Annual patterns of xylem embolism in high-yield apple cultivars
Barbara Beikircher A B and Stefan Mayr AA University of Innsbruck, Institute of Botany, Sternwartestrasse 15, 6020 Innsbruck, Austria.
B Corresponding author. Email: barbara.beikircher@uibk.ac.at
Functional Plant Biology 44(6) 587-596 https://doi.org/10.1071/FP16048
Submitted: 5 February 2016 Accepted: 20 February 2017 Published: 31 March 2017
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
Temperate angiosperm species show pronounced annual patterns in xylem embolism. In this study, we investigated whether high-yield cultivars of Malus domestica Borkh. growing under optimised soil water conditions follow similar patterns to wild-type plants, and evaluated crucial factors for the formation of winter embolism and the subsequent restoration of the hydraulic system in spring. In five different cultivars growing at three different sites, various hydraulic and microclimatic parameters were monitored over three successive years. In all cultivars on all sites and in all years, the percentage loss of hydraulic conductivity (PLC) increased in autumn with freeze–thaw events and accumulated over winter. Maximum values were reached in late winter and differed significantly among cultivars. In spring, the hydraulic system was restored and PLC remained negligible during summer. Embolism formation in autumn was significantly correlated with the occurrence of freeze–thaw events, whereas further conductivity losses over winter were related to winter desiccation and influenced by climatic and cultivar-specific parameters. Restoration of the hydraulic system in spring was strongly linked to a decrease in the starch content of wood and buds, and soil temperature. Despite high soil water availability, hydraulic recovery took several weeks and was not completed before bud break. Spring is thus a critical phase for temperate angiosperms, especially for high-yield cultivars with risky hydraulic strategies.
Additional keywords: conductance, Malus domestica, refilling, starch, xylem pressure.
Introduction
A functioning water transport system is essential for plant growth and survival, especially in long-lived woody plants. Long-distance water transport is driven by a pressure gradient transduced from leaves to roots and requires hydraulically continuous water columns in xylem conduits (e.g. Tyree and Ewers 1991; Tyree and Cochard 1996; Tyree and Zimmermann 2002). Drought and/or freeze–thaw events can cause breakage (cavitation) of these water columns, resulting in air-filled conduits (embolism) and impaired xylem water transport (Sperry and Tyree 1990; Tyree and Ewers 1991; Tyree and Zimmermann 2002). Drought-induced embolism occurs when the evaporative demand cannot be satisfied by water uptake from the soil, and the xylem pressure thus decreases below species-specific thresholds (e.g. Tyree and Zimmermann 2002). Reduced or inhibited water uptake from the soil is not only provoked by soil drought but also by frozen (frost drought) or cold soils. The latter is related to the increased transfer resistance between soil and roots, the reduced permeability of roots to water as well as the reduced growth of fine roots, which are mainly responsible for water uptake (Kozlowski et al. 1991; Larcher 2003; Pallardy 2008; Beikircher et al. 2016). In several temperate trees, 6°C was found to be a critical temperature limit for root growth (Alvarez-Uria and Körner 2007). Freeze–thaw-induced embolism develops when air bubbles enclosed in frozen xylem sap expand during thawing and cause cavitation (Sperry and Sullivan 1992; Tyree et al. 1994; Hacke and Sauter 1996; Cochard et al. 2001; Mayr et al. 2007; Charrier et al. 2014). Two critical factors to avoid freeze–thaw-induced embolism are therefore conduit diameter and xylem tension: wider conduits allow the formation of larger bubbles, which shrink more slowly than smaller ones, and nucleate embolism at lower (i.e. less negative) tension (Pittermann and Sperry 2006). Furthermore, freezing velocity and the minimal temperature of the frost cycle also seem to play a role (Sevanto et al. 2012; Charrier et al. 2014). Thus the extent to which a tree’s hydraulic system is impaired during winter strongly depends on anatomical parameters of the xylem (Sperry and Sullivan 1992; Choat et al. 2011), the protection against evaporative water losses (leaf shedding, cuticular and peridermal conductance (gp); Larcher 2003; Beikircher and Mayr 2013) and, with regard to climatic parameters, air and soil temperature, vapour pressure deficit and soil water availability. Previous studies have shown that trees in temperate regions can suffer a considerable loss of hydraulic conductance in winter (Sperry et al. 1988b, 1994; Cochard and Tyree 1990; Sperry and Sullivan 1992; Hacke and Sauter 1996; Cochard et al. 2001; Ameglio et al. 2002; Mayr et al. 2003; Christensen-Dalsgaard and Tyree 2014). In deciduous species, though, a full water transport capacity is not required in winter and even a high level of native embolism is not particularly risky for the plant. This changes suddenly in spring, when transpirational water demand increases in response to warmer temperatures, increased daylength and bud break (e.g. Sperry et al. 1994; Ameglio et al. 2002; Hao et al. 2013). In spring, the functionality of the water transport system has to be restored rapidly to avoid growth limitation or desiccation-related dieback of shoots. Restoration of the hydraulic system in spring is related to the differentiation of new xylem conduits and, in many diffuse-porous angiosperms, to the refilling of embolised ones (Sperry et al. 1988b, 1994; Sperry and Sullivan 1992; Hacke and Sauter 1996; Utsumi et al. 1998; Cochard et al. 2001; Cobb et al. 2007; Christensen-Dalsgaard and Tyree 2014). One possible mechanism for hydraulic recovery is the generation of positive xylem pressures in roots or stems that is sufficient to dissolve embolisms before leaf flushing (Sperry et al. 1987, 1994; Cochard et al. 2001; Ewers et al. 2001; Ameglio et al. 2002; Cobb et al. 2007; Brodersen and McElrone 2013; Hao et al. 2013). Refilling has also been observed in the absence of root or stem pressure (Tyree et al. 1999; Cochard et al. 2001; Salleo et al. 2006; Nardini et al. 2011; Brodersen and McElrone 2013; Christensen-Dalsgaard and Tyree 2014). The mechanisms involved in refilling without tension are still under debate (see Zwieniecki and Holbrook (2009) and Brodersen and McElrone (2013) for a detailed review). However, there is strong evidence that sugars derived from starch polymerisation in xylem parenchyma generate an osmotic gradient that then drives water inflow from adjacent parenchyma cells into conduits (Salleo et al. 2004; Zwieniecki and Holbrook 2009; Nardini et al. 2011; Secchi and Zwieniecki 2012). Two important prerequisites therefore are: (I) adequate water supply, with living cells in the xylem and phloem potentially playing a major role (Salleo et al. 2004; Brodersen et al. 2010; Nardini et al. 2011); (II) hydraulic isolation of embolised conduits from water-filled conduits under tension. If air-filled conduits are not isolated, any inflowing water would immediately be drawn away. Hydraulic isolation is probably achieved by special morphological and biochemical features of interconduit pits (Holbrook and Zwieniecki 1999; Nardini et al. 2011).
