the coleoptile of rice seedlings during prolonged anoxia. Journal of Experimental Botany 52, 1507–1517.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Colmer TD,
Cox MCH, Voesenek LACJ
(2006) Root aeration in rice (Oryza sativa L.): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytologist 170, 767–778.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cox MCH,
Millenaar FF,
de Jong van Berkel YEM,
Peeters AJM, Voesenek LACJ
(2003) Plant movement; submergence-induced petiole elongation in Rumex palustris depends on hyponastic growth. Plant Physiology 132, 282–291.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cox MCH,
Benschop JJ,
Vreeburg RAM,
Wagemaker CAM,
Moritz T,
Peeters AJM, Voesenek LACJ
(2004) The roles of ethylene, auxin, abscisic acid and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiology 136, 2948–2960.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Crawford RMM, Brändle R
(1996) Oxygen deprivation stress in a changing environment. Journal of Experimental Botany 47, 145–159.
| Crossref | GoogleScholarGoogle Scholar |

Dacey JWH
(1981) Pressurized ventilation in the yellow waterlily. Ecology 62, 1137–1147.
| Crossref | GoogleScholarGoogle Scholar |

Dixon MH,
Hill SA,
Jackson MB,
Ratcliffe RG, Sweetlove LJ
(2006) Physiological and metabolic adaptations of Potamogeton pectinatus L. tubers support rapid elongation of stem tissue in the absence of oxygen. Plant & Cell Physiology 47, 128–140.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Drew MC,
He CJ, Morgan PW
(2000) Programmed cell death and aerenchyma formation in roots. Trends in Plant Science 5, 123–127.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ella ES,
Kawano N, Ito O
(2003) Importance of active oxygen-scavenging system in the recovery of rice seedlings after submergence. Plant Science 165, 85–93.
| Crossref | GoogleScholarGoogle Scholar |

Fukao T,
Xu K,
Ronald PC, Bailey-Serres J
(2006) A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell 18, 2021–2034.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fukao T,
Harris T, Bailey-Serres J
(2009) Evolutionary analysis of the Sub1 gene cluster that confers submergence tolerance to domesticated rice. Annals of Botany 103, 143–150.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Garthwaite AJ,
von Bothmer R, Colmer TD
(2003) Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum. Functional Plant Biology 30, 875–889.
| Crossref | GoogleScholarGoogle Scholar |

Geigenberger P
(2003) Response of plant metabolism to too little oxygen. Current Opinion in Plant Biology 6, 247–256.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gibbs J, Greenway H
(2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30, 1–47.
| Crossref | GoogleScholarGoogle Scholar |

Goggin DE, Colmer TD
(2005) Intermittent anoxia induces oxidative stress in wheat seminal roots: assessment of the antioxidant defence system, lipid peroxidation and tissue solutes. Functional Plant Biology 32, 495–506.
| Crossref | GoogleScholarGoogle Scholar |

Greenway H, Gibbs J
(2003) Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology 30, 999–1036.
| Crossref | GoogleScholarGoogle Scholar |

Greenway H,
Armstrong W, Colmer TD
(2006) Conditions leading to high CO2 (>5 kPa) in waterlogged-flooded soils and possible effects on root growth and metabolism. Annals of Botany 98, 9–32.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Grimoldi AA,
Insauti P,
Roitman GG, Soriano A
(1999) Responses to flooding intensity in Leontodon taraxacoides. New Phytologist 141, 119–128.
| Crossref | GoogleScholarGoogle Scholar |

Groeneveld HW, Voesenek LACJ
(2003) Submergence-induced petiole elongation in Rumex palustris is controlled by developmental stage and storage compounds. Plant and Soil 253, 115–123.
| Crossref | GoogleScholarGoogle Scholar |

Hattori Y,
Miura K,
Asano K,
Yamamoto E,
Mori H,
Kitano H,
Matsuoka M, Ashikari M
(2007) A major QTL confers rapid internode elongation in response to water rise in deepwater rice. Breeding Science 57, 305–314.
| Crossref | GoogleScholarGoogle Scholar |

He JB,
Bogemann GM,
Van de Steeg HM,
Rijnders JGHM,
Voesenek LACJ, Blom CWPM
(1999) Survival tactics of Ranunculus species in river floodplains. Oecologia 118, 1–8.
| Crossref | GoogleScholarGoogle Scholar |

