Cytokinins – recent news and views of evolutionally old molecules
Lukáš SpíchalA Centre of the Region Haná for Biotechnological and Agricultural Research, Faculty of Science, Palacký University, Šlechtitelů 11, CZ-78371 Olomouc, Czech Republic. Email: lukas.spichal@upol.cz
This paper is part of an ongoing series: ‘The Evolution of Plant Functions’.
Functional Plant Biology 39(4) 267-284 https://doi.org/10.1071/FP11276
Submitted: 14 December 2011 Accepted: 6 March 2012 Published: 24 April 2012
Abstract
Cytokinins (CKs) are evolutionally old and highly conserved low-mass molecules that have been identified in almost all known organisms. In plants, they evolved into an important group of plant hormones controlling many physiological and developmental processes throughout the whole lifespan of the plant. CKs and their functions are, however, not unique to plants. In this review, the strategies and mechanisms of plants – and phylogenetically distinct plant-interacting organisms such as bacteria, fungi, nematodes and insects employing CKs or regulation of CK status in plants – are described and put into their evolutionary context. The major breakthroughs made in the last decade in the fields of CK biosynthesis, degradation and signalling are also summarised.
Additional keywords: biosynthesis, degradation, metabolism, signaling.
Introduction
At the beginning of the last century, Gottlieb Haberlandt introduced the idea that there are diffusible factors in potato (Solanum tuberosum L.) tuber phloem that positively regulate cell division or cytokinesis. The search for such compounds, named cytokinins (CKs), became intensive in the 1950s when Miller and Skoog discovered the first CK, kinetin, in autoclaved herring sperm (Miller et al. 1955). Since then, many natural and synthetic compounds have been classified as CKs, which have become an intensively studied group of plant hormones and an inseparable part of plant physiology. In plants, CKs have been shown to regulate cell division, seed dormancy and germination, senescence, release of buds from apical dominance and de novo bud formation, and stimulation of leaf expansion (reviewed in Mok 1994); to control plant organ development; to mediate the responses to variable extrinsic factors, such as light conditions in the shoot and availability of nutrients and water in the root; and to have a role in the response to biotic and abiotic stress (reviewed in Werner and Schmülling 2009). CKs and their functions are not unique to plants. CKs are involved in RNA translation in phyllogeneticaly distinct organisms from bacteria to humans (Persson et al. 1994; Golovko et al. 2000), and various plant-interacting organisms employ or manipulate CKs to sequester plant developmental programmes to their own ends (Schultz 2002).
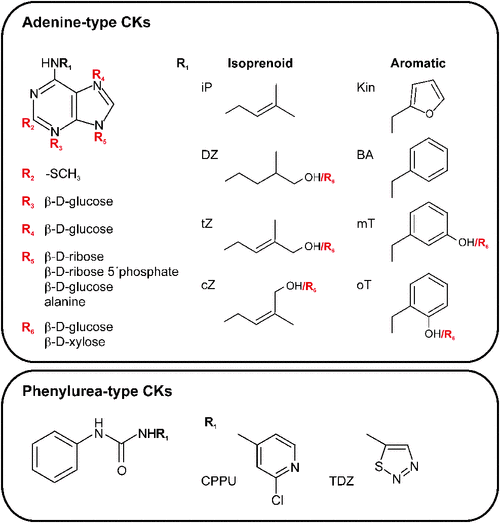
Chemically, natural CKs are derivatives of adenine with N6-side chains that differ in the structure of the N6 substitutent (Mok and Mok 2001). Isoprenoid CKs have saturated or unsaturated aliphatic side chains of isoprenoid origin (Fig. 1). A second group carries an aromatic side chain that can be further substituted in different positions of an aromatic ring (Fig. 1). Almost all natural CKs are present in living organisms as free bases and corresponding nucleosides and nucleotides, and conjugates with glucose, xylose or amino acid residues such as alanine (Fig. 1). After the discovery of kinetin, isoprenoid CKs and their derivatives modified at the C2 position with a methylthiol group (2-MeS CKs), both were identified as functional components of specific tRNAs in all living organisms except for the Archea (Burrows et al. 1968; reviewed in Persson et al. 1994). The first free CK identified from plants was zeatin, isolated from maize (Zea mays L.) endosperm in the 1970s (Letham 1973). The aromatic CK N6-benzyladenine (BA), one of the most highly active and easily obtainable synthetic CKs, and its hydroxylated derivatives (topolins) and methoxy derivatives were also identified as natural CKs in several plant species (reviewed in Strnad 1997; Tarkowská et al. 2003). Other compounds with CK activity are synthetic derivatives of phenylurea (Fig. 1; Shantz and Steward 1955; Iwamura 1994). Despite their structural divergence, both the adenine-type and phenylurea-type CKs exhibit activity in different CK bioassays and are effectively recognised by CK receptors, as will be described later (Yamada et al. 2001; Spíchal et al. 2004; Mok et al. 2005).
|
Since the beginning of the new millennium, substantial progress has been made in understanding the molecular basis of the CK function, biosynthesis, metabolism, degradation, signalling and evolution, which has been summarised in several comprehensive reviews focussed on CK’s role in plants. This review presents a compilation of up-to-date perspectives on CK evolution, biochemistry and function not only in plants but also in other living organisms.
CKs are evolutionally highly conserved molecules with broad phylogenetic occurrence
CKs as a part of the tRNA of phylogenetically distinct organisms from bacteria to vertebrates
CKs are evolutionally highly conserved molecules and, in their free or tRNA-bound form, are present in various organisms such as bacteria, plants, fungi, nematodes, insects and humans. Certain tRNAs that bind to codons starting with an uracil contain, at position 37, an adenine residue modified by an isopentenyl moiety (Golovko et al. 2000). Such prenylation of tRNA belongs to nucleotide hypermodifications that are important for the efficiency and fidelity of protein translation by the ribosome (reviewed by Persson et al. 1994). The enzyme involved in the adjustment is tRNA-specific isopentenyltransferase (tRNA-IPT). Appropriate genes coding the enzyme have been experimentally identified in, for example, Escherichia coli (Caillet and Droogmans 1988), Saccharomyces cerrevisiae (Dihanich et al. 1987), Arabidopsis thaliana (L.) Heynh. (Takei et al. 2001; Kakimoto 2001), Homo sapiens (Golovko et al. 2000) and others. The lack of CK modification in tRNA-IPT mutants causes the destabilisation of the codon–anticodon interactions and codon context sensitivity (Caillet and Droogmans 1988). In mammals, the alterations in tRNA modification have been related to cancer, and the human tRNA-IPT gene (TRIT1) was identified as a candidate tumour suppressor negatively regulating lung carcinogenesis (Spinola et al. 2005).
From the evolutionally fixed function of improving translation as the modified nucleosides of specific tRNAs (Persson et al. 1994) free CKs evolved to have further various specific functions as signalling molecules in plants and organisms interacting with plants. Decomposition of tRNA was thus originaly thought to be a source of free CKs. Although, this has been considered to be insufficient to establish the significant amount of CKs required by seed plants (Barnes et al. 1980), multiplication of genes coding for tRNA-IPT in basal land plants (see Frébort et al. 2011) seems to be the first evolutionary step to increase the source of free CKs as described later.
CK-related genes originate from bacteria
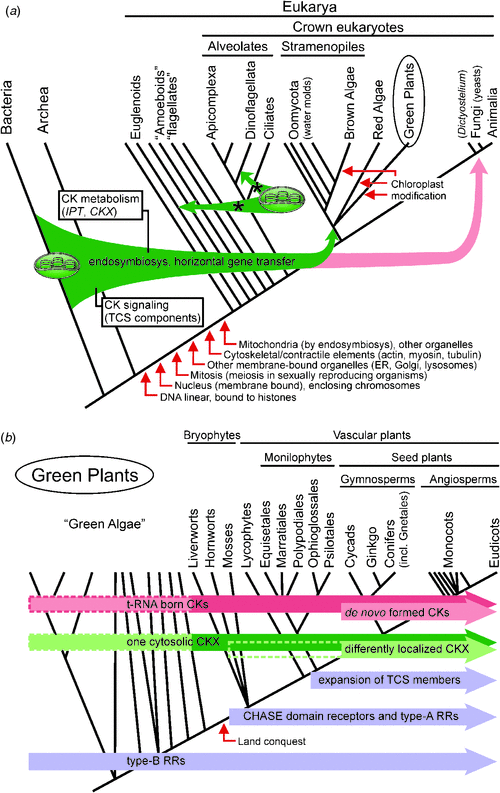
Plants develop effective machinery for the sensing of CKs and management of their levels (detailed information about genes and biochemistry is given in the next section). From the information available, this machinery employs genes that are similar to those existing already in the genomes of the lowest organisms such as bacteria and thus could be of bacterial origin. A possible means of bacterial gene acquisition in plants is via horizontal gene transfer (HGT) through endosymbiosis of a cyanobacterial ancestor, giving rise to chloroplasts (Fig. 2a) and subsequent translocation of genes from the ancestral organelles to the nucleus. Such a gene flow is well documented by analyses of 16S and 23S rRNA, tufA, atpB, rpoC1 and HSP60, each indicating strongly that plastids are derived from cyanobacterial ancestors (Delwiche and Palmer 1996). HGT (combined with gene duplication) played a role in the evolution of Rubisco (Delwiche and Palmer 1996), the key enzyme employed by green plants. Recent genome-wide studies identified CK-related genes coding for the key enzymes of CK biosynthesis (isopentenyltransferase, IPT) and degradation (CK oxidase–dehydrogenase, CKX), respectively, in cyanobacteria and other bacteria (Frébort et al. 2011). Although the function of cyanobacterial IPT and CKX in CK metabolism has not been confirmed yet (Frébort et al. 2011), CKs were identified in several cyanobacteria species such as Synechocystis, Chroococcidiopsis, Anabaena, Phormidium and Oscillatoria (Hussain et al. 2010). This evidence implies that CK metabolism in plants evolved through HGT from bacteria to plants via the chloroplast, which is of cyanobacterial origin (Fig. 2a; Schmülling et al. 2003).
|
Comparative analyses identified several gene families with cyanobacterial ancestry in the genomes of a taxonomically wide range of plastid-lacking eukaryotes (Phytophthora (Chromalveolata), Naegleria (Excavata), Dictyostelium (Amoebozoa), Saccharomyces and Monosiga (Opisthokonta); Maruyama et al. 2009). This demonstrates the potentially significant contributions of ancestral or extant cyanobacteria to the eukaryotic genomes, which probably occurred via HGT or ancient primary endosymbiotic gene transfer events, followed by retention of the transferred genes in the nuclear genomes after the plastid loss (Maruyama et al. 2009). This can explain the presence of CK-related genes in Dictyostelium discoideum, an early eukaryote slime mould that diverged from the line leading to animals shortly after the separation of plants and animals but which retained characteristics of both kingdoms (Anjard and Loomis 2008). Dictyostelium has three IPT genes – two coding proteins closely related to the bacterial and eukaryotic tRNA-IPTs and one IPT involved in de novo CK synthesis – and two genes coding for proteins similar to members of a two-component system (TCS; Anjard and Loomis 2008). TCS is a well described signal transduction pathway in prokaryotes and lower eukaryotes; in plants, it forms a CK signalling pathway (West and Stock 2001). TCS is shared among bacteria, plants and early diverging eukaryotes such as Saccharomyces cerevisiae or Dictyostelium discoideum but not animals. Interestingly, Dictyostelium codes for two cyclases–histidine-kinase-associated sensory extracellular (CHASE) domains that contain proteins that are similar to receptors sensing CK signals in plants. However, neither appears to be a cytokinin receptor and CKs that are essential inducers of Dictyostelium sporulation act through another pathway that is as yet unknown (Anjard and Loomis 2008). No other gene with significant homology to the CK-sensing CHASE domain has been found anywhere apart from land plants, including in algal or cyanobacterial genomes, except for the genome of the virus Ectocarpus siliculosus virus 1 (Pils and Heyl 2009; Frébort et al. 2011). This virus encoding a CHASE domain-containing histidine (His) kinase integrates itself into the genome of the brown algae Ectocarpus siliculosus (which produces CKs) and thus may have acted as a possible vector (Pils and Heyl 2009). In mammals, two enzymes have been found to share sequence homology with the TCS His kinases (Tan et al. 2002). However, the absence of a CHASE domain disqualifies them from this role in CK signalling.
In conclusion, it can be speculated that (1) the individual components of the TCS were most probably acquired by early eukaryotes via HGT from bacteria (Fig. 2a; Anantharaman et al. 2007), (2) during evolution, the pathway was not acquired multiple times but was rather lost in animals (Pils and Heyl 2009).
Evolution of CK machinery in plants
Although CKs have been identified in representatives of brown (Phaeophyta) and red (Rhodophyta) seaweeds as well as many green soil-dwelling microalgae (Chlorophyta) (reviewed in Stirk and Van Staden 2010), the early evolution of CK sensing and metabolism in plants is poorly understood. The algal biosynthetic pathway has not been confirmed yet, no CK-metabolic genes have been identified so far in algal genomes (Gu et al. 2010), and only Type B response regulators (RRs) – but not the cytokinin receptor – have been found in the green unicellular microalgae Chlamydomonas reinhardtii (Pils and Heyl 2009). Two scenarios were suggested for the evolution of CK signalling in algae by Pils and Heyl (2009): (1) algae that were not confronted with the new stress conditions faced by the land conquesting plants and did not need more elaborate developmental programmes requiring a new or more complex regulation of CKs could have lost the CHASE domain His kinase fixed in the last common ancestor of amoebae and algae over time, or (2) CHASE domain His kinase exists in the charaphytes that are ancestral to land plants but the fact that their genome sequences have not been mapped yet has prevented the identification of CK receptors in these algae lineages.
As illustrated in Fig. 2b the different factors of the TCS were gradually acquired during plant evolution. His-containing phospho-transmitter proteins (HPts) and Type B RRs had already appeared in the green algae, whereas a CHASE domain-containing His kinase, a CK receptor and Type A RRs that serve as negative feedback regulators of the pathway were fixed later during the conquest of land in response to new stress stimuli (Pils and Heyl 2009). Phylogenetic analysis has revealed that the hormone-binding receptor and a class of negative regulators first appeared in land plants, as shown in the example of the moss Physcomitrella patens (Pils and Heyl 2009). Interestingly, the number of receptors remained fairly constant from mosses to flowering plants, and the common ancestor probably likely already had three CK receptors before the split of the monocots and the dicots, whereas the other protein families expanded (Fig. 2b; Pils and Heyl 2009; Heyl et al. 2012).