Most studies dealing with annual patterns of xylem embolism have focussed on native species growing in forests under natural conditions or in experimental plots (Sperry et al. 1988b, 1994; Cochard and Tyree 1990; Sperry and Sullivan 1992; Hacke and Sauter 1996; Utsumi et al. 1998; Cochard et al. 2001). Only Ameglio et al. (2002) made measurements on orchard-grown walnut (Juglans spp.) and peach (Prunus persica (L.) Batsch) trees. Artificial systems, such as commercial apple (Malus domestica Borkh) orchards are interesting in two aspects: First, the plants are high-yield cultivars, evolved from millennia-long crossing of wild forms as well as specific breeding. Second, plants are usually grown under ideal conditions with regard to nutrient and water supply to minimise hydraulic limitation of growth and productivity (Naor and Girona 2012; Beikircher et al. 2013). A recent study found that this combination can lead to risky hydraulic strategies (Beikircher et al. 2013).
In this study, we followed the loss of hydraulic conductivity (i.e. native embolism; percentage loss of hydraulic conductivity (PLC)) over three consecutive years in M. domestica. We also monitored the water content of bark, wood and buds; the starch content of bark and wood; and gp. Plant hydraulic aspects were then related to microclimatic conditions and phenology. The main research questions were as follows: (I) Given the optimised water supply throughout the year, do high-yield cultivars show pronounced patterns in native embolism, similar to those observed in native plants at natural sites? (II) Which climatic and species-related factors are crucial for the level of potential winter embolism as well as refilling in spring? (III) Do cultivars show specific patterns in embolism formation and refilling? To answer these questions, we analysed five different Malus cultivars growing in different orchards.
Material and methods
Plant material and study sites
This study was carried out on different apple cultivars in commercial apple orchards in northern Italy. Malus domestica Borkh. cv. Golden Delicious specimens were grown on south-exposed orchards near Latsch (643 m above sea leavel (a.s.l.); 46°37N,10°52ʹE), north-exposed orchards near Tschengls (950 m a.s.l.; 46°37ʹN,10°38ʹE) and near Tarsch (854 m a.s.l.; 46°36ʹN,10°53ʹE). In Latsch, additional cultivars included Braeburn, Gala, Nicoter and Red Delicious. All plants were of similar age and height (for details, see Beikircher et al. 2013; Beikircher and Mayr 2013).
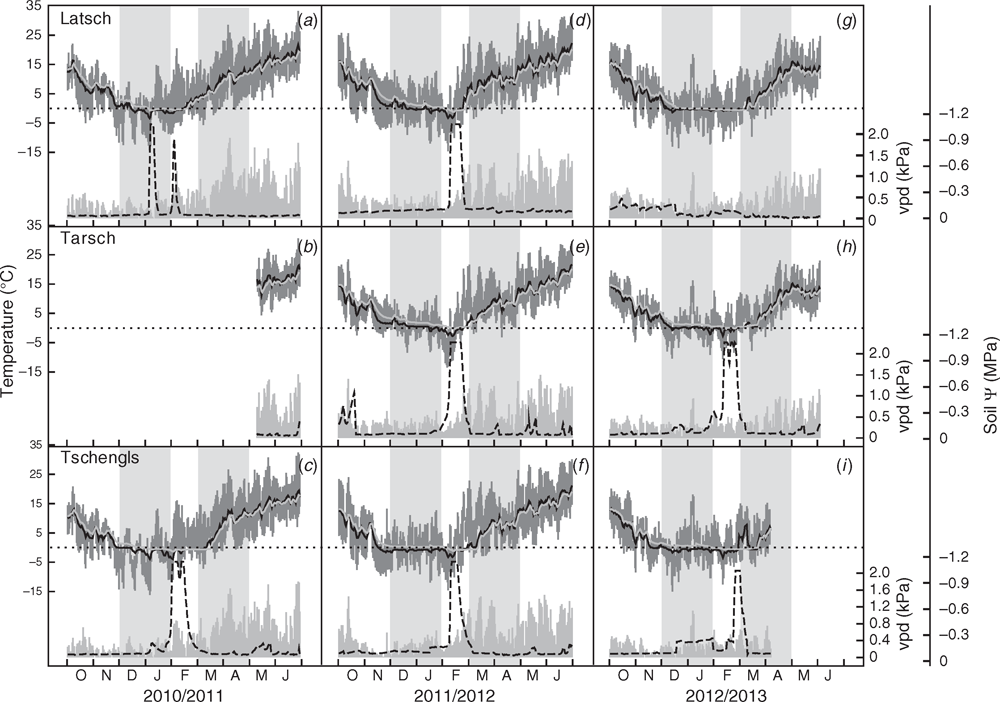
The study sites are located in the Vinschgau Valley, which is a dry inner alpine valley with exceptionally high sunshine duration (315 sunny days per year), a high annual mean temperature (9.6°C) and low precipitation (450–550 mm). Elaborate fertilisation protocols and daily irrigation during the growing season ensure optimal plant growth and productivity. At the study sites, air temperature and relative humidity at 250 cm height (measured at the upper crown; EMS 33 sensor); soil temperature (PT 100 sensor) at 5, 15 and 25 cm depths; and soil water potential (GB sensor) at 25 cm depth were recorded at 1-min intervals. Every 15 min, means of these values were stored in a datalogger (Modulog 3029 sensors and datalogger,Environmental Measuring Systems EMS).