Holbrook NM, Zwieniecki MA
(2003) Plant biology – water gate. Nature 425, 361.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Huang S,
Greenway H, Colmer TD
(2003) Responses by coleoptiles of intact seedlings to anoxia: K+ net uptake from the external solution and translocation from the caryopses. Annals of Botany 91, 271–278.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Huang S,
Greenway H,
Colmer TD, Millar AH
(2005) Protein synthesis by rice coleoptiles during prolonged anoxia: implications for glycolysis, growth and energy utilization. Annals of Botany 96, 703–715.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Huang S,
Colmer TD, Millar AH
(2008) Does anoxia tolerance involve altering the energy currency towards PPi? Trends in Plant Science 13, 221–227.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Igamberdiev AU, Hill RD
(2009) Plant mitochondrial function during anaerobiosis. Annals of Botany 103, 259–268.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Insausti P,
Grimoldi AA,
Chaneton EJ, Vasellati V
(2001) Flooding induces a suite of adaptive plastic responses in the grass Paspalum dilatatum. New Phytologist 152, 291–299.
| Crossref | GoogleScholarGoogle Scholar |

Ismail AM,
Ella ES,
Vergara GV, Mackill DJ
(2009) Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Annals of Botany 103, 197–209.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jackson MB
(2002) Long-distance signalling from roots to shoots assessed: the flooding story. Journal of Experimental Botany 53, 175–181.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jackson MB
(2008) Ethylene-promoted elongation: an adaptation to submergence stress. Annals of Botany 101, 229–248.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jackson MB, Ram PC
(2003) Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Annals of Botany 91, 227–241.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jackson MB,
Ishizawa K, Ito O
(2009) Evolution and mechanisms of plant tolerance to flooding stress. Annals of Botany 103, 137–142.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Justin SHFW, Armstrong W
(1987) The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106, 465–495.

Kawano N,
Ella E,
Ito O,
Yamauchi Y, Tanaka K
(2002) Metabolic changes in rice seedlings with different submergence tolerance after desubmergence. Environmental and Experimental Botany 47, 195–203.
| Crossref | GoogleScholarGoogle Scholar |

Kende H,
van der Knaap E, Cho H-T
(1998) Deepwater rice: a model plant to study stem elongation. Plant Physiology 118, 1105–1110.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Klok EJ,
Wilson IW,
Wilson D,
Chapman SC,
Ewing RM,
Sommerville SC,
Peacock WJ,
Dolferus R, Dennis ES
(2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. The Plant Cell 14, 2481–2494.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Konings H
(1982) Ethylene-promoted formation of aerenchyma in seedling roots of Zea mays L. under aerated and non-aerated conditions. Physiologia Plantarum 54, 119–124.
| Crossref | GoogleScholarGoogle Scholar |

Kreuzwieser J,
Hauberg J,
Howell KA,
Caroll A,
Rennenberg H,
Millar AH, Whelan J
(2009) Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiology 149, 461–473.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kulichikhin KY,
Greenway H,
Bryne L, Colmer TD
(2009) Regulation of intracellular pH during anoxia in rice coleoptiles in acid and near neutral conditions. Journal of Experimental Botany 60, 2119–2128.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Laan P,
Berrevoets MJ,
Lythe S,
Armstrong W, Blom C
(1989) Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. Journal of Ecology 77, 693–703.
| Crossref | GoogleScholarGoogle Scholar |

Laan P,
Clement JMAM, Blom CWPM
(1991) Growth and development of Rumex roots as affected by hypoxic and anoxic conditions. Plant and Soil 136, 145–151.
| Crossref | GoogleScholarGoogle Scholar |

Laanbroek HJ
(1990) Bacterial cycling of minerals that affect plant growth in waterlogged soils – a review. Aquatic Botany 38, 109–125.
| Crossref | GoogleScholarGoogle Scholar |

Lasanthi-Kudahettige R,
Magneschi L,
Loreti E,
Gonzali S,
Licausi F,
Novi G,
Beretta O,
Vitulli F,
Alpi A, Perata P
(2007) Transcript profiling of the anoxic rice coleoptile. Plant Physiology 144, 218–231.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lee KS,
Park SR, Kim YK
(2007) Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: a review. Journal of Experimental Marine Biology and Ecology 350, 144–175.
| Crossref | GoogleScholarGoogle Scholar |