Another interesting evolutionary pattern is visible in CK metabolic genes (Fig. 2b). Both the IPT and CKX genes coexist in the moss Physcomitrella patens, which points to an important evolutionary event generating fine-tuning control of CK homeostasis via biosynthesis and degradation processes (Gu et al. 2010). tRNA-IPTs are evolutionally conserved throughout the whole phylogenetic tree, whereas adenylate IPTs that are capable of de novo CK synthesis occurred later during the evolution of seed plants (Fig. 2b). The basal land plants Physcomitrella patens and Selaginella moellendorffii contain only tRNA-IPTs (Yevdakova and von Schwartzenberg 2007) and decomposition of modified tRNA represent the only source of CKs for them. Interestingly, six tRNA-IPT genes were identified in the Physcomitrella patens genome but only two such genes are present in higher land plants (Frébort et al. 2011). The first evolutionary adaptation of the land plants satisfiing demands for the higher CK production demands connected to the completely new conditions was thus duplication of tRNA-IPTs. However, another strategy – the divergence of the biosynthetic pathway and acquirement of adenylate IPTs capable of de novo CK synthesis – was fixed later during the evolution of seed plants (Frébort et al. 2011; Fig. 2b). Both tRNA-IPTs and adenylate IPTs can be found in the cyanobacteria, pointing again to the possible cyanobacterial origin of the plant genes that might have been acquired through HGT via the chloroplast (Schmülling et al. 2003; Frébort et al. 2011). Plant tRNAs have typically been reported to contain cis-zeatin ribosides (cZR) as the most abundant cytokinin (Taller 1994) and indeed cis-zeatin CKs have been found to be dominant in representatives of liverworts, mosses and ferns (Gajdošová et al. 2011). Although the tRNA pathway is generally considered to be insufficient to establish the significant amount of CKs required by seed plants (Barnes et al. 1980), analysis of atipt2 and atipt9 (both encoding tRNA-IPTs) mutants of Arabidopsis thaliana suggested that tRNA may still represent a significant source of cis-zeatin in higher plants (Miyawaki et al. 2006). A similar situation can be described for CKX genes. In all genomes sequenced so far, one cytosolic CKX is always conserved (Frébort et al. 2011). However, in the basal land plants Physcomitrella and Selaginella, other putative CKXs have been identified (Gu et al. 2010; Frébort et al. 2011). This already implies the drastic expansion of CKX genes into multigene families with a varying number of members with the diverse functions, expression patterns and (sub)cellular localisations that are typical of higher plants (Gu et al. 2010).
Evolution and use of CKs by plant-interacting organisms as tools for plant growth management
CKs have been identified in a broad spectrum of bacterial taxons including Cyanobacteria, Proteobacteria, Actinobacteria and Firmicutes, and also in various phylogenetically very distinct organisms such as fungi, insects and nematodes. Why have these organisms evolved and fixed mechanisms controlling CK production? In most cases, it is linked to the life strategies of plant pathogens or symbionts that actively use CKs for manipulation of plant host growth and development. Diazotrophic cyanobacteria of the genus Nostoc colonise cavities in the host plant’s surface developed by the plant in the absence of the cyanobiont; however, after colonisation, the cyanobacteria can rearrange the plant tissue to form differentiated, elongated host cells increasing the cyanobiont–host surface contact (Beattie 2007). This activity may involve CKs as morphogenetic tools. Gall-forming plant pathogenic bacteria such as Rhodococcus fascians and Agrobacterium tumefaciens produce auxin and CKs as tools to enhance their pathogenicity and to modulate the physiology of host plants (Choi et al. 2011). Unlike Agrobacterium tumefaciens, which engineers the host plant to locally overproduce CKs from transfer DNA (T-DNA) integrated into the plant chromosome (Sakakibara et al. 2005), Rhodococcus fascians produces the efficient mix of CKs (Pertry et al. 2009) involved in enhancement of sensitivity to pathogen-derived CKs and in symptom maintenance (Pertry et al. 2010). CK biosynthesis is also a virulence determinant in other gall-producing bacteria, such as Erwinia herbicola pv. gypsophilae, Pseudomonas syringae pv. savastanoi and Streptomyces turgidiscabies (Joshi and Loria 2007). The CKs produced by the bacteria are mostly N6-isopentenyladenine (iP) and trans-zeatin; however, recent studies indicate the high importance of cis-zeatin and especially the 2-MeS CKs that can serve as symptom maintainers due to their differentiated affinities to the host plants’ sensing and degradation apparatus (Pertry et al. 2009). Symbiotic nitrogen-fixing bacteria such as Rhizobium leguminarosum trigger the formation of a new root-derived organ in their legume hosts, the nitrogen-fixing nodule (reviewed in, for example, Frugier et al. 2008). In this process, CKs act downstream of Nod factor perception, as the key differentiation signal and the proper functioning of the CK receptor was found essential for nodule organogenesis (Gonzalez-Rizzo et al. 2006; Murray et al. 2007; Tirichine et al. 2007). Interestingly, some photosynthetic bradyrhizobia form nodules on their plant hosts, although they lack the canonical nodABC genes and Nod factors (Giraud et al. 2007). Based on the findings that Rhizobium Nod strains expressing bacterial IPT induce nodule formation (Cooper and Long 1994) and that CKs can mimic the effects of Nod factors even when applied exogenously (Lorteau et al. 2001), it is speculated that bradyrhizobia can use CKs as an alternative signal triggering nodule organogenesis (Giraud et al. 2007). The question was addressed whether CK could be the key player in the molecular mechanism used also by other CK-producing symbiotic organisms, such as Frankia, to induce nodulation on nonleguminous plants (Giraud et al. 2007). Plant growth-promoting bacteria of the genera Azospirillum, Bacillus and Pseudomonas are known to have beneficial effects on bacterial–legume or fungal–plant symbioses, probably through the production of phytohormones (including CK) that enhance root hair proliferation and elongation (Beattie 2007).
The same effects are even more enhanced in the mutualistic tripartite relationship of rhizosphere bacteria, plants and arbuscular micorrhizal fungi, such as Glomus, that increase phosphorus content in plants (Beattie 2007). CKs were also identified in some ectomycorrhizal fungi, e.g. Rhizopogon spp. (Miller 1967). CKs are also produced by the phytopathogenic fungi Puccinia thlaspeos, Fusarium moniliforme and Ustilago maydis, and most probably, in concert with other phytohormones, serve to alter the plant host’s morphology (Stirk and Van Staden 2010; Bruce et al. 2011). A putative tRNA-IPT gene was predicted in Ustilago maydis (Bölker et al. 2008) and it was hypothesised to release sufficient CK levels to play a significant role during the development of corn smut disease (Bruce et al. 2011). A soilborne fungal pathogen, Verticillium longisporum, causes vascular disease in oilseed rape (Brassica napus L.) and other members of the Brassicaceae. Preceding the disease symptoms (stunted growth, early senescence), the fungus actively decreases plant CK levels by upregulation of plant CKX by an unknown mechanism that can be reverted by upregulation of the CK biosynthetic gene or application of a CKX inhibitor (TTeichmann and L Spíchal, unpubl. data). Another root parasite, the obligate biotrophic protist Plasmodiophora brassicae (Rhizaria), besides its own CK production, modulates the CK status of a host plant during infection by downregulation of CK degradation and upregulation of CK perception to cause clubroot disease (Siemens et al. 2006).
Although the utilisation of CKs as plant managing signals by fungi, insects and nematodes is a very interesting field to study, recent knowledge is still limited to the analysis of CK content in some species (reviewed in Stirk and Van Staden 2010) and, except for the predicted broad presence of tRNA-IPT genes in all eukaryotes, less is known about this part of CK evolution in nature. In general, the evolution of CK machinery in insects and nematodes seems to be highly connected to the evolution of their parasitism. Insects and nematodes share a common ancestor as members of the high-level taxon Ecdysozoa (Aguinaldo et al. 1997) and both induce cellular modifications in host tissues, including the formation of galls, implying a role for phytohormones (Bird and Koltai 2000). Progress in the sequencing of nematode genomes has revolutionised the understanding of these organisms at the molecular level (Mitreva et al. 2005) and can also help in understanding the aquisition of CK mechanisms by eukaryotes in general. It was shown that besides intrachromosomal rearrangements and modifications to secretory proteins, some parasitic nematodes might rely on virulence factors acquired by HGT from prokaryotes (Mitreva et al. 2005). HGT thus represents an important route of evolution from free-living nematodes to the parasitic lifestyle (Mitreva et al. 2005). In support of this, no striking cases of potential HGT from prokaryotes have been identified in either free-living nematodes or animal parasitic nematodes, although candidate transfers from a fungal genome into Caenorhabditis elegans have been identified (Mitreva et al. 2005). Candidate donors are usually soil-dwelling and are either plant-pathogenic or plant-associated microorganisms, hence occupying the same ecological niche as the nematodes (Haegeman et al. 2011). The most striking examples of HGT between bacteria and root knot nematodes in relation to CKs are the genes with the highest similarity to those involved in the rhizobial nodulation pathway (e.g. NodL). Phylogenetically, rhizobia appear to be the predominant group of ‘donor’ bacteria (Scholl et al. 2003). Moreover, it was suggested that root knot nematodes and bacterial Nod factors elicit common signal transduction events in host plants (Weerasinghe et al. 2005). Nematodes causing galls (Meloidogyne spp.) and cysts (Heterodera spp.) formation in roots are able to reshape the initial feeding cell of a host plant into a functional giant cell or syncytium (Stirk and Van Staden 2010). In this process, CKs, which are possibly secreted by the nematode to the host tissue, are implicated as triggers of cell proliferation, resulting in gall formation and establishment of a nutrient sink at the nematodes’ feeding site (De Meutter et al. 2003).
A similar scenario could also work in the case of gall-inducing insects whose larvae produce CKs in the infected tissue and, by maintaining high CK levels, keep the gall as an active nutrient sink (Stirk and Van Staden 2010). Galling sawfly (Pontania proxima, Hymenoptera) injects the CK-enriched contents of an accessory gland into the host plant tissues with the egg to initiate a tumour-like swelling to accommodate developing larvae (Schultz 2002). CKs or CK-like activity have been identified in larvae of the fly Procecidochares utilis inducing galls in crofton weed (Ageratina adenophora (Spreng.) R. M. King & H. Rob.), the jumping plant louse Pachypsylla celtidis-mamma that causes hackberry nipple galls on Celtis occidentalis L., the Eurytoma wasp that induces galls in Erythrina latissima E. Mey, the fly Eurosta solidaginis that makes ball galls in the stem of Solidago altissima L. and the wasp Dryocosmus kuriphilus that forms galls in the buds of Japanese chestnut (Castanea crenata Siebold & Zucc.; reviewed in Stirk and Van Staden 2010). Interestingly, differences in CK profiles and different rates of CK biosynthesis were found in the male and female larvae of the wasp Trichilogaster acaciaelongifoliae, which form sexually dimorphic galls in developing inflorescence of Acacia longifolia (Andrews) Willd., supporting the idea that larvae can synthesise CKs independently (Dorchin et al. 2009). It can be assumed that these species adopted CK biosynthetic machinery and produce their own CKs to influence the growth and development of their host plants. However, a recent study showed that the phytophagous leaf-mining moth Phyllonorycter blancardella (Lepidoptera) relies on CK-producing bacterial endosymbionts, most probably Wolbachia, to manipulate the physiology of its host plant, resulting in photosynthetically active green patches in otherwise senescent leaves and to increase its fitness (Kaiser et al. 2010). It is also worth mentioning the work by Tsoupras et al. (1983), who published an identification of the predominating maternal conjugate of ecdysone with CK, N6-isopentenyladenosine-5′-monophosphate (iPRMP) in newly laid eggs of Locusta migratoria. The role in embryonic development of CK liberated on the process of hydrolysis of the maternal ecdyson is however not known (Tsoupras et al. 1983).
CK biosynthesis, metabolism, degradation and perception
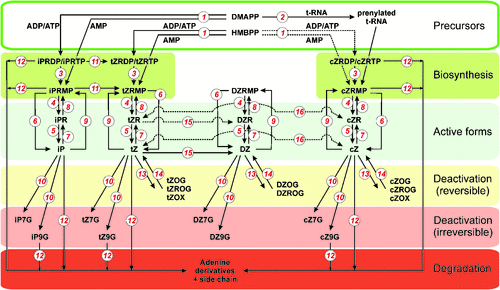
In this part a of the review, a survey of known genes and enzymes regulating CK biosynthesis, metabolism and signalling will be described to give basic knowledge of the machinery involved in CK homeostasis in living organisms. A scheme of CK biosynthesis, interconversions and degradation in plants is shown in Fig. 3.
|
CK biosynthesis
The first and rate-limiting step in CK biosynthesis is the transfer of an isoprenoid moiety to the N6-position of the adenine nucleotide catalysed by IPT. This enzyme activity was first described in extracts of the slime mould Dictyostelium discoideum (Taya et al. 1978), which produces a spore germination inhibitor discadenine, a compound that is structurally related to the CKs and is active in a CK bioassay (Nomura et al. 1977). Two types of structurally related IPT enzymes derived from a common ancestral gene differing in their target of isopentenylation are known to produce CKs in living organisms. The tRNA-IPT enzyme (EC 2.5.1.8) modifies some tRNAs by adding the dimethylallylpyrophosphate (DMAPP) isopentenyl moiety to the adenine residue adjacent to the anticodon. The broad phylogenetical appearance of tRNA-IPTs and the significance of the tRNA isopentenylation have been discussed in the previous text.
Another IPT, adenylate IPT (EC 2.5.1.27) is a free nucleotide-forming enzyme known to be a main contributor to the CK pool. The first identification of a gene encoding a CK de novo biosynthetic enzyme was carried out in the gall-forming bacteria Agrobacterium tumefaciens (Akiyoshi et al. 1984; Barry et al. 1984). This gene, designated Tmr (tumour morphology root), is located in the T-DNA of the tumour-inducing (Ti) plasmid and encodes an IPT that is primarly associated with synthesis of iPRMP from DMAPP and AMP (Blackwell and Horgan 1991), but with the same Km value that also recognises 4-hydroxy-3-methyl-2-(E)-butenyl diphosphate (HMBPP) as the side chain substrate (Sakakibara et al. 2005). In the virulence region of the Agrobacterium tumefaciens Ti-plasmid, which is not translocated to the plant cells, another gene encoding the enzyme of trans-zeatin synthesis (Tzs) is present, utilising HMBPP as a prenyl donor and AMP as the acceptor (Krall et al. 2002). Recently, Tzs was crystallised and critical amino acid residues responsible for differences in the prenyl-donor substrate specificity of Tzs and plant IPTs were determined in its substrate binding pocket (Sugawara et al. 2008). CK biosynthesis is also a virulence determinant of other gall-producing bacteria, and IPTs were identified and functionally proved in, for example, the collinear fas operons of Streptomyces turgidiscabies (Fas4; Joshi and Loria 2007) and Rhodococcus fascians (FasD; Pertry et al. 2010).