Temperature decreases below –2°C and subsequent increases above 2°C within 24 h were defined as freeze–thaw events. From the minimum air temperature (Tmin), minimum relative humidity (RHmin), maximum air temperature (Tmax) and maximum relative humidity (RHmax), vapour pressure deficit (VPD) was calculated as:
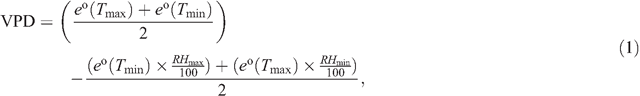
where e° is the saturation vapour pressure at minimum or maximum temperature, respectively (Allen et al. 1998).
Sampling and preparation of branches
From June 2010 to June 2013, at irregular intervals throughout the year, five east- or west-exposed branches (orchard rows were aligned north–south) of five different trees per cultivar and site were chosen at random. In the late morning, branches were cut at the base, immediately recut under water and rehydrated for 30 min before they were wrapped in dark plastic bags with wet paper towels and transported to the laboratory. In the laboratory, branches were repeatedly cut from both sites alternatively under water. In an earlier study (Beikircher and Mayr 2015), this harvesting and sampling protocol proved to be appropriate for preserving the hydraulic state and avoid artefactual embolisation (Wheeler et al. 2013) as well as refilling (Trifilò et al. 2014). Before June, shoots developed in the previous season were used for measurements, whereas from June onward, we used the respective current-year shoots.
Native embolism, water content, midday leaf water potential and stomatal conductance
For the measurement of native embolism, ~5-cm-long samples were debarked, the ends recut several times with a sharp wood-carving knife (Beikircher and Mayr 2015) and sealed into tubes connected to a ‘Xyl’em’ hydraulic conductance and embolism measurement system (Bronkhorst). Hydraulic conductance at 4.5 kPa was measured before (ki) and after (kmax) removal of embolism by repeated high pressure flushes at 95 kPa for 20 min. Percentage loss of hydraulic conductivity was calculated as:

For measurements, we used distilled, filtered (0.22 µm) and degassed water, containing 0.005% (v/v) ‘Micropur Forte MF 1000F’ (a mixture containing Ag+ and NaClO for water sterilisation and preservation; Katadyn Products Inc.) to prevent microbial growth (Sperry et al. 1988a; Beikircher and Mayr 2008).
Measurements of water content were made on bark and wood of ~5-cm-long stem samples as well as on terminal buds. Fresh weights and DW (after oven drying at 80°C for 48 h) were measured with an analytical balance (Sartorius BP61S, 0.0001 g precision, Sartorius AG) and the water content (expressed as percentage of DW; WC%DW) calculated as:

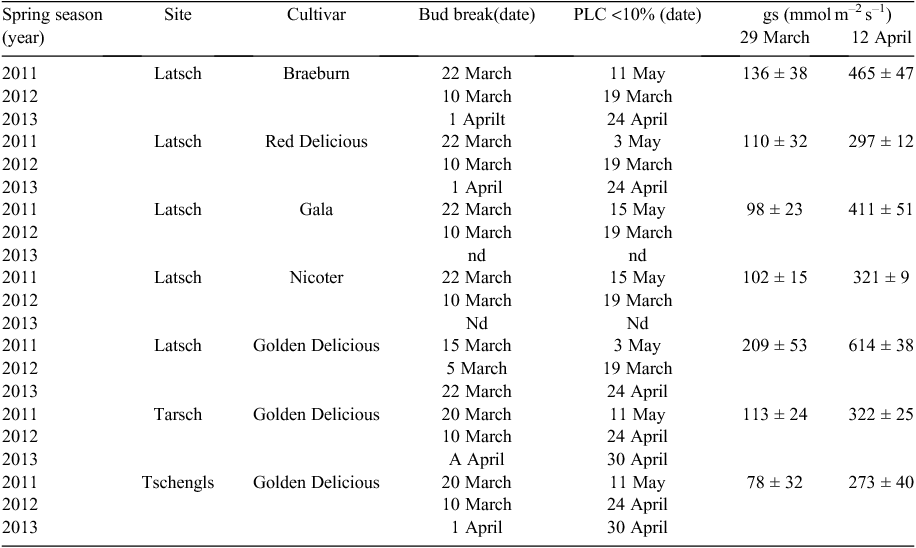
Leaf water potential measurements were made on selected days between 1100 and 1200 hours Central European Time on at least five leaves per cultivar and site, using a pressure chamber (Model 1000 Presssure Chamber, PMS Instrument Co.). Between leaf fall and bud break, measurement of xylem water potential was not possible because of the small portion of living tissues. Stomatal conductance (gs) was measured between 1030 and 1130 hours CET on sunny days in March and April 2011, using a steady-state leaf porometer (SC-1, Decagon Devices).
Starch contents of wood and bark
Analyses of starch contents were carried out on the cultivars Braeburn, Golden Delicious and Red Delicious in the orchard near Latsch. Therefore, the bark was removed from ~4-cm-long samples and cut into thin strips with scissors. The wood was thinly (~10 µm) sliced using a microtome (Sledge Microtome G.S.L. 1, Schenkung Dapples). After oven drying at 80°C for 48 h, samples were finely ground with a microdismembrator (Braun Biotech). The obtained material was incubated twice in 80% ethanol (v/v) at 75°C with polyvinylpyrolidon (PVP 40, Sigma Chemicals) added to bind polyphenols during the first incubation. The supernatants were then removed and the solvent evaporated in an oven. The dry residue containing the starch was incubated with NaOH (0.5 N) at 60°C for 1 h, neutralised with hydrochloric acid (0.5 N), treated with amylase (amyloglucosidase, Sigma-Aldrich) dissolved in a citrate buffer (pH 4.6) to break the starch down to glucose, and incubated for 30 min at 60°C. The supernatants were then mixed with a NADP/ATP and HK/G6P-DH-solution (Enzytec E1210, R-Biopharm) and the absorption of NADPH (dependent on starch content) measured with a UV–visible light spectrophotometer (Lambda 20, Perkin Elmer) at 340 nm before and after the addition of the latter solution. Starch content (SC; %) was calculated as:

where c is the starch concentration (g L–1) in the measured solution, m is the mass of plant material and V is the volume of the solution (L) in the spectrophotometer (see also Mayr et al. 2014).