Liu F,
Van Toai T,
Moy L,
Bock G,
Linford LD, Quackenbush J
(2005) Global transcription profiling reveals novel insights into hypoxic response in Arabidopsis. Plant Physiology 137, 1115–1129.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lorbiecke R, Sauter M
(1999) Adventitious root growth and cell-cycle induction in Deep water rice. Plant Physiology 119, 21–30.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Loreti E,
Poggi A,
Novi G,
Alpi A, Perata P
(2005) Genome-wide analysis of gene expression in Arabidopsis seedlings under anoxia. Plant Physiology 137, 1130–1138.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lynn DE, Waldren S
(2001) Morphological variation in populations of Ranunculus repens from the temporary limestone lakes (turloughs) in the west of Ireland. Annals of Botany 87, 9–17.
| Crossref | GoogleScholarGoogle Scholar |

Lynn DE, Waldren S
(2003) Survival of Ranunculus repens L. (creeping buttercup) in an amphibious habitat. Annals of Botany 91, 75–84.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Magneschi L, Perata P
(2009) Rice germination and seedling growth in the absence of oxygen. Annals of Botany 103, 181–196.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Malik AI,
Colmer TD,
Lambers H, Schortemeyer M
(2001) Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Australian Journal of Plant Physiology 28, 1121–1131.
| Crossref | GoogleScholarGoogle Scholar |

Malik AI,
Colmer TD,
Lambers H,
Setter TL, Schortemeyer M
(2002) Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytologist 153, 225–236.
| Crossref | GoogleScholarGoogle Scholar |

Malik AI,
Colmer TD,
Lambers H,
Setter TL, Schortemeyer M
(2003) Aerenchyma formation and radial O2 loss along adventitious roots of wheat with only the apical root portion exposed to O2-deficiency. Plant, Cell & Environment 26, 1713–1722.
| Crossref | GoogleScholarGoogle Scholar |

McDonald MP,
Galwey NW, Colmer TD
(2002) Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wet- and dry-land grass species. Plant, Cell & Environment 25, 441–451.
| Crossref | GoogleScholarGoogle Scholar |

Millenaar FF,
Cox MCH,
de Jong van Berkel YEM,
Welschen RAM,
Pierik R,
Voesenek LACJ, Peeters AJM
(2005) Ethylene-induced differential growth of petioles in Arabidopsis thaliana; analyzing natural variation, response kinetics and regulation. Plant Physiology 137, 998–1008.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mommer L, Visser EJW
(2005) Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Annals of Botany 96, 581–589.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mommer L,
Pedersen O, Visser EJW
(2004) Acclimation of a terrestrial plant to submergence facilitates gas exchange under water. Plant, Cell & Environment 27, 1281–1287.
| Crossref | GoogleScholarGoogle Scholar |

Mommer L,
Pons TL,
Wolters-Arts M,
Venema JH, Visser EJW
(2005) Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affects gas diffusion resistance and photosynthetic performance. Plant Physiology 139, 497–508.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mommer L,
Pons TL, Visser EJW
(2006a) Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. Journal of Experimental Botany 57, 283–290.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mommer L,
Lenssen JPM,
Huber H,
Visser EWJW, De Kroon H
(2006b) Ecophysiological determinants of plant performance under flooding: a comparative study of seven plant families. Journal of Ecology 94, 1117–1129.
| Crossref | GoogleScholarGoogle Scholar |

Mommer L,
Wolters-Arts M,
Andersen C,
Visser EJW, Pedersen O
(2007) Submergence-induced leaf acclimation in terrestrial species varying in flooding tolerance. New Phytologist 176, 337–345.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Monk LS,
Fagerstedt KV, Crawford RMM
(1987) Superoxide dismutase as an anaerobic polypeptide – a key factor in recovery from oxygen deprivation in Iris pseudacorus? Plant Physiology 85, 1016–1020.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Munns R
(2009) Charles Darwin: an inspiring plant biologist and author. Functional Plant Biology 36, iii.
| Crossref | GoogleScholarGoogle Scholar |

Mustroph A,
Boamfa EI,
Laarhoven LJJ,
Harren FJM,
Albrecht G, Grimm B
(2006) Organ-specific analysis of the anaerobic primary metabolism in rice and wheat seedlings. I. Dark ethanol production is dominated by the shoots. Planta 225, 103–114.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Parolin P
(2009) Submerged in darkness: adaptations to prolonged submergence by woody species of the Amazonian floodplains. Annals of Botany 103, 359–376.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pedersen O
(1993) Long-distance water transport in aquatic plants. Plant Physiology 103, 1369–1375.
| PubMed |