A family of plant DMAPP:ATP/ADP IPT genes were first identified in Arabidopsis thaliana (Takei et al. 2001; Kakimoto 2001). Arabidopsis thaliana contains AtIPT genes that are involved in de novo biosynthesis (AtIPT1 and AtIPT3–AtIPT8) and two tRNA IPTs (AtIPT2 and AtIPT9; Takei et al. 2001; Kakimoto 2001). IPTs were identified and functionally proved in other plants such as hop (Humulus lupulus L.)(HlIPT; Sakano et al. 2004), mulberry (Morus alba L.; Abe et al. 2007), maize (ZmIPT2; Brugière et al. 2008) and rice (Oryza sativa L.) (OsIPT1–OsIPT8; Sakamoto et al. 2006). Biochemical characterisations showed that all plant IPTs described so far prefer DMAPP as the side chain donor, and ATP or ADP, but not AMP, as the accepting moiety to form N6-isopentenyladenosine-5′-triphosphate (iPTP) or iPDP as the precursors of active CKs. In contrast to bacterial Tmr, Arabidopsis IPTs exclusively use DMAPP as a substrate (Sakakibara et al. 2005). However, Morus alba IPT was shown to also utilise HMBPP, supporting the idea of trans-zeatin formation in plants via the iPRMP-independent pathway suggested by Åstot et al. (2000). Interestingly, this enzyme also accepts dADP, dATP, CDP and GDP as the prenyl acceptors (Sakamoto et al. 2006). Binding affinity of nucleotides in the order of ATP > dATP~ADP > GTP > CTP > UTP was recently reported for HlIPT (Chu et al. 2010). Crystallisation studies on Agrobacterium Tzs revealed that the carbon–nitrogen-based prenylation proceeds by the SN2-reaction mechanism (Sugawara et al. 2008) and, together with structural studies of HlIPT (Chu et al. 2010) and yeast tRNA-IPT (Zhou and Huang 2008), have given insight into substrate recognition.
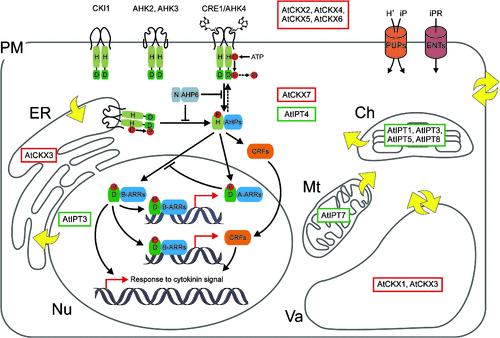
AtIPT-GFP fusions were used for localisation of AtIPT isoforms at the subcellular level showing plastid (AtIPT1, -3, -5 and -8), cytosolic (AtIPT2 and -4) and mitochondrial (AtIPT7) localisations (Fig. 3; Kasahara et al. 2004). However, localisation of AtIPT3 is dependent on its farnesylation status and, despite the presence of a chloroplast transit peptide, farnesylation appears to direct most of the protein to the nucleus (Galichet et al. 2008). This finding, together with the endoplasmic reticulum (ER) localisation of AtCKX3 (Werner et al. 2003), now seems to be very relevant due to new evidence of CK receptor localisation to the ER membrane, with the CK sensor domain most probably oriented into the lumen of the ER (Caesar et al. 2011). Interestingly, farnesylation also substantially influences the catalytic activity of AtIPT3 (Galichet et al. 2008). AtIPT::GUS expression analysis revealed wide distribution of expression patterns throughout the plant during its developmental stages (Miyawaki et al. 2004). AtIPT expression is affected by application of nutrient, CK and auxin (Miyawaki et al. 2004; Takei et al. 2004a; Tanaka et al. 2006). A study on multiple AtIPT knockout mutants for AtIPT1, AtIPT3, AtIPT5, AtIPT6 and AtIPT7 reported by Miyawaki et al. (2004) showed that plants with reduced CK biosynthesis display the CK deficiency phenotypes described for plants overexpressing CKX (Werner et al. 2001; Werner et al. 2003).
CK metabolism
CKs are metabolised in living systems by modification of their purine moiety, or by N6-side chain modifications or cleavage. These structural changes lead to reversible or irreversible loss of CK activity, and determine the function and compartmentalisation of the respective CK metabolite.
The enzymes of general purine metabolism can convert the adenine ring of CKs to the respective nucleoside and nucleotide. Several enzymes involved in these interconversions as 5′-nucleotidase (EC 3.1.3.5), adenosine nucleosidase (EC 3.2.2.7), adenine phosphoribosyltransferase (EC 2.4.2.7) and adenosine kinase (ADK, EC 2.7.1.20) were found in different plant species (reviewed in Mok and Mok 2001). Although these enzymes have broad substrate specificity and recognise adenosines in general, an ADK was isolated from tobacco (Nicotiana tabacum L.) BY-2 suspension culture using CK-based affinity purification (Laukens et al. 2003), and ADKs cloned from Nicotiana tabacum have been shown to have high affinity to CKs (Kwade et al. 2005). Recently, Arabidopsis thaliana lines silenced in ADK expression proved that despite its generally high Km for CK ribosides, ADK contributes significantly to CK homeostasis in vivo (Schoor et al. 2011).
The list of the enzymes modifying CKs at the adenine part of the molecule was extended in 2007 with the CK-specific phosphoribohydrolase ‘lonely guy’ (LOG; Kurakawa et al. 2007) that directly converts inactive CK nucleotides to their free-base forms. The role of LOG in CK metabolism was confirmed in Arabidopsis thaliana (Kuroha et al. 2009; Tokunaga et al. 2012) and is also supported by localisation of the LOG homologue in the fas operon involved in the production of CKs by Rhodococcus fascians (Pertry et al. 2010).
Next to ribosylation and phosphoribosylation, other frequent modifications of the purine ring are glucosylation of N-3, N-7 and N-9, and the formation of alanyl conjugates (Duke et al. 1978; Shaw 1994). The enzyme involved in N-glucosylation of N-7 and N-9, a glucosyltransferase (EC 2.4.1.118), uses uridine diphosphate glucose (UDPG) and uridine triphosphate glucose (TDPG) as glucosyl donors. With the exception of N-3 glucosides, N-glucosides are generally not active in bioassays and it has been assumed that N-7 and N-9 glucosylation irreversibly inactivates CKs (Mok and Mok 2001), whereas N-3 glucoside can be converted to the free base by β-glucosidases (Brzobohatý et al. 1993). Recently, CK N-9 glucosides were found to be the preferred substrates of some CKX isoforms (Galuszka et al. 2007; Kowalska et al. 2010).
Other types of glycosylation affect the N6-side chain of hydroxylated forms of CKs and contribute to the modulation of CK activity through the side chain modifications. Genes encoding the O-glucosyltransferase (ZOG, EC 2.4.1.203; Dixon et al. 1989) and O-xylosyltransferase (EC 2.4.1.204; Turner et al. 1987) were cloned from Phaseolus lunatus L. and Phaseolus vulgaris L., respectively. Their biochemical characterisation showed differences in CK substrate and sugar donor recognition. ZOG uses both UDPG and uridine diphosphate xylose (UDPX) as donor substrates, whereas O-xylosyltransferase uses only UDPX. Both the transferases recognise the CK substrate in a highly specific manner and only the trans-isomer of zeatin and partial dihydrozeatin are the substrates. The strict substrate specificity of the enzymes suggests that O-glycosylation is a precisely regulated process. This is supported by identification of a maize gene encoding a cis-zeatin O-glucosyltransferase (cisZOG) that does not recognise either trans-zeatin or dihydrozeatin, and uses UPDG as the only sugar donor (Martin et al. 2001). Besides trans-zeatin and cis-zeatin, ZOG and cisZOG also recognise topolins and hydroxylated phenylureas as substrates, and the substrate specificities correlate with CK recognition by the Arabidopsis thaliana and maize CK receptors (Mok et al. 2005). O-glycosylation has been shown to preserve the CKs from N6-side chain cleavage by CK oxidase (Armstrong 1994). In addition, O-glycosylated CKs can be easily converted to the active form by β-glucosidases (EC 3.2.1.21; Brzobohatý et al. 1993), which explains the biological activity of O-glucosylated CKs in different bioassays, although they are not recognised by CK receptors (Spíchal et al. 2004). Based on the facts mentioned above and the finding that O-glucosylated dihydrozeatin is localised in vacuoles (Fusseder and Ziegler 1988), it is believed that O-glycosylation produces an inactive, stable storage form of CKs and thus plays an important role in balancing CK levels (Mok and Mok 2001).
Another important N6-side chain modification is hydroxylation of the isopentenyladenine side chain. This modification represents important step of trans-zeatin biosynthesis and is catalysed by cytochrome P450 mono-oxygenases CYP735A1 and CYP735A2 (Takei et al. 2004b). The double bond of zeatin can be reduced by a zeatin reductase (EC 1.3.1.69) to form dihydrozeatin with an unsaturated trans-zeatin side chain. Zeatin reductase isolated from immature seeds of Phaseolus vulgaris is highly specific to trans-zeatin base and does not reduce the cis-form of zeatin or isopentenyladenine (Martin et al. 1989). The enzyme converting zeatin from the trans- to the cis- isoform was partially purified from Phaseolus (Bassil et al. 1993). However, such conversion slowly proceeds in vitro and without any enzymatic activity, and no other works so far have been published that exploit the physiological significance of zeatin isomerisation.
In some organisms, the key metabolic regulation of CK contents is provided by an irreversible cleavage of the N6-side chain. This enzymatic activity was first reported by Pačes et al. (1971), who demonstrated conversion of radiolabelled N6-isopentenyladenine to adenine in a crude tobacco tissue. The enzyme was named CK oxidase (shortened to CKO by the original author; nowadays, the acronym CKX is more often used) and for a long time was classified as a copper amine oxidase (EC 1.4.3.6) utilising oxygen as the electron acceptor (Whitty and Hall 1974; Hare and Van Staden 1994). To date, the sequences of genes encoding CKX proteins have been identified in many plant species such as Arabidopsis, maize, rice, orchid (Dendrobium Sonia), barley (Hordeum vulgare L.) and wheat (Triticum aestivum L.)(review in Frébort et al. 2011). CKX genes were also identified in several prokaryotic organisms such as the bacteria Rhodococcus fascians (the FasE gene), the function of which was confirmed by studies of the substrate specificity of a corresponding recombinant enzyme (Pertry et al. 2010) and the presence of homologous genes was found in cyanobacterial genomes (e.g. of Nostoc and Anabaena; (reviewed in Schmülling et al. 2003; Frébort et al. 2011). Cloning of the first CKX gene from maize (ZmCKX1) and its expression in a yeast and a moss (Houba-Hérin et al. 1999; Morris et al. 1999) launched detailed biochemical studies on CKX. The enzyme was found to contain a covalently bound flavin adenine dinucleotide (FAD) cofactor (Houba-Hérin et al. 1999; Morris et al. 1999; Bilyeu et al. 2001). The reaction proceeds through a two-electron transfer from the CK substrate to an FAD cofactor accompanied by the formation of an imine intermediate (Laloue and Fox 1985) that hydrolyses to adenine and unsaturated aldehyde (Brownlee et al. 1975; Popelková et al. 2006). The finding that CKX can effectively use a variety of artificial electron acceptors under anaerobic conditions (Galuszka et al. 2001; Frébort et al. 2002) led to the reclassification of the enzyme as CK dehydrogenase (EC 1.5.99.12). Biochemical studies on the ZmCKX1 reaction mechanism suggested the preference of quinones as electron acceptors (Frébortová et al. 2004) generated from plant phenolics by peroxidise, and either tyrosine, laccase or catechol oxidase (Galuszka et al. 2005). Recently, free radicals generated by enzymatic oxidation of natural benzoxazinones were shown to be the most efficient electron acceptors of CKX (Frébortová et al. 2010).
So far, two CKXs have been crystallised. The crystal structures of ZmCKX1 and AtCKX7 reveal a highly conserved active site in terms of both architecture and amino acid composition (Malito et al. 2004; Bae et al. 2008). The enzyme does not undergo any conformational changes upon substrate binding. The substrate binds above the isoalloxazine plane of the FAD cofactor and its amino group forms a hydrogen bond with an aspartic acid–glutamic acid (Asp–Glu) pair that facilitates the oxidation (Malito et al. 2004). Studies on the substrate specificity of Arabidopsis thaliana and maize CKX isoforms revealed that not only free bases and ribosides of isoprenoid CKs, including cis-zeatin (Šmehilová et al. 2009; Pertry et al. 2010; Gajdošová et al. 2011), but also CK 9-glucosides and nucleotides are effectively cleaved by individual isoforms (Galuszka et al. 2007; Kowalska et al. 2010). It was also presented that aromatic CKs are degraded by CKX as well, although with lower reaction rates (Frébortová et al. 2004; Galuszka et al. 2007). The individual CKX isoforms also differ in their cellular localisation (Fig. 4). In Arabidopsis thaliana, CKX proteins targeted either the vacuoles (AtCKX1 and AtCKX3) or the apoplast (AtCKX2, AtCKX4, AtCKX5 and AtCKX6), except for AtCKX7, which lacks the signal peptide, indicating its cytosolic localisation (reviewed in Frébort et al. 2011). The vacuolar localisation of AtCKX1 and AtCKX3, the ER localisation of AtCKX3 and secretion of AtCKX2 were confirmed by protein–GFP fusions (Werner et al. 2003). It is expected that all secreted CKX enzymes are post-transcriptionaly modified by glycosylation (Galuszka et al. 2008). Glycosylation most probably contributes to the enzyme localisation, and also to translocation and protein stability (Schmülling et al. 2003), as the glycosylated CKXs were shown to have different pH optima and higher activity compared with the nonglycosylated forms (Kamínek and Armstrong 1990; Motyka et al. 2003).
|
CK perception and signal transduction
CKs act in plants as signalling molecules at only nanomolar concentrations and their interaction with a specific receptor represents a crucial step leading to conversion of the signal into the specific response. Several authors report on biochemical approaches leading to identification of CK-binding proteins (CBPs) in several plant species such as maize, barley, wheat, oats (Avena sativa L.), tobacco, cucumber (Cucumis sativus L.), carrot (Daucus carota L.) and Vigna radiata (L.) R. Wilczek (Brinegar 1994; Brault et al. 1999; Kamínek et al. 2003; Pasternak et al. 2006). Despite the many CBPs that exhibited binding of CKs in vitro, their Kd values were too low to qualify them as receptors. However, what was interesting was the preference in binding of aromatic CKs found for CBPs localised in wheat and oat grains, suggesting that CBPs may regulate the levels of CK that has an aromatic side chain by their immobilisation during grain development (Kamínek et al. 2003).
Initial attempts to identify the genuine CK receptor resulted in the discovery of the CKI1 (CK-independent 1) protein homologous to the His kinases and receiver domains of the bacterial TCS pathway (Kakimoto 1996). Although CKI1 does not contain the CK-binding domain and does not bind CKs in vitro (Yamada et al. 2001), it shares at least some of the signalling proteins with the two-component phosphorelay system in the CK signalling pathway (Hejátko et al. 2009). CKI1 is critical in female gametophyte development (Pischke et al. 2002; Hejátko et al. 2003) and is important for vascular development via the regulation of procambium proliferation and the maintenance of its identity (Hejátko et al. 2009).
In 2001, CRE1 (CK response 1; Inoue et al. 2001) and AHK2, 3, 4 (Arabidopsis histidine kinase 2, 3, 4; Suzuki et al. 2001) were identified in Arabidopsis thaliana as genes coding for putative CK receptors. CRE1 was found to be identical to AHK4 and to Wooden Leg (WOL), which was described as being essential for root growth and normal cell division during initial embryonic vascular formation (Mähönen et al. 2000). AHK2, AHK3 and CRE1/AHK4/WOL are His kinases that are homologous to proteins of the TCS pathway, which is a common sensor and intracellular signalling system among prokaryotes, lower eukaryotes and plants (Parkinson and Kofoid 1992; Mizuno 1998). Their protein structure consists of two (CRE1/AHK4) to three (AHK2 and AHK3) hydrophobic membrane-spanning domains at the N-terminal part, followed by transmitter and receiver domains possessing an invariant His or Asp residue, respectively (Ueguchi et al. 2001a). The N-terminal loop contains a ligand-binding region (the CHASE domain) that is common among prokaryotes and lower eukaryotes, where it serves as ligand-binding domain for low molecular weight ligands and small peptides (Mougel and Zhulin 2001).