Peridermal conductance to water vapour
For measurement of gp, eight to 10 end shoots per cultivar and site were harvested in January 2011. Shoots were debarked at the basal end (~1 cm), recut with a sharp wood-carving knife and sealed in tubes connected to a ‘Xyl’em’ apparatus (see above). The distal part of the shoot with the terminal bud was then cut off under water and the shoot flushed at a pressure of 95 kPa for 45 min to remove embolisms. Afterwards, the wet parts on both sample ends were cut off and the ends were tightly sealed with Parafilm ‘M’ (Pechiney Plastic Packaging). Immediately after sealing, the sample weight was measured with an analytical balance (Sartorius BP61S, Sartorius). Samples were put in a test tube rack for bench dehydration and FW was measured at 1-min intervals. Simultaneously, air humidity and temperature as well as barometric pressure were monitored with a thermohygrometer (RS Components, Handelsges.mbH.) and a temperature-compensated pressure sensor (SCX15ANC, Honeywell Sensing and Control), respectively. Afterwards, the transpiring surface area was calculated from shoot length and diameter, assuming a cylindrical shape for each shoot. Peridermal conductance for each time interval was calculated from evaporation (E), saturated vapour pressure (SVP; Pa), actual vapour pressure (VP; Pa) and barometric pressure (P; Pa; see Beikircher and Mayr 2013) as:

For maximum gp, the highest values of each shoot were averaged per cultivar and site.
Statistics
Differences in PLC and gp among cultivars, sites and study years were tested with an ANOVA using the Bonferroni correction (normal distribution, equal variances). Correlation coefficients between embolism levels and number of freeze–thaw cycles experienced were calculated with Pearson’s product–moment coefficient. All tests were made at a probability level of 5% using SPSS (ver. 21, SPSS).
Results
Climatic conditions varied considerably between orchards and between study years (Table 1, Fig. 1). The warmest site was Latsch. The coldest one was Tschengls because of the lack of direct sunshine from mid-November until the end of January. Tarsch is situated ~200 m higher than Latsch. It is characterised by moderate temperatures in winter, caused by frequent temperature inversions, but cold temperatures in spring. The relatively low number of days with soil frost at this site (Table 1) is related to the protection of the soil by a constant snow cover during the winter months. The earliest springtime increase in soil temperature to values above 5°C was observed at the beginning of March 2012 in Latsch. At the other study sites and in the other study years, it occurred in April (Fig. 1).
Percentage loss of hydraulic conductivity and water and starch contents followed pronounced annual patterns. For the sake of convenience, we only present data for the cultivar Braeburn in the main text (Fig. 2). For (comparable) data for other cultivars and other sites, see the figures in the supplementary material (Figs S1–S4, available as Supplementary Material to this paper). With the occurrence of the first freeze–thaw cycles in autumn, embolism levels started to increase and achieved their maxima in late winter. Overall, PLC in November corresponded to the number of freeze–thaw cycles experienced. In Tarsch only, PLC was significantly higher despite having fewer freeze–thaw cycles (Fig. 3).
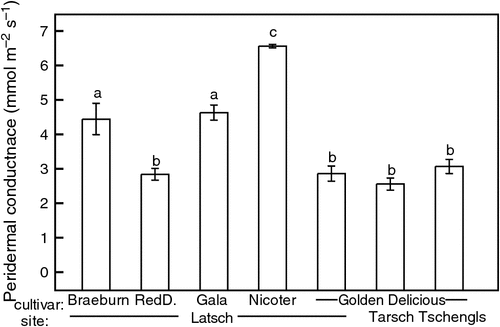
In mid-winter (January), embolism levels differed significantly between study years, cultivars and sites. Among the three study sites of Golden Delicious, the lowest PLC was found in Latsch (Fig. 3). In Tschengls, significant differences among study years were observed. In January 2012, PLC was about twofold higher than in 2010 and 2013. This was in accordance with a considerably greater number of days with soil frost (105) compared with the other years (~85) and sites (Table 1). Midwinter embolism was also found to vary considerably among different cultivars subjected to similar conditions in Latsch. In all study years, significantly lower PLC was found in Golden and Red Delicious than in other cultivars. Golden and Red Delicious also showed lower gp than Braeburn, Gala and Nicoter (Fig. 4).
|
Despite the differences in winter embolism, all cultivars at all sites were able to fully restore their hydraulic system in spring and levels of native embolism were negligible throughout summer (Fig. 2, Figs S1–S4). Concurrently with the decrease in PLC, a decrease in starch content in wood and bark was observed (Fig. 2, Figs S1–S2). Depending on the study year, considerable differences in restoration speed were found among study sites. In the warm spring of 2012, soil warming over 5°C occurred about 1 month earlier in Latsch. Accordingly, restoration of the hydraulic system was also completed earlier than at the other sites, whereas bud break occurred almost simultaneously at all sites (Table 2). In spring 2011 and 2013, differences in soil temperature and thus also restoration were less pronounced (Fig. 1, Table 2). In contrast to midwinter embolism, no obvious differences in restoration speed and bud break were observed among different cultivars growing at different sites (Table 2).
Discussion
High-yielding cultivars of M. domestica growing in optimally-managed orchards showed pronounced annual patterns in embolism, similar to those reported previously for various temperate angiosperm species in natural or seminatural sites. In the following, we focus on two main topics: the formation and the patterns of winter embolism, and restoration of the hydraulic system in spring. Thereby, we first provide a short overview about general patterns found and then emphasise the outstanding observations on intra- and intercultivar levels.