Pedersen O,
Vos H, Colmer TD
(2006) Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant, Cell & Environment 29, 1388–1399.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pedersen O,
Rich SM, Colmer TD
(2009) Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. The Plant Journal 58, 147–156.
| Crossref | GoogleScholarGoogle Scholar |

Perata P, Voesenek LACJ
(2007) Submergence tolerance in rice requires Sub1A, an ethylene-response-factor-like gene. Trends in Plant Science 12, 43–46.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Piedade MTF,
Junk WJ, Long SP
(1991) The productivity of the C4 grass Echinochloa polystachya on the Amazon floodplain. Ecology 72, 1456–1463.
| Crossref | GoogleScholarGoogle Scholar |

Pierik R,
van Aken JM, Voesenek LACJ
(2009) Is elongation-induced leaf emergence beneficial for submerged Rumex species? Annals of Botany 103, 353–357.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Raven JA
(2008) Not drowning but photosynthesizing: probing plant plastrons. New Phytologist 177, 841–845.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rich SM,
Ludwig M, Colmer TD
(2008) Photosynthesis in aquatic adventitious roots of the halophytic stem-succulent Tecticornia pergranulata (formerly Halosarcia pergranulata). Plant, Cell & Environment 31, 1007–1016.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Saab IN, Sachs MM
(1996) A flooding-induced xyloglucan endotransglycosylase homolog in maize is responsive to ethylene and associated with aerenchyma. Plant Physiology 112, 385–391.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sachs MM,
Subbaiah CC, Saab IN
(1996) Anaerobic gene expression and flooding tolerance in maize. Journal of Experimental Botany 47, 1–15.
| Crossref | GoogleScholarGoogle Scholar |

Sato T,
Harada T, Ishizawa K
(2002) Stimulation of glycolysis in anaerobic elongation of pondweed (Potamogeton distinctus) turions. Journal of Experimental Botany 53, 1847–1856.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Setter TL, Laureles EV
(1996) The beneficial effect of reduced elongation growth on submergence tolerance of rice. Journal of Experimental Botany 47, 1551–1559.
| Crossref | GoogleScholarGoogle Scholar |

Setter TL, Waters I
(2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant and Soil 253, 1–34.
| Crossref | GoogleScholarGoogle Scholar |

Setter TL,
Kupkanchanakul T,
Pakinnaka L,
Aguru Y, Greenway H
(1987) Mineral nutrients in floodwater and floating rice growing at water depths up to two metres. Plant and Soil 104, 147–150.
| Crossref | GoogleScholarGoogle Scholar |

Setter TL,
Waters I,
Sharma SK,
Singh KN, Kulshreshtha N ,
et al
.
(2009) Review of wheat improvement for waterlogging tolerance in Australia and India: the importance of anaerobiosis and element toxicities associated with different soils. Annals of Botany 103, 221–235.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Shiono K,
Takahashi H,
Colmer TD, Nakazono M
(2008) Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Science 175, 52–58.
| Crossref | GoogleScholarGoogle Scholar |

Silvertown J,
Dodd ME,
Gowing DJG, Mountford JO
(1999) Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 400, 61–63.
| Crossref | GoogleScholarGoogle Scholar |

Silvestri S,
Defina A, Marani M
(2005) Tidal regime, salinity and salt marsh plant zonation. Estuarine, Coastal and Shelf Science 62, 119–130.
| Crossref | GoogleScholarGoogle Scholar |

Singh S,
Mackill DJ, Ismail AM
(2009) Responses of SUB1 rice introgression lines to submergence in the field: yield and grain quality. Field Crops Research 113, 12–23.
| Crossref | GoogleScholarGoogle Scholar |

Sorrell BK, Dromgoole FI
(1987) Oxygen transport in the submerged freshwater macrophyte Egeria densa Planch. I. Oxygen production, storage and release. Aquatic Botany 28, 63–80.
| Crossref | GoogleScholarGoogle Scholar |

Steffens B, Sauter M
(2009) Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. The Plant Cell 21, 184–196.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Strand VV
(2002) The influence of ventilation systems on water depth penetration of emergent macrophytes. Freshwater Biology 47, 1097–1105.
| Crossref | GoogleScholarGoogle Scholar |

Summers JE,
Ratcliffe RG, Jackson MB
(2000) Anoxia tolerance in the aquatic monocot Potamogeton pectinatus: absence of oxygen stimulates elongation in association with an unusually large Pasteur effect. Journal of Experimental Botany 51, 1413–1422.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tanaka A,
Ranjit P,
Mulleriyawa RP, Yaus T
(1968) Possibility of hydrogen sulphide induced iron toxicity of the rice plant. Soil Science and Plant Nutrition 14, 1–6.