The function of AHKs as CK receptors was proved by heterologous complementation of His kinase-dependent pathways of yeast (Inoue et al. 2001; Ueguchi et al. 2001a; Suzuki et al. 2001) and bacteria (Suzuki et al. 2001; Yamada et al. 2001) mutants engineered to express CK receptors and for easy scoring of the CK response (reviewed in Schmülling 2001). Expression of CRE1/AHK4, AHK3 and AHK2-CHASE-TM (AHK2-CHASE including the two adjacent transmembrane domains) in Escherichia coli ΔrcsC and cps::lacZ mutant strains producing β-galactosidase in reaction to CK (Suzuki et al. 2001), and direct receptor binding assays (Romanov et al. 2005) were used for molecular and biochemical characterisation of the receptors (Suzuki et al. 2001; Yamada et al. 2001; Spíchal et al. 2004; Romanov et al. 2006). It has been shown that the receptors bind isoprenoid and aromatic CKs, and also thidiazuron (a derivative of phenylurea), in a highly specific manner with Kd in the nanomolar range (Yamada et al. 2001; Romanov et al. 2005; Romanov et al. 2006; Lomin et al. 2011; Stolz et al. 2011). Individual receptors of Arabidopsis thaliana are most sensitive to the bases of the isoprenoid-type CKs and thidiazuron, but differ in their recognition of CK metabolites. In contrast to CRE1/AHK4 and AHK2, which effectively discriminate between CK basis and their conjugates, AHK3 has broader specificity and, alongside cis-zeatin and dihydrozeatin (DZ) (Spíchal et al. 2004; Stolz et al. 2011) noticeably recognises CK ribosides and nucleotides (Spíchal et al. 2004). AHK3 shows a stronger preference towards trans-zeatin-type CKs than iP-type CKs, whereas CRE1/AHK4 recognises both trans-zeatin and iP similarly (Spíchal et al. 2004; Romanov et al. 2006). Based on the opposite predominant occurrences of trans-zeatin- and iP-type CKs in xylem and phloem sap, respectively, and the fact that AHK3 is predominantly expressed in the shoot but CRE1/AHK4 is expressed in the root (Ueguchi et al. 2001b; Higuchi et al. 2004), the specific function of AHK3 of responding to a long-distance signal coming from the roots was suggested (Romanov et al. 2006). A recent study by Stolz et al. (2011) showed that AHK2 is highly similar to CRE1/AHK4 in ligand preference and function, as proved by the promoter-swap experiments. Interestingly, in contrast to AHKs, all maize His kinases have very high affinity to both isoprenoid and aromatic CKs (Lomin et al. 2011) and are effectively activated by cis-zeatin (Yonekura-Sakakibara et al. 2004). These findings helped to refute the long-held picture of cis-zeatin as a nonactive CK and, together with new findings of the phylogenetic and ontogenetic distribution, biological activities and metabolism of cis-zeatin in plants (Gajdošová et al. 2011), reopened the discussions about the true physiological role of cis-zeatin. The molecular basis of the ligand recognition by CK receptors has been unravelled only recently thanks to crystallisation of the sensor domain of CRE1/AHK4 (Hothorn et al. 2011). Superposition of CRE1/AHK4 sensor domain structures accommodating chemically distinct adenine-type CKs and thidiazuron revealed that the basic design principles for active CKs are the presence of a planar ring structure occupying the adenine-binding pocket, followed by a linker competent to establish hydrogen bonds with Asp262 and a planar aliphatic or aromatic tail group (Hothorn et al. 2011). To determine the relevant biological functions of individual receptors, the loss-of-function single (Ueguchi et al. 2001b), double and triple mutants of AHKs were studied (Nishimura et al. 2004; Higuchi et al. 2004; Riefler et al. 2006). The analyses revealed partially redundant but differentiated functions for the individual receptors and prominent roles for the AHK2–AHK3 receptor combination in quantitative control of organ growth, with opposite regulatory functions in roots and shoots (Riefler et al. 2006). The physiological consequences of the lowered CK perception are described below. Three very recent studies reported the localisation of the Arabidopsis and maize CK receptors in the ER (Fig. 4; Wulfetange et al. 2011; Lomin et al. 2011; Caesar et al. 2011). Although the general concept of CK signalling assumed that CK receptors are located in the plasma membrane (Kim et al. 2006) and sense the extracellular hormone (Inoue et al. 2001; Ueguchi et al. 2001a), data on the pH dependence of CK binding by AHK3 and CRE1/AHK4 support the function of the receptors inside the plant cell (Romanov et al. 2006). The CK signalling pathway consists of three elements interacting via phosphorelay from the phospho-accepting His to Asp residues of conserved domains. Binding of the CK ligand into the receptor active site triggers kinase activity that autophosphorylates the His residue of the receptor transmitter domain with subsequent release of the phosphate to the Asp of the receiver domain. Downstream from the receptors, the phosphate is transferred to HPts, which transfer the phosphate to the Asp residues of the response regulators localised in the nucleus (Fig. 4; Hwang and Sheen 2001). Interestingly, CRE1/AHK4, exclusively, has a dual function and works in the presence of CK as a kinase phosphorylating Arabidopsis histidine phosphotransfer proteins (AHPs) but, conversely, in the absence of CK, acts as a phosphatase dephosphorylating AHPs (Fig. 4; Mähönen et al. 2006).
The AHP family of CK signal transducers contains five HPts (AHP1–AHP5), small proteins with phosphate-accepting His domain ssimilar to the His kinase transmitter domain of the AHK receptors, and one pseudo-HPt (AHP6) in which the conserved His residue essential for the His–Asp phosphorelay is replaced by asparagine (Asn) (Suzuki et al. 2000; Mähönen et al. 2006). The function of AHPs to transmit the signal from the receptor to RRs localised in the nucleus was demonstrated by functional complementation of yeast HPts mutants by AHP1, AHP2 and AHP3 (Suzuki et al. 2000), and the function of some AHPs as cytoplasmatic–nuclear shuttles in response to exogenously applied CK was suggested (Hwang and Sheen 2001). However, a recent study by Punwani et al. (2010) suggested that the overall distribution of AHPs remains nucleo-cytosolic independently of CK signalling, and proposed that the AHP proteins undergo constant, active, bidirectional nuclear–cytosolic transport regardless of their phosphorylation status and that this movement serves to shuttle phosphoryl groups into and out of the nucleus. Analysis of AHP1–5 multiple insertion mutants indicated that most of the AHPs are redundant, positive regulators of CK signalling and affect multiple aspects of plant development (Hutchison et al. 2006), whereas the pseudo-HPT AHP6 works as the inhibitor of CK signalling (Mähönen et al. 2006).
HPts phosphorylate RRs as the target points of the CK signalling pathway. Based on their protein structure, all 22 Arabidopsis thaliana RRs (ARRs) were divided into two major groups, Type A and Type B (D’Agostino and Kieber 1999; Imamura et al. 1999). Type B ARRs contain a receiver domain followed by an extended C-terminus with an output domain including a GARP motif that is related to the DNA binding motifs of MYB transcription factors (Riechmann et al. 2000). These RRs are localised in the nucleus and act as transcriptional activators (Lohrmann et al. 1999; Sakai et al. 2000, 2001). The Type A RRs are relatively small and contain a receiver domain followed by short C-terminal extensions (Mason et al. 2004). Type A, but not Type B, ARRs are very rapidly induced in response to CK application (D’Agostino et al. 2000) and their protein levels are stabilised via phosphorylation upon stimulation of the pathway (To et al. 2007). After phosphorylation by AHP, the sequestered Type B ARRs are released from a negative regulator protein and activate transcription of target genes via binding to cis elements in their promoters (Hwang and Sheen 2001). Such target genes may be Type A ARRs or CK response factors (CRFs; Rashotte et al. 2006) that both further positively regulate transcription of CK response genes (Fig. 4). In parallel, CRFs are induced by phosphorylated AHPs and relocalise to the nucleus (Rashotte et al. 2006). Transient overexpression of some of Type A ARRs caused the repression of other Type A ARRs, indicating negative feedback control of the CK signalling pathway (Hwang and Sheen 2001). The negative regulation of CK signalling by the Type A ARRs most probably involves phosphorylation-dependent interactions (To et al. 2007), although it may also involve competition with the Type B ARRs for phosphorylation by the AHP proteins (Kieber and Schaller 2010). Studies of multiple knockouts of the both groups of RRs indicated functional overlap of their members and confirmed the roles of positive regulators of Type B RRs and negative regulators of Type A RRs in CK signalling, respectively (To et al. 2004; Mason et al. 2005).
Transgenic plants with altered CK status as tools for understanding CK functions
After the discovery of CKs, only exogenous applications were available for studies of their biological function. Progress in plant molecular genetics offered transformation with bacterial ipt as a tool for manipulation of endogenous CK levels. Several works described how the expression of ipt under the control of specific promoters leads to massively increased CK concentrations causing the so-called general CK syndrome, which comprises reduced apical dominance followed by release of axillary buds, reduced stem and leaf area, reduced root growth and retarded leaf senescence (Medford et al. 1989; Schmülling et al. 1989; Beinsberger et al. 1991; Hewelt et al. 1994; Zhang et al. 1995; Gan and Amasino 1995; Redig et al. 1996; Faiss et al. 1997). Anther-specific expression of ipt in tobacco pointed to the effect of CKs on floral organ systems and reproductivity as well (Sa et al. 2002). Further roles of CKs in regulation of plant growth and development were discovered using transgenic plants with altered CK metabolism and perception. Cloning of the first CKX genes in 2001 enabled studies of endogenous CK functions in plants by creating their deficiency through CKX overproduction. Transgenic tobacco and Arabidopsis thaliana plants constitutively expressing the CKX genes from the 35S promoter exhibited reduced CK contents, leading to the CK deficiency phenotype, which is characterised by formation of slow-growing, stunted shoots with small leaves; an enlarged root system; and distinct changes in plant sink and source parameters (Werner et al. 2001, 2003; 2008; Yang et al. 2003). The developmental consequences of CK deficiency were later confirmed by studies of Arabidopsis thaliana ipt (CK biosynthetic) loss-of-function mutants (Miyawaki et al. 2006). Construction of Arabidopsis thaliana CK receptor mutants revealed a high level of redundancy of CRE1/AHK4, AHK3 and AHK2; however, analyses of the multiple mutants has assigned functions to CKs in the control of shoot growth, leaf senescence, seed size and germination, root elongation and branching, and CK metabolism (Higuchi et al. 2004; Nishimura et al. 2004; Riefler et al. 2006). Surprisingly, the lack of all three receptors is not lethal and the plants develop normally, although they are markedly limited in growth and reproduction (Higuchi et al. 2004; Nishimura et al. 2004; Riefler et al. 2006). This raises the question whether CKs are indeed essential hormones for plant development or if any other recognition system for CK operates in plants. In legumes, analysis of loss-of-function and gain-of-function mutations of CK receptors has revealed the direct and essential involvement of CK perception in nodule organogenesis (Gonzalez-Rizzo et al. 2006; Murray et al. 2007; Tirichine et al. 2007). Similar phenotypes to the plants with genetically reduced CK perception have also been described for plants with reduced CK transduction and response (i.e. higher-order mutants of His phosphotransfer proteins (ahp1, -2,- 3, -4 and -5; Hutchison et al. 2006) and Type B response regulators (arr1, -10 and -12; Mason et al. 2005), or plants expressing 35S:ARR1-SRDX, a dominant repressor of response regulator ARR1 (Heyl et al. 2008)). Mutation of particular CKX isoforms leading to flower organ-specific enhancement of CK levels confirmed the role of CK in regulation of flower and reproductive development through control of the size and activity of floral meristems and placenta (Bartrina et al. 2011). Ectopic overexpression of the CK-activating enzyme LOG showed pleiotropic phenotypes, such as promotion of cell division in embryos and leaf vascular tissues, reduced apical dominance and a delay of leaf senescence (Kuroha et al. 2009). Very recently, analyses of Arabidopsis thaliana LOG multiple knockout mutants suggested the dominant role of the single-step activation pathway mediated by LOGs for CK production (Tokunaga et al. 2012). Tokunaga et al. (2012) showed that some LOG gene family members have overlapping but differentiated functions in growth and development, and are required for the maintenance of shoot apical meristem size and normal primary root growth.
Conclusions and perspectives
The last century ended with the knowledge about CK function covering the identification of many natural CKs and their metabolites in various organisms, synthesis of a great number of CK analogues and descriptions of their function (in classical CK bioassays) or effects (after exogenous application to plants). The enzymatic activity connected to CK biosynthesis and degradation was extensively studied in plants and the first biosynthetic gene (ipt) was identified in bacteria. The era of molecular genetics offered the possibility to use the gene to investigate the effects of CK overproduction in plants through ectopic expression of ipt, and various physiological roles have been attributed to CKs. Many basic aspects of CKs’ mode of action were, however, poorly understood. Symbolically, the dawn of the new millennium also brought a new day for CK research. The first genes involved in CK degradation (CKX) were cloned and were used for generation of CK-deficient plants as tools for understanding the genuine roles of endogenous CK in plants and the basic concept of CKs as positive regulators of the shoot and negative regulators of the root was confirmed (Werner et al. 2001). In 2001, the enigma of CK biosynthesis and signalling in plants was unravelled, the relevant gene families were cloned and their proteins were functionally described. Great collections of (mainly Arabidopsis thaliana) mutants have been prepared and have helped us understand the molecular basis of CK function, CKs’ interaction with other hormones and their adaptation to different conditions and stresses.
However, many questions still remain to be answered. There is, for example, simple evidence missing regarding the biosynthesis of aromatic CKs. Although aromatic CKs have been identified as natural compounds, and the ability of plants to recognise them by their signalling and metabolic machinery has been described, the biosynthetic pathway of aromatic CKs is not known. CKs work both as paracrine and long-distance signalling molecules. Thus the transport inside and outside the cell as well as across the membranes of the subcellular compartments should represent an important means of regulating CK levels and function in plants. The proteins capable of transporting CK bases (purine permeases; Bürkle et al. 2003) and CK nucleosides (equilibrative nucleoside transporters; Hirose et al. 2005) have been reported; however, their affinities to CKs and selectivity do not seem sufficient to consider them specific CK transporters. Hence, CK transport seems to be one of the next topics to be solved. What are the other directions for future CK research? The subcellular localisation of CK receptors that was recently discoverd calls for the revision of the CK signalling model and promises new exciting plots within the CK story. Structural insights into how CK bindis to the CRE1/AHK4 receptor (Hothorn et al. 2011) should accelerate the search for CK receptor antagonists that might have interesting applications in agriculture (Spíchal et al. 2009; Nisler et al. 2010) as root-growth promoting substances (Arata et al. 2010). Similarly, due to the finding that CKX activity is a determinant of generative organ development and yield (Ashikari et al. 2005; Bartrina et al. 2011), great potential lies ahead for the practical use of deregulation of CK degradation by transgenic approaches or by chemical inhibition of CKX activity. CK perception and metabolism are involved in response to various biotic and abiotic stresses, which opens new possibilities for agricultural applications to increase the stress tolerance. Finally, it will be very interesting to continue in tracking CK evolution and the missing connections and relationships may be unravelled with the sequencing of new genomes.