Winter embolism
When night temperatures decreased below freezing in autumn, in all cultivars and on all sites, PLC started to increase. During winter, conductivity losses further increased and maximum values were achieved in late winter (Fig. 2, Figs S1–S4). Similar patterns have been reported for several other species growing under natural (Sperry and Sullivan 1992; Sperry et al. 1994) or semi-natural (Cochard and Tyree 1990; Hacke and Sauter 1996; Utsumi et al. 1998; Cochard et al. 2001; Ameglio et al. 2002; Cobb et al. 2007) conditions. Our findings show that a complex combination of climatic and cultivar-specific factors is decisive for the extent of hydraulic impairment of trees during the cold season. Therefore, winter desiccation plays a major role.
The first PLC increase in autumn can mainly be attributed to freeze–thaw-induced embolism. Accordingly, embolism levels in late autumn (November) differed considerably among sites and years (and thus the respective number of freeze–thaw events). The intracultivar comparison of Golden Delicious growing on different sites, though, provides evidence that the relationship between embolism and freeze–thaw cycles is complex. For instance, at Tarsch, PLC was significantly higher in the latter two study years despite a much lower number of freeze–thaw cycles compared with Latsch and Tschengls (Fig. 3). An explanation for this finding might be the higher air temperatures in autumn at this site (Table 1), as higher evaporative demands and lower minimal temperatures of frost cycles increase the risk of freeze–thaw-induced cavitation (Sperry and Sullivan 1992; Ameglio et al. 2002; Charrier et al. 2014). In contrast, in Latsch, where five different cultivars were subjected to similar conditions, within a given study year, no statistically significant differences in PLC among cultivars were observed. Besides similar environmental conditions, this may also be caused by similar xylem anatomy. At least for the cultivars Braeburn, Golden and Red Delicious, a previous study (Beikircher et al. 2013) found no significant differences in conduit diameters. Nevertheless, in an annual comparison, PLC in November was significantly correlated with the number of freeze–thaw cycles (R2 = 0.762; Fig. 3).
In midwinter (January), we observed high variations in PLC among sites and among cultivars. Within the three study years, embolism levels were lower overall in the winter season of 2011–12 despite a considerable higher number of freeze–thaw cycles compared with the preceding winter season. The only exception was Golden Delicious in Tschengls, which had significantly more embolism (Fig. 3). The winter season of 2011–12 was remarkably warm with mean monthly air temperatures above 0°C in December and January (Table 1). Accordingly, the soils in Latsch and Tarsch were not permanently frozen until late January. In contrast to those sites, Tschengls is north-exposed with no direct sunshine from mid-November until the end of January. Therefore, the upper soil layer (5 cm) was constantly frozen from mid-November until late February (Fig. 1). Blocked water supply because of the frozen soil and concurrently high evaporative demands (Table 1) thus explains the observed high conductivity losses in midwinter of 2011–12 in Tschengls. At Latsch and Tarsch, plants profited from the unfrozen soil. Surprisingly, in the following winter season (2012–13), higher levels of native embolism were found in Tarsch despite the significantly lower number of days with soil frost (Table 1, Fig. 3). This was because the soil in Tarsch was covered with snow from mid-December to mid-February 2013. Therefore, soil temperatures remained around 0°C but water uptake was blocked by the frozen stem base (data not shown). This demonstrates that blockage of water uptake is not simply a matter of soil temperature. Thus, for a detailed analysis of winter hydraulics, soil temperature at different depths as well as other parameters influencing soil and xylem temperature have to be considered.
Midwinter embolism did not only vary significantly among sites but also among cultivars. In January 2012, for instance, PLC in Latsch varied from 16% (Golden Delicious) to 51% (Gala; Fig. 3). Measurements on gp revealed that cultivars with higher observed PLC were also less protected against water loss through the periderm (i.e. higher gp; Figs 3 and 4). Furthermore, Gala and Nicoter are known to develop lammas shoots, which, unlike normal shoots, develop late in the growing season. Because of the incomplete maturation of the periderm and cuticle, these shoots pose a high risk for uncontrolled water loss during winter (Beikircher and Mayr 2013). Besides protection against water losses, water storage capacity can also play a role in preventing embolism. This was demonstrated in additional measurements in the winter of 2011–12 on young Golden Delicious plants growing directly near the main study plants in Tarsch. In midwinter, embolism values in young trees, which have lower water storage capacity (e.g. Mayr and Charra-Vaskou 2007), were significantly higher than in older trees (Fig. S3).
Spring recovery
Regardless of the amount of winter embolism, in every study year all cultivars on all sites were able to restore their hydraulic system. As early as late winter (February), when soils were still partly frozen, an increase in the water content of buds was observed. As soon as soil temperatures increased above 0°C, the water contents of wood and bark also increased (Fig. 2, Figs S1–S4). Initially, the level of embolism remained high but the remarkably high s.e. in PLC pointed to a high variation among branches and indicated that first refilling processes had already started. Despite optimal soil water conditions, complete restoration of the hydraulic system took several weeks and was not completed before bud break (Table 2, Figs S1–S4). This may be a typical pattern for species in which positive pressure plays no or only a limited role for refilling. In species with pronounced bleeding, refilling is completed before bud break (e.g. Hacke and Sauter 1996). The genus Malus is known to develop root pressure in spring but to a much lesser extent than in, for example, maple (Acer spp.), walnut or birch (Betula spp.), ceasing before or around bud break (Wiegand 1906). Thus positive pressures might have been involved initially but cannot explain the full recovery, which only occurred several weeks after bud break. If water uptake in spring is still impaired by frozen or cold soils, the resulting late recovery may increase the risk for hydraulic failure, as transpirational water losses can occur already at early phenological stages (Beikircher et al. 2016). In March 2011, for instance, we measured midday stomatal conductance values between 78 and 209 mmol m–2 s–1 on newly developed leaves (phenological stage: ‘half-inch green’, i.e. >10 mm of leaf tissue is protruding from the buds). In Golden Delicious, by April, stomatal conductance was almost in the range of fully developed leaves in summer (see Beikircher et al. 2013; Table 2). Because of daily irrigation and thus optimal water supply though, these transpirational losses did not affect our study plants.