Thomson CJ, Greenway H
(1991) Metabolic evidence for stellar anoxia in maize roots exposed to low O2 concentrations. Plant Physiology 96, 1294–1301.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Thomson CJ,
Armstrong W,
Waters I, Greenway H
(1990) Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant, Cell & Environment 13, 395–403.
| Crossref | GoogleScholarGoogle Scholar |

Tournaire-Roux C,
Sutka M,
Javot H,
Gout E,
Gerbeau P,
Luu DT,
Richard Bligny R, Maurel C
(2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425, 393–397.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Van der Sman AJM,
Blom CWPM, Barendse GWM
(1993) Flooding resistance and shoot elongation in relation to developmental stage and environmental conditions in Rumex maritimus L. and Rumex palustris Sm. New Phytologist 125, 73–84.
| Crossref | GoogleScholarGoogle Scholar |

van Dongen JT,
Frohlich A,
Ramirez-Aguilar SJ,
Schauer N,
Fernie AR,
Erban A,
Kopka J,
Clarke J,
Langer A, Geigenberger P
(2009) Transcript and metabolite profiling of the adaptive response to mild decreases in oxygen concentration in the roots of Arabidopsis plants. Annals of Botany 103, 269–280.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vandeleur R,
Niemietz C,
Tilbrook J, Tyerman SD
(2005) Roles of aquaporins in root responses to irrigation. Plant and Soil 274, 141–161.
| Crossref | GoogleScholarGoogle Scholar |

Verdoucq L,
Grondin A, Maurel C
(2008) Structure–function analyses of plant aquaporin AtPIP2;1 gating by divalent cations and protons. The Biochemical Journal 415, 409–416.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vervuren PJA,
Blom CWPM, de Kroon H
(2003) Extreme flooding events on the Rhine and the survival and distribution of riparian plant species. Journal of Ecology 91, 135–146.
| Crossref | GoogleScholarGoogle Scholar |

Visser EJW,
Cohen JD,
Barendse GWM,
Blom CWPM, Voesenek LACJ
(1996) An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiology 112, 1687–1692.
| PubMed |

Visser EJW,
Colmer TD,
Blom CWPM, Voesenek LACJ
(2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and di-cotyledonous wetland species with contrasting types of aerenchyma. Plant, Cell & Environment 23, 1237–1245.
| Crossref | GoogleScholarGoogle Scholar |

Voesenek LACJ, Pierik R
(2008) Plant stress profiles. Science 320, 880–881.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Voesenek L,
Rijnders J,
Peeters AJM,
Van de Steeg HMV, De Kroon H
(2004) Plant hormones regulate fast shoot elongation under water: from genes to communities. Ecology 85, 16–27.
| Crossref | GoogleScholarGoogle Scholar |

Voesenek LACJ,
Colmer TD,
Pierik R,
Millenaar FF, Peeters AJM
(2006) How plants cope with complete submergence. New Phytologist 170, 213–226.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vreeburg RAM,
Benschop JJ,
Peeters AJM,
Colmer TD, Ammerlaan AHM ,
et al
.
(2005) Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. The Plant Journal 43, 597–610.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Waters I,
Armstrong W,
Thomson CJ,
Setter TL,
Adkins S,
Gibbs J, Greenway H
(1989) Diurnal changes in radial oxygen loss and ethanol metabolism in roots of submerged and non-submerged rice seedlings. New Phytologist 113, 439–451.
| Crossref | GoogleScholarGoogle Scholar |

Xia JH, Roberts JKM
(1996) Regulation of H+ extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment. Plant Physiology 111, 227–233.
| PubMed |

Xu K,
Xu X,
Fukao T,
Canlas P,
Marghirang-Rodriguez R,
Heuer S,
Ismail AM,
Bailey-Serres J,
Ronald PC, Mackill DJ
(2006)
Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442, 705–708.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zabalza A,
van Dongen JT,
Froehlich A,
Oliver SN, Faix B ,
et al
.
(2009) Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiology 149, 1087–1098.
| Crossref | GoogleScholarGoogle Scholar | PubMed |