Acknowledgements
I thank Dr Petr Galuszka and Professor Miroslav Strnad for their helpful suggestions and critical reading of the manuscript. This work was supported by the Czech Ministry of Education Youth and Sports (MSM 6198959216), and by Grant no. ED0007/01/01, Centre of the Region Hana for Biotechnological and Agricultural Research.
References
Abe I, Tanaka H, Abe T, Noguchi H (2007) Enzymatic formation of unnatural cytokinin analogs by adenylate isopentenyltransferase from mulberry. Biochemical and Biophysical Research Communications 355, 795–800.| Enzymatic formation of unnatural cytokinin analogs by adenylate isopentenyltransferase from mulberry.Crossref | GoogleScholarGoogle Scholar |
Aguinaldo AMA, Turbeville JM, Linford LS, Rivera MC, Garey JR, Raff RA, Lake JA (1997) Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387, 489–493.
| Evidence for a clade of nematodes, arthropods and other moulting animals.Crossref | GoogleScholarGoogle Scholar |
Akiyoshi DE, Klee H, Amasino RM, Nester EW, Gordon MP (1984) T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 81, 5994–5998.
| T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis.Crossref | GoogleScholarGoogle Scholar |
Anantharaman V, Iyer LM, Aravind L (2007) Comparative genomics of protists: new insights into the evolution of eukaryotic signal transduction and gene regulation. Annual Review of Microbiology 61, 453–475.
| Comparative genomics of protists: new insights into the evolution of eukaryotic signal transduction and gene regulation.Crossref | GoogleScholarGoogle Scholar |
Anjard C, Loomis WF (2008) Cytokinins induce sporulation in Dictyostelium. Development 135, 819–827.
| Cytokinins induce sporulation in Dictyostelium.Crossref | GoogleScholarGoogle Scholar |
Arata Y, Nagasawa-Iida A, Uneme H, Nakajima H, Kakimoto T, Sato R (2010) The phenylquinazoline compound S-4893 is a non-competitive cytokinin antagonist that targets Arabidopsis cytokinin receptor CRE1 and promotes root growth in Arabidopsis and rice. Plant & Cell Physiology 51, 2047–2059.
| The phenylquinazoline compound S-4893 is a non-competitive cytokinin antagonist that targets Arabidopsis cytokinin receptor CRE1 and promotes root growth in Arabidopsis and rice.Crossref | GoogleScholarGoogle Scholar |
Armstrong DJ (1994) Cytokinin oxidase and the regulation of cytokinin degradation. In ‘Cytokinins. Chemistry, activity and function’. (Ed. DWS Mok, MC Mok) pp. 139–154. (CRC Press: Boca Raton)
Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005) Cytokinin oxidase regulates rice grain production. Science 309, 741–745.
| Cytokinin oxidase regulates rice grain production.Crossref | GoogleScholarGoogle Scholar |
Åstot C, Doležal K, Nordström A, Wang Q, Kunkel T, Moritz T, Chua NH, Sandberg G (2000) An alternative cytokinin biosynthesis pathway. Proceedings of the National Academy of Sciences of the United States of America 97, 14778–14783.
| An alternative cytokinin biosynthesis pathway.Crossref | GoogleScholarGoogle Scholar |
Bae E, Bingman CA, Bitto E, Aceti DJ, Phillips GN (2008) Crystal structure of Arabidopsis thaliana cytokinin dehydrogenase. Proteins 70, 303–306.
| Crystal structure of Arabidopsis thaliana cytokinin dehydrogenase.Crossref | GoogleScholarGoogle Scholar |
Barnes MF, Tien CL, Gray JS (1980) Biosynthesis of cytokinins by potato cell cultures. Phytochemistry 19, 409–412.
| Biosynthesis of cytokinins by potato cell cultures.Crossref | GoogleScholarGoogle Scholar |
Barry GF, Rogers SG, Fraley RT, Brand L (1984) Identification of a cloned cytokinin biosynthetic gene. Proceedings of the National Academy of Sciences of the United States of America 81, 4776–4780.
| Identification of a cloned cytokinin biosynthetic gene.Crossref | GoogleScholarGoogle Scholar |
Bartrina I, Otto E, Strnad M, Werner T, Schmülling T (2011) Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. The Plant Cell 23, 69–80.
| Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Bassil NV, Mok D, Mok MC (1993) Partial purification of a cis-trans-isomerase of zeatin from immature seed of Phaseolus vulgaris L. Plant Physiology 102, 867–872.
Beattie GA (2007) Plant-associated bacteria: survey, molecular phylogeny, genomics and recent advances. In ‘Plant-associated bacteria’. (Ed. SS Gnanamanickam) pp. 1–56. (Springer: Berlin)
Beinsberger SEI, Valcke RLM, Deblaere RY, Clijsters HMM, De Greef JA, Van Onckelen H (1991) Effects of the introduction of Agrobacterium tumefaciens T-DNA ipt gene in Nicotiana tabaccum L. cv. Petit Havana Sr1 plant cels. Plant & Cell Physiology 32, 489–496.
Bilyeu KD, Cole JL, Laskey JG, Riekhof WR, Esparza TJ, Kramer MD, Morris RO (2001) Molecular and biochemical characterization of a cytokinin oxidase from maize. Plant Physiology 125, 378–386.
| Molecular and biochemical characterization of a cytokinin oxidase from maize.Crossref | GoogleScholarGoogle Scholar |
Bird DMcK, Koltai H (2000) Plant parasitic nematodes: habitats, hormones, and horizontally-acquired genes. Journal of Plant Growth Regulation 19, 183–194.
Blackwell JR, Horgan R (1991) A novel strategy for production of a highly expressed in an active form. FEBS Letters 295, 10–12.
| A novel strategy for production of a highly expressed in an active form.Crossref | GoogleScholarGoogle Scholar |
Bölker M, Basse CW, Schirawski J (2008) Ustilago maydis secondary metabolism – from genomics to biochemistry. Fungal Genetics and Biology 45, S88–S93.
| Ustilago maydis secondary metabolism – from genomics to biochemistry.Crossref | GoogleScholarGoogle Scholar |
Brault M, Caiveau O, Pédron J, Maldiney R, Sotta B, Miginiac E (1999) Detection of membrane-bound cytokinin-binding proteins in Arabidopsis thaliana cells. European Journal of Biochemistry 260, 512–519.
| Detection of membrane-bound cytokinin-binding proteins in Arabidopsis thaliana cells.Crossref | GoogleScholarGoogle Scholar |
Brinegar C (1994) Cytokinin-binding proteins and receptors. In ‘Cytokinins. Chemistry, activity and function’. (Ed. DWS Mok, MC Mok) pp. 217–232. (CRC Press: Boca Raton)
Brownlee BG, Hall RH, Whitty CD (1975) 3-Methyl-2-butenal: an enzymatic degradation product of the cytokinin, N-6-(delta-2 isopentenyl)adenine. Canadian Journal of Biochemistry 53, 37–41.
| 3-Methyl-2-butenal: an enzymatic degradation product of the cytokinin, N-6-(delta-2 isopentenyl)adenine.Crossref | GoogleScholarGoogle Scholar |
Bruce SA, Saville BJ, Emery RJN (2011) Ustilago maydis produces cytokinins and abscisic acid for potential regulation of tumor formation in maize. Journal of Plant Growth Regulation 30, 51–63.
| Ustilago maydis produces cytokinins and abscisic acid for potential regulation of tumor formation in maize.Crossref | GoogleScholarGoogle Scholar |
Brugière N, Humbert S, Rizzo N, Bohn J, Habben JE (2008) A member of the maize isopentenyl transferase gene family, Zea mays isopentenyl transferase 2 (ZmIPT2), encodes a cytokinin biosynthetic enzyme expressed during kernel development. Cytokinin biosynthesis in maize. Plant Molecular Biology 67, 215–229.
| A member of the maize isopentenyl transferase gene family, Zea mays isopentenyl transferase 2 (ZmIPT2), encodes a cytokinin biosynthetic enzyme expressed during kernel development. Cytokinin biosynthesis in maize.Crossref | GoogleScholarGoogle Scholar |
Brzobohatý B, Moore I, Kristoffersen P, Bako L, Campos N, Schell J, Palme K (1993) Release of active cytokinin by a beta-glucosidase localized to the maize root meristem. Science 262, 1051–1054.
| Release of active cytokinin by a beta-glucosidase localized to the maize root meristem.Crossref | GoogleScholarGoogle Scholar |
Bürkle L, Cedzich A, Döpke C, Stransky H, Okumoto S, Gillissen B, Kühn C, Frommer WB (2003) Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. The Plant Journal 34, 13–26.
| Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Burrows WJ, Armstrong DJ, Skoog F, Hecht SM, Boyle JT, Leonard NJ, Occolowitz J (1968) Cytokinin from soluble RNA of Escherichia coli: 6-(3-methyl-2-butenylamino)-2-methylthio-9-beta-D-ribofuranosylpurine. Science 161, 691–693.
| Cytokinin from soluble RNA of Escherichia coli: 6-(3-methyl-2-butenylamino)-2-methylthio-9-beta-D-ribofuranosylpurine.Crossref | GoogleScholarGoogle Scholar |
Caesar K, Thamm AMK, Witthöft J, Elgass K, Huppenberger P, Grefen C, Horák J, Harter K (2011) Evidence for the localization of the Arabidopsis cytokinin receptors AHK3 and AHK4 in the endoplasmic reticulum. Journal of Experimental Botany 62, 5571–5580.
| Evidence for the localization of the Arabidopsis cytokinin receptors AHK3 and AHK4 in the endoplasmic reticulum.Crossref | GoogleScholarGoogle Scholar |
Caillet J, Droogmans L (1988) Molecular cloning of the Escherichia coli miaA gene involved in the formation of delta 2-isopentenyl adenosine in tRNA. Journal of Bacteriology 170, 4147–4152.
Choi J, Choi D, Lee S, Ryu C-M, Hwang I (2011) Cytokinins and plant immunity: old foes or new friends? Trends in Plant Science 16, 388–394.
| Cytokinins and plant immunity: old foes or new friends?Crossref | GoogleScholarGoogle Scholar |
Chu H-M, Ko T-P, Wang AH-J (2010) Crystal structure and substrate specificity of plant adenylate isopentenyltransferase from Humulus lupulus: distinctive binding affinity for purine and pyrimidine nucleotides. Nucleic Acids Research 38, 1738–1748.
| Crystal structure and substrate specificity of plant adenylate isopentenyltransferase from Humulus lupulus: distinctive binding affinity for purine and pyrimidine nucleotides.Crossref | GoogleScholarGoogle Scholar |
Cooper JB, Long SR (1994) Morphogenetic rescue of Rhizobium meliloti nodulation mutants by trans-zeatin secretion. The Plant Cell 6, 215–225.
D’Agostino IB, Kieber JJ (1999) Phosphorelay signal transduction: the emerging family of plant response regulators. Trends in Biochemical Sciences 24, 452–456.
| Phosphorelay signal transduction: the emerging family of plant response regulators.Crossref | GoogleScholarGoogle Scholar |
D’Agostino IB, Deruere J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiology 124, 1706–1717.
| Characterization of the response of the Arabidopsis response regulator gene family to cytokinin.Crossref | GoogleScholarGoogle Scholar |
De Meutter J, Tytgat T, Witters E, Gheysen G, van Onckelen H, Gheysen G (2003) Identification of cytokinins produced by the plant parasitic nematodes Heterodera schachtii and Meloidogyne incognita. Molecular Plant Pathology 4, 271–277.
| Identification of cytokinins produced by the plant parasitic nematodes Heterodera schachtii and Meloidogyne incognita.Crossref | GoogleScholarGoogle Scholar |
Delwiche CF, Palmer JD (1996) Rampant horizontal transfer and duplication of Rubisco genes in eubacteria and plastids. Molecular Biology and Evolution 13, 873–882.
Dihanich ME, Najarian D, Clark R, Gillman EC, Martin NC, Hopper AK (1987) Isolation and characterization of MOD5, a gene required for isopentenylation of cytoplasmic and mitochondrial tRNAs of Saccharomyces cerevisiae. Molecular and Cellular Biology 7, 177–184.
Dixon SC, Martin RC, Mok MC, Shaw G, Mok DWS (1989) Zeatin glycosylation enzymes in Phaseolus. Isolation of O-glucosyltransferase from P. lunatus and comparison to O-xylosyltransferase from P. vulgaris. Plant Physiology 90, 1316–1321.
| Zeatin glycosylation enzymes in Phaseolus. Isolation of O-glucosyltransferase from P. lunatus and comparison to O-xylosyltransferase from P. vulgaris.Crossref | GoogleScholarGoogle Scholar |
Dorchin N, Hoffman JH, Stirk WA, Novák O, Strnad M, van Staden J (2009) Sexually dimorphic gall structures correspond to differential phytohormone contents in male and female wasp larvae. Physiological Entomology 34, 359–369.
| Sexually dimorphic gall structures correspond to differential phytohormone contents in male and female wasp larvae.Crossref | GoogleScholarGoogle Scholar |
Duke CC, MacLeod JK, Summons RE, Letham DS, Parker CW (1978) The structure and synthesis of cytokinin metabolites II. Lupinic acid and O-β-D-glucopyranosyl zeatin from Lupinus augustifolius. Australian Journal of Chemistry 31, 1291–1301.
| The structure and synthesis of cytokinin metabolites II. Lupinic acid and O-β-D-glucopyranosyl zeatin from Lupinus augustifolius.Crossref | GoogleScholarGoogle Scholar |
Faiss M, Zalubilová J, Strnad M, Schmülling T (1997) Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. The Plant Journal 12, 401–415.
| Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants.Crossref | GoogleScholarGoogle Scholar |
Frébort I, Šebela M, Galuszka P, Werner T, Schmülling T, Peč P (2002) Cytokinin oxidase/cytokinin dehydrogenase assay: optimized procedures and applications. Analytical Biochemistry 306, 1–7.
| Cytokinin oxidase/cytokinin dehydrogenase assay: optimized procedures and applications.Crossref | GoogleScholarGoogle Scholar |
Frébort I, Kowalska M, Hluska T, Frébortová J, Galuszka P (2011) Evolution of cytokinin biosynthesis and degradation. Journal of Experimental Botany 62, 2431–2452.
| Evolution of cytokinin biosynthesis and degradation.Crossref | GoogleScholarGoogle Scholar |
Frébortová J, Fraaije MW, Galuszka P, Šebela M, Peč P, Hrbáč J, Novák O, Bilyeu KD, English JT, Frébort I (2004) Catalytic reaction of cytokinin dehydrogenase: preference for quinones as electron acceptors. Biochemical Journal 380, 121–130.