Concurrently with the decrease in PLC, we observed a decrease in the starch contents of wood and bark (Fig. 2, Figs S1–S2), which is in accordance with the hypothesis that starch depolymerisation plays a major role in refilling processes (see Introduction). A similar pattern in starch content has been reported for several other species (Essiamah and Eschrich 1985). All cultivars on all sites had restored their hydraulic system by the end of spring in all years. The residual (<10%) loss of hydraulic conductivity in previous-year shoots is probably related to the cavitation or frost fatigue of conduits (Hacke et al. 2001; Christensen-Dalsgaard and Tyree 2014). The speed of restoration of the hydraulic system was found to be strongly related to climatic conditions. As demonstrated below, the crucial factors, therefore, were air and soil temperatures influencing tree phenology and water uptake.
Across all cultivars and all sites, the earliest completion of hydraulic recovery was observed in the spring season of 2012. Following an exceptional mild winter (see above), February and March 2012 were also extraordinarily warm, with mean air temperatures ~3.2°C above the long-term average. This led to an early onset of growth and one of the earliest bud breaks ever observed in the study area. In Golden Delicious specimens growing at different sites, bud break occurred more or less simultaneously at the beginning of March 2012, despite considerably differing soil warming and embolism recovery (Fig. 1, Table 2). In the favourably situated orchard near Latsch, soil temperature increased to above 5°C already in early March, but this was delayed by several weeks at Tarsch and Tschengls (Table 1, Fig. 1). Thus in the latter orchards, water uptake could probably not yet cover the demand for refilling and growth processes as well as transpirational water losses. The differences in soil temperature were reflected in restoration speed: In Latsch, PLC decreased below 10% by the end of March, whereas recovery in Tarsch and Tschengls took until the end of April (Table 2). In contrast, in spring 2011 and 2013, soil warming above 5°C on all study sites occurred more or less simultaneously at the beginning of April (Table 1, Fig. 2), and restoration of the hydraulic system occurred around the same time (Table 2). On the intercultivar level, differences in bud break as well as refilling were astonishingly small. Despite significant differences in midwinter embolism among cultivars (see above), in all three study years, leaf flushing and completion of hydraulic restoration occurred more or less simultaneously in all five cultivars. This indicates that these processes are rather environmentally-driven than cultivar-specific.
Summa summarum, high-yield cultivars of M. domestica growing under optimised conditions followed similar annual patterns in embolism and related parameters, as have been reported for native species. The formation of winter embolism was related to various climatic and cultivar-specific factors influencing the trees’ water status. Restoration of the hydraulic system in spring depended on climatic rather than cultivar-specific parameters, and was not completed before bud break. Although this is common in deciduous plants, it may pose a considerable risk in overbred plants growing in artificial ecosystems like in our study. Three out of the five studied cultivars have been found to exhibit a very risky hydraulic behaviour with high vulnerability to drought-induced embolism and late stomatal closure (Beikircher et al. 2013). In combination with a partly impaired water transport system in spring, when water demand for growth and refilling processes as well as transpiration is high, this could lead to aggravation of the trees’ water status and subsequently to plant damage. Indeed, in the study area a dieback of plant parts or even whole plants in spring has been observed at irregular intervals in the past, which we assume to be related to winter desiccation and failed recovery in spring (also see Beikircher and Mayr 2013). For the management of such orchards, we thus recommend (I) avoidance of sites with prolonged soil frost, (II) an elaborate irrigation scheme in spring and (III) careful selection of cultivars.
Acknowledgements
We thank the staff of the South Tyrolean Advisory Service for Fruit and Wine-growing, especially Martin Abler, for helpful support as well as the farmers who enabled the study in their orchards. We also thank Mag. Birgit Dämon for helpful assistance with starch analyses. This study was financed by the Austrian Science Fund (FWF; project L556-B16; ‘Winter Damage on Apple Trees’ and project T667-B16 ‘Hydraulics of Juvenile Trees’) and is linked to the COST Action FP1106 ‘STReESS’.
References
Allen RG, Pereira LS, Raes D, Smith M (1998) ‘Crop evapotranspiration – guidelines for computing crop water requirements.’ (Food and Agricultural Organisation of the United Nations: Rome)Alvarez-Uria P, Körner C (2007) Low temperature limits of root growth in deciduous and evergreen temperate tree species. Functional Ecology 21, 211–218.
| Low temperature limits of root growth in deciduous and evergreen temperate tree species.Crossref | GoogleScholarGoogle Scholar |
Ameglio T, Bodet C, Lacointe A, Cochard H (2002) Winter embolism, mechanism of xylem hydraulic conductivity recovery and springtime growth patterns in walnut and peach trees. Tree Physiology 22, 1211–1220.
| Winter embolism, mechanism of xylem hydraulic conductivity recovery and springtime growth patterns in walnut and peach trees.Crossref | GoogleScholarGoogle Scholar |
Beikircher B, Mayr S (2008) The hydraulic architecture of Juniperus communis L. ssp. communis: shrubs and trees compared. Plant, Cell & Environment 31, 1545–1556.
| The hydraulic architecture of Juniperus communis L. ssp. communis: shrubs and trees compared.Crossref | GoogleScholarGoogle Scholar |
Beikircher B, Mayr S (2013) Winter peridermal conductance of apple trees: lammas shoots and spring shoots compared. Trees 27, 707–715.
| Winter peridermal conductance of apple trees: lammas shoots and spring shoots compared.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2srntV2jug%3D%3D&md5=70bc8dd7f26e700d1363862f3a8af145CAS |
Beikircher B, Mayr S (2015) Avoidance of harvesting and sampling artefacts in hydraulic analyses: a protocol tested on Malus domestica. Tree Physiology 36, 797–803.
| Avoidance of harvesting and sampling artefacts in hydraulic analyses: a protocol tested on Malus domestica.Crossref | GoogleScholarGoogle Scholar |
Beikircher B, De Cesare C, Mayr S (2013) Hydraulics of high-yield orchard trees: a case study of three Malus domestica cultivars. Tree Physiology 33, 1296–1307.