| Catalytic reaction of cytokinin dehydrogenase: preference for quinones as electron acceptors.Crossref | GoogleScholarGoogle Scholar |
Frébortová J, Novák O, Frébort I, Jorda R (2010) Degradation of cytokinins by maize cytokinin dehydrogenase is mediated by free radicals generated by enzymatic oxidation of natural benzoxazinones. The Plant Journal 61, 467–481.
| Degradation of cytokinins by maize cytokinin dehydrogenase is mediated by free radicals generated by enzymatic oxidation of natural benzoxazinones.Crossref | GoogleScholarGoogle Scholar |
Frugier F, Kosuta S, Murray JD, Crespi M, Szczyglowski K (2008) Cytokinin: secret agent of symbiosis. Trends in Plant Science 13, 115–120.
| Cytokinin: secret agent of symbiosis.Crossref | GoogleScholarGoogle Scholar |
Fusseder A, Ziegler P (1988) Metabolism and compartmentation of dihydrozeatin exogenously supplied to photoautotrophic suspension cultures of Chenopodium rubrum. Planta 173, 104–109.
| Metabolism and compartmentation of dihydrozeatin exogenously supplied to photoautotrophic suspension cultures of Chenopodium rubrum.Crossref | GoogleScholarGoogle Scholar |
Gajdošová S, Spíchal L, Kamínek M, Hoyerová K, Novák O, Dobrev PI, Galuszka P, Klíma P, Gaudinová A, Žižková E, Hanuš J, Dančák M, Trávníček B, Pešek B, Krupička M, Vaňková R, Strnad M, Motyka V (2011) Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. Journal of Experimental Botany 62, 2827–2840.
| Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants.Crossref | GoogleScholarGoogle Scholar |
Galichet A, Hoyerová K, Kamínek M, Gruissem W (2008) Farnesylation directs AtIPT3 subcellular localization and modulates cytokinin biosynthesis in Arabidopsis. Plant Physiology 146, 1155–1164.
| Farnesylation directs AtIPT3 subcellular localization and modulates cytokinin biosynthesis in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Galuszka P, Frébort I, Šebela M, Sauer P, Jacobsen S, Peč P (2001) Cytokinin oxidase or dehydrogenase? Mechanism of cytokinin degradation in cereals. European Journal of Biochemistry 268, 450–461.
| Cytokinin oxidase or dehydrogenase? Mechanism of cytokinin degradation in cereals.Crossref | GoogleScholarGoogle Scholar |
Galuszka P, Frébortová J, Luhová L, Bilyeu KD, English JT, Frébort I (2005) Tissue localization of cytokinin dehydrogenase in maize: possible involvement of quinone species generated from plant phenolics by other enzymatic systems in the catalytic reaction. Plant & Cell Physiology 46, 716–728.
| Tissue localization of cytokinin dehydrogenase in maize: possible involvement of quinone species generated from plant phenolics by other enzymatic systems in the catalytic reaction.Crossref | GoogleScholarGoogle Scholar |
Galuszka P, Popelková H, Werner T, Frébortová J, Pospíšilová H, Mik V, Köllmer I, Schmülling T, Frébort I (2007) Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L. Journal of Plant Growth Regulation 26, 255–267.
| Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L.Crossref | GoogleScholarGoogle Scholar |
Galuszka P, Spíchal L, Kopečný D, Tarkowski P, Frébortová J, Šebela M, Frébort I (2008) Metabolism of plant hormones cytokinins and their function in signaling, cell differentiation and plant development. In ‘Studies in natural products chemistry, Vol. 34.’ (Ed. Atta-ur-Rahman) pp. 203–264. (Elsevier: Amsterdam)
Gan S, Amasino RM (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270, 1986–1988.
| Inhibition of leaf senescence by autoregulated production of cytokinin.Crossref | GoogleScholarGoogle Scholar |
Giraud E, Moulin L, Vallenet D, Barbe V, Cytryn E, Avarre JC, Jaubert M, Simon D, Cartieaux F, Prin Y, Bena G, Hannibal L, Fardoux J, Kojadinovic M, Vuillet L, Lajus A, Cruveiller S, Rouy Z, Mangenot S, Segurens B, Dossat C, Franck WL, Chang WS, Saunders E, Bruce D, Richardson P, Normand P, Dreyfus B, Pignol D, Stacey G, Emerich D, Verméglio A, Médigue C, Sadowsky M (2007) Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316, 1307–1312.
| Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia.Crossref | GoogleScholarGoogle Scholar |
Golovko A, Hjälm G, Sitbon F, Nicander B (2000) Cloning of a human tRNA isopentenyl transferase. Gene 258, 85–93.
| Cloning of a human tRNA isopentenyl transferase.Crossref | GoogleScholarGoogle Scholar |
Gonzalez-Rizzo S, Crespi M, Frugier F (2006) The Medicago truncatula CRE1 CK receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. The Plant Cell 18, 2680–2693.
| The Medicago truncatula CRE1 CK receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti.Crossref | GoogleScholarGoogle Scholar |
Gu R, Fu J, Guo S, Duan F, Wang Z, Mi G, Yuan L (2010) Comparative expression and phylogenetic analysis of maize cytokinin dehydrogenase/oxidase (CKX) gene family. Journal of Plant Growth Regulation 29, 428–440.
| Comparative expression and phylogenetic analysis of maize cytokinin dehydrogenase/oxidase (CKX) gene family.Crossref | GoogleScholarGoogle Scholar |
Haegeman A, Jones JT, Danchin EG (2011) Horizontal gene transfer in nematodes: a catalyst for plant parasitism? Molecular Plant—Microbe Interactions 24, 879–887.
| Horizontal gene transfer in nematodes: a catalyst for plant parasitism?Crossref | GoogleScholarGoogle Scholar |
Hare PD, Van Staden J (1994) Cytokinin oxidase: biochemical features and physiological significance. Physiologia Plantarum 91, 128–136.
| Cytokinin oxidase: biochemical features and physiological significance.Crossref | GoogleScholarGoogle Scholar |
Hejátko J, Pernišová M, Eneva T, Palme K, Brzobohatý B (2003) The putative sensor histidine kinase CKI1 is involved in female gametophyte development in Arabidopsis. Molecular Genetics and Genomics 269, 443–453.
| The putative sensor histidine kinase CKI1 is involved in female gametophyte development in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Hejátko J, Ryu H, Kim G-T, Dobešová R, Choi S, Choi SM, Souček P, Horák J, Pekárová B, Palme K, Brzobohaty B, Hwang I (2009) The histidine kinases CYTOKININ-INDEPENDENT1 and ARABIDOPSIS HISTIDINE KINASE2 and 3 regulate vascular tissue development in Arabidopsis shoots. The Plant Cell 21, 2008–2021.
| The histidine kinases CYTOKININ-INDEPENDENT1 and ARABIDOPSIS HISTIDINE KINASE2 and 3 regulate vascular tissue development in Arabidopsis shoots.Crossref | GoogleScholarGoogle Scholar |
Hewelt A, Prinsen E, Schell J, Van Onckelen H, Schmülling T (1994) Promoter tagging with a promoterless ipt gene leads to cytokinin-induced phenotypic variability in transgenic tobacco plants: implications of gene dosage effects. The Plant Journal 6, 879–891.
| Promoter tagging with a promoterless ipt gene leads to cytokinin-induced phenotypic variability in transgenic tobacco plants: implications of gene dosage effects.Crossref | GoogleScholarGoogle Scholar |
Heyl A, Ramireddy E, Brenner WG, Riefler M, Allemeersch J, Schmülling T (2008) The transcriptional repressor ARR1-SRDX suppresses pleiotropic cytokinin activities in Arabidopsis. Plant Physiology 147, 1380–1395.
| The transcriptional repressor ARR1-SRDX suppresses pleiotropic cytokinin activities in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Heyl A, Riefler M, Romanov GA, Schmülling T (2012) Properties, functions and evolution of cytokinin receptors. European Journal of Cell Biology 91, 246–256.
| Properties, functions and evolution of cytokinin receptors.Crossref | GoogleScholarGoogle Scholar |
Higuchi M, Pischke MS, Mähönen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, Helariutta Y, Sussman MR, Kakimoto T (2004) In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences of the United States of America 101, 8821–8826.
| In planta functions of the Arabidopsis cytokinin receptor family.Crossref | GoogleScholarGoogle Scholar |
Hirose N, Makita N, Yamaya T, Sakakibara H (2005) Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside transporter in rice suggest a function in cytokinin transport. Plant Physiology 138, 196–206.
| Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside transporter in rice suggest a function in cytokinin transport.Crossref | GoogleScholarGoogle Scholar |
Hothorn M, Dabi T, Chory J (2011) Structural basis for cytokinin recognition by Arabidopsis thaliana histidine kinase 4. Nature Chemical Biology 7, 766–768.
| Structural basis for cytokinin recognition by Arabidopsis thaliana histidine kinase 4.Crossref | GoogleScholarGoogle Scholar |
Houba-Hérin N, Pethe C, d’Alayer J, Laloue M (1999) Cytokinin oxidase from Zea mays: purification, cDNA cloning and expression in moss protoplasts. The Plant Journal 17, 615–626.
| Cytokinin oxidase from Zea mays: purification, cDNA cloning and expression in moss protoplasts.Crossref | GoogleScholarGoogle Scholar |
Hussain A, Krischke M, Roitsch T, Hasnain S (2010) Rapid determination of cytokinins and auxin in cyanobacteria. Current Microbiology 61, 361–369.
| Rapid determination of cytokinins and auxin in cyanobacteria.Crossref | GoogleScholarGoogle Scholar |
Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM, Ecker JR, Kieber JJ (2006) The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. The Plant Cell 18, 3073–3087.
| The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling.Crossref | GoogleScholarGoogle Scholar |
Hwang I, Sheen J (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413, 383–389.
| Two-component circuitry in Arabidopsis cytokinin signal transduction.Crossref | GoogleScholarGoogle Scholar |
Imamura A, Hanaki N, Nakamura A, Suzuki T, Taniguchi M, Kiba T, Ueguchi C, Sugiyama T, Mizuno T (1999) Compilation and characterization of Arabidopsis thaliana response regulators implicated in His–Asp phosphorelay signal transduction. Plant & Cell Physiology 40, 733–742.
Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409, 1060–1063.
| Identification of CRE1 as a cytokinin receptor from Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Iwamura H (1994) Cytokinin antagonists: synthesis and biological activity. In ‘Cytokinins. Chemistry, activity and function’. (Ed. DWS Mok, MC Mok) pp. 43–55. (CRC Press: Boca Raton)
Joshi MV, Loria R (2007) Streptomyces turgidiscabies possesses a functional cytokinin biosynthetic pathway and produces leafy galls. Molecular Plant—Microbe Interactions 20, 751–758.
| Streptomyces turgidiscabies possesses a functional cytokinin biosynthetic pathway and produces leafy galls.Crossref | GoogleScholarGoogle Scholar |
Kaiser W, Huguet E, Casas J, Commin C, Giron D (2010) Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proceedings. Biological Sciences 277, 2311–2319.
| Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts.Crossref | GoogleScholarGoogle Scholar |
Kakimoto T (1996) CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science 274, 982–985.
| CKI1, a histidine kinase homolog implicated in cytokinin signal transduction.Crossref | GoogleScholarGoogle Scholar |
Kakimoto T (2001) Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases. Plant & Cell Physiology 42, 677–685.
| Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases.Crossref | GoogleScholarGoogle Scholar |
Kamínek M, Armstrong DJ (1990) Genotypic variation in cytokinin oxidase from Phaseolus callus cultures. Plant Physiology 93, 1530–1538.
| Genotypic variation in cytokinin oxidase from Phaseolus callus cultures.Crossref | GoogleScholarGoogle Scholar |
Kamínek M, Trčková M, Fox JE, Gaudinová A (2003) Comparison of cytokinin-binding proteins from wheat and oat grains. Physiologia Plantarum 117, 453–458.
| Comparison of cytokinin-binding proteins from wheat and oat grains.Crossref | GoogleScholarGoogle Scholar |
Kasahara H, Takei K, Ueda N, Hishiyama S, Yamaya T, Kamiya Y, Yamaguchi S, Sakakibara H (2004) Distinct isoprenoid origins of cis- and trans-zeatin biosyntheses in Arabidopsis. The Journal of Biological Chemistry 279, 14049–14054.
| Distinct isoprenoid origins of cis- and trans-zeatin biosyntheses in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Kieber JJ, Schaller GE (2010) The perception of cytokinin: a story 50 years in the making. Plant Physiology 154, 487–492.
| The perception of cytokinin: a story 50 years in the making.Crossref | GoogleScholarGoogle Scholar |
Kim HJ, Ryu H, Hong SH, Woo HR, Lim PO, Lee IC, Sheen J, Nam HG, Hwang I (2006) Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 103, 814–819.
| Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Kowalska M, Galuszka P, Frébortová J, Šebela M, Béres T, Hluska T, Šmehilová M, Bilyeu KD, Frébort I (2010) Vacuolar and cytosolic cytokinin dehydrogenases of Arabidopsis thaliana: heterologous expression, purification and properties. Phytochemistry 71, 1970–1978.
| Vacuolar and cytosolic cytokinin dehydrogenases of Arabidopsis thaliana: heterologous expression, purification and properties.Crossref | GoogleScholarGoogle Scholar |
Krall L, Raschke M, Zenk MH, Baron C (2002) The Tzs protein from Agrobacterium tumefaciens C58 produces zeatin riboside 5′-phosphate from 4-hydroxy-3-methyl-2-(E)-butenyl diphosphate and AMP. FEBS Letters 527, 315–318.
| The Tzs protein from Agrobacterium tumefaciens C58 produces zeatin riboside 5′-phosphate from 4-hydroxy-3-methyl-2-(E)-butenyl diphosphate and AMP.Crossref | GoogleScholarGoogle Scholar |
Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J (2007) Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445, 652–655.
| Direct control of shoot meristem activity by a cytokinin-activating enzyme.Crossref | GoogleScholarGoogle Scholar |
Kuroha T, Tokunaga H, Kojima M, Ueda N, Ishida T, Nagawa S, Fukuda H, Sugimoto K, Sakakibara H (2009) Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. The Plant Cell 21, 3152–3169.
| Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Kwade Z, Swiatek A, Azmi A, Goossens A, Inzé D, Van Onckelen H, Roef L (2005) Identification of four adenosine kinase isoforms in tobacco By-2 cells and their putative role in the cell cycle-regulated cytokinin metabolism. The Journal of Biological Chemistry 280, 17512–17519.
| Identification of four adenosine kinase isoforms in tobacco By-2 cells and their putative role in the cell cycle-regulated cytokinin metabolism.Crossref | GoogleScholarGoogle Scholar |
Laloue M, Fox JE (1985) Characterization of an imine intermediate in the degradation of isopentenylated cytokinins by a cytokinin oxidase from wheat. In ‘Abstracts of the 12th International Conference on Plant Growth Substances’. (Ed. M Bopp) pp. 23. (Springer: Berlin)
Laukens K, Lenobel R, Strnad M, Van Onckelen H, Witters E (2003) Cytokinin affinity purification and identification of a tobacco BY-2 adenosine kinase. FEBS Letters 533, 63–66.