| Hydraulics of high-yield orchard trees: a case study of three Malus domestica cultivars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsl2msQ%3D%3D&md5=867b026fdfb9b9700360235f682593beCAS |
Beikircher B, Mittmann C, Mayr S (2016) Prolonged soil frost affects hydraulics and phenology of apple trees. Frontiers in Plant Science 7, 867
| Prolonged soil frost affects hydraulics and phenology of apple trees.Crossref | GoogleScholarGoogle Scholar |
Brodersen CR, McElrone AJ (2013) Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Frontiers in Plant Science 4, 108
| Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants.Crossref | GoogleScholarGoogle Scholar |
Brodersen CR, McElrone AJ, Choat B, Matthews MA, Shackel KA (2010) The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography. Plant Physiology 154, 1088–1095.
| The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsV2nsbfL&md5=6a19490f4780f2dcd63235d0df7e6771CAS |
Charrier G, Charra-Vaskou K, Kasuga J, Cochard H, Mayr S, Ameglio T (2014) Freeze–thaw stress: effects of temperature on hydraulic conductivity and ultrasonic activity in ten woody angiosperms. Plant Physiology 164, 992–998.
| Freeze–thaw stress: effects of temperature on hydraulic conductivity and ultrasonic activity in ten woody angiosperms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXks1Smu7k%3D&md5=249f27c454a61e82fe4ec837a2e1e467CAS |
Choat B, Medek DE, Stuart SA, Pasquet-Kok J, Egerton JJG, Salari H, Sack L, Ball MC (2011) Xylem traits mediate a trade-off between resistance to freeze–thaw-induced embolism and photosynthetic capacity in overwintering evergreens. New Phytologist 191, 996–1005.
| Xylem traits mediate a trade-off between resistance to freeze–thaw-induced embolism and photosynthetic capacity in overwintering evergreens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1WmsbbM&md5=865289d2a39894b6656f8b43a25a69d0CAS |
Christensen-Dalsgaard KK, Tyree MT (2014) Frost fatigue and spring recovery of xylem vessels in three diffuse-porous trees in situ. Plant, Cell & Environment 37, 1074–1085.
| Frost fatigue and spring recovery of xylem vessels in three diffuse-porous trees in situ.Crossref | GoogleScholarGoogle Scholar |
Cobb AR, Choat B, Holbrook NM (2007) Dynamics of freeze–thaw embolism in Smilax rotundifolia (Smilacaceae). American Journal of Botany 94, 640–649.
| Dynamics of freeze–thaw embolism in Smilax rotundifolia (Smilacaceae).Crossref | GoogleScholarGoogle Scholar |
Cochard H, Tyree MT (1990) Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiology 6, 393–407.
| Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c%2FosVWqtA%3D%3D&md5=78b8f2ca94ce6429fb85c559292ac3bbCAS |
Cochard H, Lemoine D, Ameglio T, Granier A (2001) Mechanism of xylem recovery from winter embolism in Fagus sylvatica. Tree Physiology 21, 27–33.
| Mechanism of xylem recovery from winter embolism in Fagus sylvatica.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvksleisA%3D%3D&md5=f42623ba2bdfbf2a9e867145d59066e6CAS |
Essiamah S, Eschrich W (1985) Changes of starch content in the storage tissues of deciduous trees during winter and spring. IAWA Journal 6, 97–106.
| Changes of starch content in the storage tissues of deciduous trees during winter and spring.Crossref | GoogleScholarGoogle Scholar |
Ewers FW, Ameglio T, Cochard H, Beaujard F, Martignac M, Vandame M, Bodet C, Cruiziat P (2001) Seasonal variation in xylem pressure of walnut trees: root and stem pressures. Tree Physiology 21, 1123–1132.
| Seasonal variation in xylem pressure of walnut trees: root and stem pressures.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MrjsV2rtw%3D%3D&md5=6c8f47fc8dcec278b26fed8b2ca692cfCAS |
Hacke U, Sauter JJ (1996) Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia 105, 435–439.
| Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC1cznsFKntg%3D%3D&md5=6919721215c322f8a41a5c96950fa3ceCAS |
Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA (2001) Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiology 125, 779–786.
| Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhs1Klsb8%3D&md5=4811ef4784566b72e9ea49f3991a8d5eCAS |
Hao GY, Wheeler JK, Holbrook NM, Goldstein G (2013) Investigating xylem embolism formation, refilling and water storage in tree trunks using frequency domain reflectometry. Journal of Experimental Botany 64, 2321–2332.
| Investigating xylem embolism formation, refilling and water storage in tree trunks using frequency domain reflectometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnvV2ntbs%3D&md5=9938324a7a3bd647f87667263259b93dCAS |
Holbrook NM, Zwieniecki MA (1999) Embolism repair and xylem tension: do we need a miracle? Plant Physiology 120, 7–10.
| Embolism repair and xylem tension: do we need a miracle?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjt1Sru74%3D&md5=a35976b460e52436200482473bce753aCAS |
Kozlowski TT, Kramer PJ, Pallardy SG (1991) ‘The physiological ecology of woody plants.’ (Academic Press: San Diego)
Larcher W (2003) ‘Physiological plant ecology.’ (Springer-Verlag: Berlin)
Mayr S, Charra-Vaskou K (2007) Winter at the alpine timberline causes complex within-tree patterns of water potential and embolism in Picea abies. Physiologia Plantarum 131, 131–139.
| Winter at the alpine timberline causes complex within-tree patterns of water potential and embolism in Picea abies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVSqtLjE&md5=9c49b68b0b54c3150e137c9ef20102b1CAS |
Mayr S, Schwienbacher F, Bauer H (2003) Winter at the alpine timberline. Why does embolism occur in Norway spruce but not in stone pine? Plant Physiology 131, 780–792.
| Winter at the alpine timberline. Why does embolism occur in Norway spruce but not in stone pine?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtlyjsLo%3D&md5=afad1dacf301686755c47e73be054891CAS |
Mayr S, Cochard H, Ameglio T, Kikuta SB (2007) Embolism formation during freezing in the wood of Picea abies. Plant Physiology 143, 60–67.
| Embolism formation during freezing in the wood of Picea abies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpt1Ogsw%3D%3D&md5=37741d5e4a1a6ef826708a4b122600a0CAS |
Mayr S, Schmid P, Laur J, Rosner S, Charra-Vaskou K, Dämon B, Hacke UG (2014) Uptake of water via branches helps timberline conifers refill embolized xylem in late winter. Plant Physiology 164, 1731–1740.