| Cytokinin affinity purification and identification of a tobacco BY-2 adenosine kinase.Crossref | GoogleScholarGoogle Scholar |
Letham DS (1973) Cytokinins from Zea mays. Phytochemistry 12, 2445–2455.
| Cytokinins from Zea mays.Crossref | GoogleScholarGoogle Scholar |
Lohrmann J, Buchholz G, Keitel C, Sweere U, Kircher S, Bäurle I, Kudla J, Schäfer E, Harter K (1999) Differential expression and nuclear localization of response regulator-like proteins from Arabidopsis thaliana. Plant Biology 1, 495–505.
| Differential expression and nuclear localization of response regulator-like proteins from Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Lomin SN, Yonekura-Sakakibara K, Romanov GA, Sakakibara H (2011) Ligand-binding properties and subcellular localization of maize cytokinin receptors. Journal of Experimental Botany 62, 5149–5159.
| Ligand-binding properties and subcellular localization of maize cytokinin receptors.Crossref | GoogleScholarGoogle Scholar |
Lorteau MA, Ferguson BJ, Guinel FC (2001) Effects of CK on ethylene production and nodulation in pea (Pisum sativum) cv. Sparkle. Physiologia Plantarum 112, 421–428.
| Effects of CK on ethylene production and nodulation in pea (Pisum sativum) cv. Sparkle.Crossref | GoogleScholarGoogle Scholar |
Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y (2000) A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes & Development 14, 2938–2943.
| A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root.Crossref | GoogleScholarGoogle Scholar |
Mähönen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Törmäkangas K, Ikeda Y, Oka A, Kakimoto T, Helariutta Y (2006) Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 311, 94–98.
| Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development.Crossref | GoogleScholarGoogle Scholar |
Malito E, Coda A, Bilyeu KD, Fraaije MW, Mattevi A (2004) Structures of Michaelis and product complexes of plant cytokinin dehydrogenase: implications for flavoenzyme catalysis. Journal of Molecular Biology 341, 1237–1249.
| Structures of Michaelis and product complexes of plant cytokinin dehydrogenase: implications for flavoenzyme catalysis.Crossref | GoogleScholarGoogle Scholar |
Martin RC, Mok MC, Shaw G, Mok DWS (1989) An enzyme mediating the conversion of zeatin to dihydrozeatin in Phaseolus embryos. Plant Physiology 90, 1630–1635.
| An enzyme mediating the conversion of zeatin to dihydrozeatin in Phaseolus embryos.Crossref | GoogleScholarGoogle Scholar |
Martin RC, Mok MC, Habben JE, Mok DW (2001) A maize cytokinin gene encoding an O-glucosyltransferase specific to cis-zeatin. Proceedings of the National Academy of Sciences of the United States of America 98, 5922–5926.
| A maize cytokinin gene encoding an O-glucosyltransferase specific to cis-zeatin.Crossref | GoogleScholarGoogle Scholar |
Maruyama S, Matsuzaki M, Misawa K, Nozaki H (2009) Cyanobacterial contribution to the genomes of the plastid-lacking protists. BMC Evolutionary Biology 9, 197–220.
| Cyanobacterial contribution to the genomes of the plastid-lacking protists.Crossref | GoogleScholarGoogle Scholar |
Mason MG, Li J, Mathews DE, Kieber JJ, Schaller GE (2004) Type-B response regulators display overlapping expression patterns in Arabidopsis. Plant Physiology 135, 927–937.
| Type-B response regulators display overlapping expression patterns in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Mason MG, Mathews DE, Argyros DA, Maxwell BB, Kieber JJ, Alonso JM, Ecker JR, Schaller GE (2005) Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. The Plant Cell 17, 3007–3018.
| Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Medford JI, Horgan R, El-Sawi Z, Klee HJ (1989) Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. The Plant Cell 1, 403–413.
Miller CO (1967) Zeatin and zeatin riboside from a mycorrhizal fungus. Science 157, 1055–1057.
| Zeatin and zeatin riboside from a mycorrhizal fungus.Crossref | GoogleScholarGoogle Scholar |
Miller CO, Skoog F, von Saltza MH, Strong FM (1955) Kinetin, a cell division factor from deoxyribonucleic acid. Journal of the American Chemical Society 77, 1392
| Kinetin, a cell division factor from deoxyribonucleic acid.Crossref | GoogleScholarGoogle Scholar |
Mitreva M, Blaxter ML, Bird DM, McCarter JP (2005) Comparative genomics of nematodes. Trends in Genetics 10, 573–581.
Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. The Plant Journal 37, 128–138.
| Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate.Crossref | GoogleScholarGoogle Scholar |
Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowská D, Tabata S, Sandberg G, Kakimoto T (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 103, 16598–16603.
| Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis.Crossref | GoogleScholarGoogle Scholar |
Mizuno T (1998) His–Asp phosphotransfer signal transduction. Journal of Biochemistry 123, 555–563.
Mok MC (1994) Cytokinins and plant development – an overview. In ‘Cytokinins. Chemistry, activity and function’. (Ed. DWS Mok, MC Mok) pp. 155–166. (CRC Press: Boca Raton)
Mok DW, Mok MC (2001) Cytokinin metabolism and action. Annual Review of Plant Physiology and Plant Molecular Biology 52, 89–118.
| Cytokinin metabolism and action.Crossref | GoogleScholarGoogle Scholar |
Mok MC, Martin RC, Dobrev PI, Vanková R, Ho PS, Yonekura-Sakakibara K, Sakakibara H, Mok DW (2005) Topolins and hydroxylated thidiazuron derivatives are substrates of cytokinin O-glucosyltransferase with position specificity related to receptor recognition. Plant Physiology 137, 1057–1066.
| Topolins and hydroxylated thidiazuron derivatives are substrates of cytokinin O-glucosyltransferase with position specificity related to receptor recognition.Crossref | GoogleScholarGoogle Scholar |
Morris RO, Bilyeu KD, Laskey JG, Cheikh NN (1999) Isolation of a gene encoding a glycosylated cytokinin oxidase from maize. Biochemical and Biophysical Research Communications 255, 328–333.
| Isolation of a gene encoding a glycosylated cytokinin oxidase from maize.Crossref | GoogleScholarGoogle Scholar |
Motyka V, Vaňková R, Čapková V, Petrášek J, Kamínek M, Schmülling T (2003) Cytokinin-induced upregulation of cytokinin oxidase activity in tobacco includes changes in enzyme glycosylation and secretion. Physiologia Plantarum 117, 11–21.
| Cytokinin-induced upregulation of cytokinin oxidase activity in tobacco includes changes in enzyme glycosylation and secretion.Crossref | GoogleScholarGoogle Scholar |
Mougel C, Zhulin IB (2001) CHASE: an extracellular sensing domain common to transmembrane receptors from prokaryotes, lower eukaryotes and plants. Trends in Biochemical Sciences 26, 582–584.
| CHASE: an extracellular sensing domain common to transmembrane receptors from prokaryotes, lower eukaryotes and plants.Crossref | GoogleScholarGoogle Scholar |
Murray JD, Karas BJ, Sato S, Tabata S, Amyly L, Szczyglowski K (2007) A CK perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315, 101–104.
| A CK perception mutant colonized by Rhizobium in the absence of nodule organogenesis.Crossref | GoogleScholarGoogle Scholar |
Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C (2004) Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. The Plant Cell 16, 1365–1377.
| Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Nisler J, Zatloukal M, Popa I, Doležal K, Strnad M, Spíchal L (2010) Cytokinin receptor antagonists derived from 6-benzylaminopurine. Phytochemistry 71, 823–830.
| Cytokinin receptor antagonists derived from 6-benzylaminopurine.Crossref | GoogleScholarGoogle Scholar |
Nomura T, Tanaka Y, Abe H, Uchiyama M (1977) Cytokinin activity of discadenine: a spore germination inhibitor of Dictyostelium discoideum. Phytochemistry 16, 1819–1820.
| Cytokinin activity of discadenine: a spore germination inhibitor of Dictyostelium discoideum.Crossref | GoogleScholarGoogle Scholar |
Pačes V, Werstiuk E, Hall RH (1971) Conversion of N 6-(Δ2-isopentenyl)adenosine to adenosine by enzyme activity in tobacco tissue. Plant Physiology 48, 775–778.
| Conversion of N 6-(Δ2-isopentenyl)adenosine to adenosine by enzyme activity in tobacco tissue.Crossref | GoogleScholarGoogle Scholar |
Parkinson JS, Kofoid EC (1992) Communication modules in bacterial signaling proteins. Annual Review of Genetics 26, 71–112.
| Communication modules in bacterial signaling proteins.Crossref | GoogleScholarGoogle Scholar |
Pasternak O, Bujacz GD, Fujimoto Y, Hashimoto Y, Jelen F, Otlewski J, Sikorski MM, Jaskolski M (2006) Crystal structure of Vigna radiata cytokinin-specific binding protein in complex with zeatin. The Plant Cell 18, 2622–2634.
| Crystal structure of Vigna radiata cytokinin-specific binding protein in complex with zeatin.Crossref | GoogleScholarGoogle Scholar |
Persson BC, Esberg B, Ólafsen Ó, Björk GR (1994) Synthesis and function of isopentenyl adenosine derivatives in tRNA. Biochimie 76, 1152–1160.
| Synthesis and function of isopentenyl adenosine derivatives in tRNA.Crossref | GoogleScholarGoogle Scholar |
Pertry I, Václavíková K, Depuydt S, Galuszka P, Spíchal L, Temmerman W, Stes E, Schmülling T, Kakimoto T, Van Montagu MCE, Strnad M, Holsters M, Tarkowski P, Vereecke D (2009) Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant. Proceedings of the National Academy of Sciences of the United States of America 106, 929–934.
| Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant.Crossref | GoogleScholarGoogle Scholar |
Pertry I, Václavíková K, Gemrotová M, Spíchal L, Galuszka P, Depuydt S, Temmerman W, Stes E, De Keyser A, Riefler M, Biondi S, Novák O, Schmülling T, Strnad M, Tarkowski P, Holsters M, Vereecke D (2010) Rhodococcus fascians impacts plant development through the dynamic fas-mediated production of a cytokinin mix. Molecular Plant—Microbe Interactions 23, 1164–1174.
| Rhodococcus fascians impacts plant development through the dynamic fas-mediated production of a cytokinin mix.Crossref | GoogleScholarGoogle Scholar |
Pils B, Heyl A (2009) Unraveling the evolution of cytokinin signaling. Plant Physiology 151, 782–791.
| Unraveling the evolution of cytokinin signaling.Crossref | GoogleScholarGoogle Scholar |
Pischke MS, Jones LG, Otsuga D, Fernandez DE, Drews GN, Sussman MR (2002) An Arabidopsis histidine kinase is essential for megagametogenesis. Proceedings of the National Academy of Sciences of the United States of America 99, 15800–15805.
| An Arabidopsis histidine kinase is essential for megagametogenesis.Crossref | GoogleScholarGoogle Scholar |
Popelková H, Fraaije MW, Novák O, Frébortová J, Bilyeu KD, Frébort I (2006) Kinetic and chemical analyses of the cytokinin dehydrogenase-catalysed reaction: correlations with the crystal structure. Biochemical Journal 398, 113–124.
| Kinetic and chemical analyses of the cytokinin dehydrogenase-catalysed reaction: correlations with the crystal structure.Crossref | GoogleScholarGoogle Scholar |
Punwani JA, Hutchison CE, Schaller GE, Kieber JJ (2010) The subcellular distribution of the Arabidopsis histidine phosphotransfer proteins is independent of cytokinin signaling. The Plant Journal 62, 473–482.
| The subcellular distribution of the Arabidopsis histidine phosphotransfer proteins is independent of cytokinin signaling.Crossref | GoogleScholarGoogle Scholar |
Rashotte AM, Mason MG, Hutchison CE, Ferreira FJ, Schaller GE, Kieber JJ (2006) A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway. Proceedings of the National Academy of Sciences of the United States of America 103, 11081–11085.
| A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway.Crossref | GoogleScholarGoogle Scholar |
Redig P, Schmülling T, Van Onckelen H (1996) Analysis of cytokinin metabolism in ipt transgenic tobacco by liquid chromatography-tandem mass spectrometry. Plant Physiology 112, 141–148.
Riechmann JL, Heard J, Martin G, Reuber L, Jiang C-Z, Keddie J, Adam L, Pineda O, Ratcliff OJ, Samaha RR, Creelman R, Pilgrim M, Broun P, Zhang JZ, Ghandehari D, Sherman BK, Yu G-L (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290, 2105–2110.
| Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes.Crossref | GoogleScholarGoogle Scholar |
Riefler M, Novak O, Strnad M, Schmülling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. The Plant Cell 18, 40–54.
| Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism.Crossref | GoogleScholarGoogle Scholar |
Romanov GA, Spíchal L, Lomin SN, Strnad M, Schmülling T (2005) A live cell hormone-binding assay on transgenic bacteria expressing a eukaryotic receptor protein. Analytical Biochemistry 347, 129–134.
| A live cell hormone-binding assay on transgenic bacteria expressing a eukaryotic receptor protein.Crossref | GoogleScholarGoogle Scholar |
Romanov GA, Lomin SN, Schmülling T (2006) Biochemical characteristics and ligand-binding properties of Arabidopsis cytokinin receptor AHK3 compared to CRE1/AHK4 as revealed by a direct binding assay. Journal of Experimental Botany 57, 4051–4058.
| Biochemical characteristics and ligand-binding properties of Arabidopsis cytokinin receptor AHK3 compared to CRE1/AHK4 as revealed by a direct binding assay.Crossref | GoogleScholarGoogle Scholar |
Sa G, Mi M, He-Chun Y, Guo-Feng L (2002) Anther-specific expression of ipt gene in transgenic tobacco and its effect on plant development. Transgenic Research 11, 269–278.
| Anther-specific expression of ipt gene in transgenic tobacco and its effect on plant development.Crossref | GoogleScholarGoogle Scholar |
Sakai H, Aoyama T, Oka A (2000) Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. The Plant Journal 24, 703–711.
| Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators.Crossref | GoogleScholarGoogle Scholar |
Sakai H, Honma T, Aoyama T, Sato S, Kato T, Tabata S, Oka A (2001) ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 294, 1519–1521.
| ARR1, a transcription factor for genes immediately responsive to cytokinins.Crossref | GoogleScholarGoogle Scholar |
Sakakibara H, Kasahara H, Ueda N, Kojima M, Takei K, Hishiyama S, Asami T, Okada K, Kamiya Y, Yamaya T, Yamaguchi S (2005) Agrobacterium tumefaciens increases cytokinin production in plastids by modifying the biosynthetic pathway in the host plant. Proceedings of the National Academy of Sciences of the United States of America 102, 9972–9977.
| Agrobacterium tumefaciens increases cytokinin production in plastids by modifying the biosynthetic pathway in the host plant.Crossref | GoogleScholarGoogle Scholar |
Sakamoto T, Sakakibara H, Kojima M, Yamamoto Y, Nagasaki H, Inukai Y, Sato Y, Matsuoka M (2006) Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiology 142, 54–62.
| Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice.Crossref | GoogleScholarGoogle Scholar |
Sakano Y, Okada Y, Matsunaga A, Suwama T, Kaneko T, Ito K, Noguchi H, Abe I (2004) Molecular cloning, expression and characterization of adenylate isopentenyltransferase from hop (Humulus lupulus L.). Phytochemistry 65, 2439–2446.
| Molecular cloning, expression and characterization of adenylate isopentenyltransferase from hop (Humulus lupulus L.).Crossref | GoogleScholarGoogle Scholar |
Schmülling T (2001) CREam of cytokinin signalling: receptor identified. Trends in Plant Science 6, 281–284.
| CREam of cytokinin signalling: receptor identified.Crossref | GoogleScholarGoogle Scholar |
Schmülling T, Beinsberger S, De Greef J, Schell J, Van Onckelen H, Spena A (1989) Construction of a heat-inducible chimeric gene to increase the cytokinin content in transgenic plant tissue. FEBS Letters 249, 401–406.
| Construction of a heat-inducible chimeric gene to increase the cytokinin content in transgenic plant tissue.Crossref | GoogleScholarGoogle Scholar |
Schmülling T, Werner T, Riefler M, Krupková E, Bartrina y Manns I (2003) Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. Journal of Plant Research 116, 241–252.
| Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species.Crossref | GoogleScholarGoogle Scholar |
Scholl EH, Thorne JL, McCarter JP, Bird DM (2003) Horizontally transferred genes in plant-parasitic nematodes: a high-throughput genomic approach. Genome Biology 4, R39
| Horizontally transferred genes in plant-parasitic nematodes: a high-throughput genomic approach.Crossref | GoogleScholarGoogle Scholar |
Schoor S, Farrow S, Blaschke H, Lee S, Perry G, von Schwartzenberg K, Emery N, Moffatt B (2011) Adenosine kinase contributes to cytokinin interconversion in Arabidopsis. Plant Physiology 157, 659–672.
| Adenosine kinase contributes to cytokinin interconversion in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Schultz JC (2002) Shared signals and the potential for phylogenetic espionage between plants and animals. Integrative and Comparative Biology 42, 454–462.
| Shared signals and the potential for phylogenetic espionage between plants and animals.Crossref | GoogleScholarGoogle Scholar |
Shantz EM, Steward FD (1955) The identification of compound A from coconut milk as 1,3-diphenylurea. Journal of the American Chemical Society 77, 6351–6353.
| The identification of compound A from coconut milk as 1,3-diphenylurea.Crossref | GoogleScholarGoogle Scholar |
Shaw G (1994) Chemistry of adenine cytokinins. In ‘Cytokinins. Chemistry, activity and function’. (Ed. DWS Mok, MC Mok) pp. 15–34. (CRC Press: Boca Raton)
Siemens J, Keller I, Sarx J, Kunz S, Schuller A, Nagel W, Schmülling T, Parniske M, Ludwig-Müller J (2006) Transcriptome analysis of Arabidopsis clubroots indicate a key role for cytokinins in disease development. Molecular Plant-Microbe Interactions 19, 480–494.
| Transcriptome analysis of Arabidopsis clubroots indicate a key role for cytokinins in disease development.Crossref | GoogleScholarGoogle Scholar |
Simpson MG (2006) ‘Plant systematic.’ (Elsevier: Amsterdam)
Šmehilová M, Galuszka P, Bilyeu KD, Jaworek P, Kowalska M, Šebela M, Sedlářová M, English JT, Frébort I (2009) Subcellular localization and biochemical comparison of cytosolic and secreted cytokinin dehydrogenase enzymes from maize. Journal of Experimental Botany 60, 2701–2712.
| Subcellular localization and biochemical comparison of cytosolic and secreted cytokinin dehydrogenase enzymes from maize.Crossref | GoogleScholarGoogle Scholar |
Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant & Cell Physiology 45, 1299–1305.
| Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay.Crossref | GoogleScholarGoogle Scholar |
Spíchal L, Werner T, Popa I, Riefler M, Schmülling T, Strnad M (2009) The purine derivative PI-55 blocks cytokinin action via receptor inhibition. The FEBS Journal 276, 244–253.
| The purine derivative PI-55 blocks cytokinin action via receptor inhibition.Crossref | GoogleScholarGoogle Scholar |
Spinola M, Galvan A, Pignatiello C, Conti B, Pastorino U, Nicander B, Paroni R, Dragani TA (2005) Identification and functional characterization of the candidate tumor suppressor gene TRIT1 in human lung cancer. Oncogene 24, 5502–5509.
| Identification and functional characterization of the candidate tumor suppressor gene TRIT1 in human lung cancer.Crossref | GoogleScholarGoogle Scholar |
Stirk WA, Van Staden J (2010) Flow of cytokinins through the environment. Plant Growth Regulation 62, 101–116.
| Flow of cytokinins through the environment.Crossref | GoogleScholarGoogle Scholar |
Stolz A, Riefler M, Lomin SN, Achazi K, Romanov GA, Schmülling T (2011) The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. The Plant Journal 67, 157–168.
| The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors.Crossref | GoogleScholarGoogle Scholar |
Strnad M (1997) The aromatic cytokinins. Physiologia Plantarum 101, 674–688.
| The aromatic cytokinins.Crossref | GoogleScholarGoogle Scholar |
Sugawara H, Ueda N, Kojima M, Makita N, Yamaya T, Sakakibara H (2008) Structural insight into the reaction mechanism and evolution of cytokinin biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 105, 2734–2739.
| Structural insight into the reaction mechanism and evolution of cytokinin biosynthesis.Crossref | GoogleScholarGoogle Scholar |
Suzuki T, Sakurai K, Imamura A, Nakamura A, Ueguchi C, Mizuno T (2000) Compilation and characterization of histidine-containing phosphotransmitters implicated in His-to-Asp phosphorelay in plants: AHP signal transducers of Arabidopsis thaliana. Bioscience, Biotechnology, and Biochemistry 64, 2486–2489.
| Compilation and characterization of histidine-containing phosphotransmitters implicated in His-to-Asp phosphorelay in plants: AHP signal transducers of Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Suzuki T, Miwa K, Ishikawa K, Yamada H, Aiba H, Mizuno T (2001) The Arabidopsis sensor His-kinase, AHK4, can respond to cytokinins. Plant & Cell Physiology 42, 107–113.
| The Arabidopsis sensor His-kinase, AHK4, can respond to cytokinins.Crossref | GoogleScholarGoogle Scholar |
Takei K, Sakakibara H, Sugiyama T (2001) Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. The Journal of Biological Chemistry 276, 26405–26410.
| Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Takei K, Ueda N, Aoki K, Kuromori T, Hirayama T, Shinozaki K, Yamaya T, Sakakibara H (2004a) AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant & Cell Physiology 45, 1053–1062.
| AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Takei K, Yamaya T, Sakakibara H (2004b) Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. The Journal of Biological Chemistry 279, 41866–41872.
| Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin.Crossref | GoogleScholarGoogle Scholar |
Taller B (1994) Distribution, biosynthesis and function of cytokinins in tRNA. In ‘Cytokinins. Chemistry, activity and function’. (Ed. DWS Mok, MC Mok) pp. 101–112. (CRC Press: Boca Raton)
Tan E, Besant PG, Attwood PV (2002) Mammalian histidine kinases: do they REALLY exist? Biochemistry 41, 3843–3851.
| Mammalian histidine kinases: do they REALLY exist?Crossref | GoogleScholarGoogle Scholar |
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. The Plant Journal 45, 1028–1036.
| Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance.Crossref | GoogleScholarGoogle Scholar |
Tarkowská D, Doležal K, Tarkowski P, Åstot C, Holub J, Fuksová K, Schmülling T, Sandberg G, Strnad M (2003) Identification of new aromatic cytokinins in Arabidopsis thaliana and Populus × canadensis leaves by LC-(+)ESI-MS and capillary liquid chromatography/frit–fast atom bombardment mass spectrometry. Physiologia Plantarum 117, 579–590.
| Identification of new aromatic cytokinins in Arabidopsis thaliana and Populus × canadensis leaves by LC-(+)ESI-MS and capillary liquid chromatography/frit–fast atom bombardment mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
Taya Y, Tanaka Y, Nishimura S (1978) 5′-AMP is a direct precursor of cytokinin in Dictyostelium discoideum. Nature 271, 545–547.
| 5′-AMP is a direct precursor of cytokinin in Dictyostelium discoideum.Crossref | GoogleScholarGoogle Scholar |
Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, Adamitu E, Tabata S, Stougaard J (2007) A gain-of-function mutation in a CK receptor triggers spontaneous root nodule organogenesis. Science 315, 104–107.
| A gain-of-function mutation in a CK receptor triggers spontaneous root nodule organogenesis.Crossref | GoogleScholarGoogle Scholar |
To JP, Haberer G, Ferreira FJ, Deruère J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kieber JJ (2004) Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. The Plant Cell 16, 658–671.
| Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling.Crossref | GoogleScholarGoogle Scholar |
To JP, Deruère J, Maxwell BB, Morris VF, Hutchison CE, Ferreira FJ, Schaller GE, Kieber JJ (2007) Cytokinin regulates type-A Arabidopsis response regulator activity and protein stability via two-component phosphorelay. The Plant Cell 19, 3901–3914.
| Cytokinin regulates type-A Arabidopsis response regulator activity and protein stability via two-component phosphorelay.Crossref | GoogleScholarGoogle Scholar |
Tokunaga H, Kojima M, Kuroha T, Ishida T, Sugimoto K, Kiba T, Sakakibara H (2012) Arabidopsis lonely guy (LOG) multiple mutants reveal a central role of the LOG-dependent pathway in cytokinin activation. The Plant Journal 69, 355–365.
| Arabidopsis lonely guy (LOG) multiple mutants reveal a central role of the LOG-dependent pathway in cytokinin activation.Crossref | GoogleScholarGoogle Scholar |
Tsoupras G, Luu B, Hoffmann JA (1983) A cytokinin (isopentenyl-adenosyl-mononucleotide) linked to ecdysone in newly laid eggs of Locusta migratoria. Science 220, 507–509.
| A cytokinin (isopentenyl-adenosyl-mononucleotide) linked to ecdysone in newly laid eggs of Locusta migratoria.Crossref | GoogleScholarGoogle Scholar |
Turner JE, Mok DWS, Mok MC, Shaw G (1987) Isolation and partial purification of an enzyme catalyzing the formation of O-xylosylzeatin in Phaseolus vulgaris embryos. Proceedings of the National Academy of Sciences of the United States of America 84, 3714–3717.
| Isolation and partial purification of an enzyme catalyzing the formation of O-xylosylzeatin in Phaseolus vulgaris embryos.Crossref | GoogleScholarGoogle Scholar |
Ueguchi C, Koizumi H, Suzuki T, Mizuno T (2001a) Novel family of sensor histidine kinase genes in Arabidopsis thaliana. Plant & Cell Physiology 42, 231–235.
| Novel family of sensor histidine kinase genes in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Ueguchi C, Sato S, Kato T, Tabata S (2001b) The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant & Cell Physiology 42, 751–755.
| The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Weerasinghe RR, Bird DM, Allen NS (2005) Root-knot nematodes and bacterial Nod factors elicit common signal transduction events in Lotus japonicus root hair cells. Proceedings of the National Academy of Sciences of the United States of America 102, 3147–3152.
| Root-knot nematodes and bacterial Nod factors elicit common signal transduction events in Lotus japonicus root hair cells.Crossref | GoogleScholarGoogle Scholar |
Werner T, Schmülling T (2009) Cytokinin action in plant development. Current Opinion in Plant Biology 12, 527–538.
| Cytokinin action in plant development.Crossref | GoogleScholarGoogle Scholar |
Werner T, Motyka V, Strnad M, Schmülling T (2001) Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences of the United States of America 98, 10487–10492.
| Regulation of plant growth by cytokinin.Crossref | GoogleScholarGoogle Scholar |
Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell 15, 2532–2550.
| Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity.Crossref | GoogleScholarGoogle Scholar |
Werner T, Holst K, Pörs Y, Guivarc’h A, Mustroph A, Chriqui D, Grimm B, Schmülling T (2008) Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. Journal of Experimental Botany 59, 2659–2672.
| Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots.Crossref | GoogleScholarGoogle Scholar |
West AH, Stock AM (2001) Histidine kinases and response regulator proteins in two-component signaling systems. Trends in Biochemical Sciences 26, 369–376.
| Histidine kinases and response regulator proteins in two-component signaling systems.Crossref | GoogleScholarGoogle Scholar |
Whitty CD, Hall RH (1974) A cytokinin oxidase in Zea mays. Canadian Journal of Biochemistry 52, 789–799.
| A cytokinin oxidase in Zea mays.Crossref | GoogleScholarGoogle Scholar |
Wulfetange K, Lomin SN, Romanov GA, Stolz A, Heyl A, Schmülling T (2011) The cytokinin receptors of Arabidopsis thaliana are locating mainly to the endoplasmic reticulum. Plant Physiology 156, 1808–1818.
| The cytokinin receptors of Arabidopsis thaliana are locating mainly to the endoplasmic reticulum.Crossref | GoogleScholarGoogle Scholar |
Yamada H, Suzuki T, Terada K, Takei K, Ishikawa K, Miwa K, Yamashino T, Mizuno T (2001) The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant & Cell Physiology 42, 1017–1023.
| The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane.Crossref | GoogleScholarGoogle Scholar |
Yang S, Yu H, Xu Y, Goh CJ (2003) Investigation of cytokinin-deficient phenotypes in Arabidopsis by ectopic expression of orchid DsCKX1. FEBS Letters 555, 291–296.
| Investigation of cytokinin-deficient phenotypes in Arabidopsis by ectopic expression of orchid DsCKX1.Crossref | GoogleScholarGoogle Scholar |
Yevdakova NA, von Schwartzenberg K (2007) Characterisation of a prokaryote-type tRNA-isopentenyltransferase gene from the moss Physcomitrella patens. Planta 226, 683–695.
| Characterisation of a prokaryote-type tRNA-isopentenyltransferase gene from the moss Physcomitrella patens.Crossref | GoogleScholarGoogle Scholar |
Yonekura-Sakakibara K, Kojima M, Yamaya T, Sakakibara H (2004) Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin. Plant Physiology 134, 1654–1661.
| Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin.Crossref | GoogleScholarGoogle Scholar |
Zhang R, Zhang X, Wang J, Letham DS, McKinney SA, Higgins TJV (1995) The effect of auxin on cytokinin levels and metabolism in transgenic tobacco tissue expressing an ipt gene. Planta 196, 84–94.
| The effect of auxin on cytokinin levels and metabolism in transgenic tobacco tissue expressing an ipt gene.Crossref | GoogleScholarGoogle Scholar |
Zhou C, Huang RH (2008) Crystallographic snapshots of eukaryotic dimethylallyltransferase acting on tRNA: insight into tRNA recognition and reaction mechanism. Proceedings of the National Academy of Sciences of the United States of America 105, 16142–16147.
| Crystallographic snapshots of eukaryotic dimethylallyltransferase acting on tRNA: insight into tRNA recognition and reaction mechanism.Crossref | GoogleScholarGoogle Scholar |