| Uptake of water via branches helps timberline conifers refill embolized xylem in late winter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsV2js74%3D&md5=5e0596dd0356feb254c792fd0e4297e8CAS |
Naor A, Girona J (2012) Apple. In ‘Crop yield response to water’. (Eds P Steduto, TC Hsiao, E Fereres, D Raes.) pp. 332–345. (Food and Agricultural Organization of the United Nations: Rome)
Nardini A, Lo Gullo MA, Salleo S (2011) Refilling embolized xylem conduits: is it a matter of phloem unloading? Plant Science 180, 604–611.
| Refilling embolized xylem conduits: is it a matter of phloem unloading?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisFyhs7g%3D&md5=28db9c438e9bc36c9ac545c538bb72dbCAS |
Pallardy SG (2008) ‘Physiology of woody plants.’ (Elsevier: London)
Pittermann J, Sperry JS (2006) Analysis of freeze–thaw embolism in conifers. The interaction between cavitation pressure and tracheid size. Plant Physiology 140, 374–382.
| Analysis of freeze–thaw embolism in conifers. The interaction between cavitation pressure and tracheid size.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVCgt7w%3D&md5=b3db21fc59df22b820d50595876a283aCAS |
Salleo S, Lo Gullo MA, Trifiló P, Nardini A (2004) New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant, Cell & Environment 27, 1065–1076.
| New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L.Crossref | GoogleScholarGoogle Scholar |
Salleo S, Trifilo P, Lo Gullo MA (2006) Phloem as a possible major determinant of rapid cavitation reversal in stems of Laurus nobilis (laurel). Functional Plant Biology 33, 1063–1074.
| Phloem as a possible major determinant of rapid cavitation reversal in stems of Laurus nobilis (laurel).Crossref | GoogleScholarGoogle Scholar |
Secchi F, Zwieniecki MA (2012) Analysis of xylem sap from functional (nonembolized) and nonfunctional (embolized) vessels of Populus nigra: chemistry of refilling. Plant Physiology 160, 955–964.
| Analysis of xylem sap from functional (nonembolized) and nonfunctional (embolized) vessels of Populus nigra: chemistry of refilling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFaksbbF&md5=98b6f7fa76d3703f541633d9dc995077CAS |
Sevanto S, Holbrook NM, Ball MC (2012) Freeze/thaw-induced embolism: probability of critical bubble formation depends on speed of ice formation. Frontiers in Plant Science 3, 107
| Freeze/thaw-induced embolism: probability of critical bubble formation depends on speed of ice formation.Crossref | GoogleScholarGoogle Scholar |
Sperry JS, Sullivan JEM (1992) Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse-porous and conifer species. Plant Physiology 100, 605–613.
| Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse-porous and conifer species.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cngvVWrsA%3D%3D&md5=b40e91eb292dbac7f923abeddc36d123CAS |
Sperry JS, Tyree MT (1990) Water-stress-induced xylem embolism in three species of conifers. Plant, Cell & Environment 13, 427–436.
| Water-stress-induced xylem embolism in three species of conifers.Crossref | GoogleScholarGoogle Scholar |
Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT (1987) Spring refilling of xylem vessels in wild grapevine. Plant Physiology 83, 414–417.
| Spring refilling of xylem vessels in wild grapevine.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cnhslChsQ%3D%3D&md5=2030a5b32dc299f14d9aca3d2e8d0626CAS |
Sperry JS, Donnelly JR, Tyree MT (1988a) A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell & Environment 11, 35–40.
| A method for measuring hydraulic conductivity and embolism in xylem.Crossref | GoogleScholarGoogle Scholar |
Sperry JS, Donnelly JR, Tyree MT (1988b) Seasonal occurence of xylem embolism in sugar maple (Acer saccharum). American Journal of Botany 75, 1212–1218.
| Seasonal occurence of xylem embolism in sugar maple (Acer saccharum).Crossref | GoogleScholarGoogle Scholar |
Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE (1994) Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 75, 1736–1752.
| Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska.Crossref | GoogleScholarGoogle Scholar |
Trifilò P, Raimondo F, Lo Gullo MA, Barbera PM, Salleo S, Nardini A (2014) Relax and refill: xylem rehydration prior to hydraulic measurements favours embolism repair in stems and generates artificially low PLC values. Plant, Cell & Environment 37, 2491–2499.
| Relax and refill: xylem rehydration prior to hydraulic measurements favours embolism repair in stems and generates artificially low PLC values.Crossref | GoogleScholarGoogle Scholar |
Tyree MT, Cochard H (1996) Summer and winter embolism in oak: impact on water relations. Annales des Sciences Forestieres 53, 173–180.
| Summer and winter embolism in oak: impact on water relations.Crossref | GoogleScholarGoogle Scholar |
Tyree MT, Ewers FW (1991) The hydraulic architecture of trees and other woody plants. New Phytologist 119, 345–360.
| The hydraulic architecture of trees and other woody plants.Crossref | GoogleScholarGoogle Scholar |
Tyree MT, Zimmermann MH (2002) ‘Xylem structure and the ascent of sap.’ (Springer-Verlag: Berlin)
Tyree MT, Davis SD, Cochard H (1994) Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA Journal 15, 335–360.
| Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction?Crossref | GoogleScholarGoogle Scholar |
Tyree MT, Salleo S, Nardini A, Lo Gullo MA, Mosca R (1999) Refilling of embolized vessels in young stems of laurel. Do we need a new paradigm? Plant Physiology 120, 11–22.
| Refilling of embolized vessels in young stems of laurel. Do we need a new paradigm?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjt1Sru78%3D&md5=92d7a4efac17ff80b8db0ac013857789CAS |
Utsumi Y, Sano Y, Fujikawa S, Funada R, Ohtani J (1998) Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiology 117, 1463–1471.
| Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsFaqu7s%3D&md5=744534a981248f93c8d89e3b1da41f16CAS |
Wheeler JK, Hugett BA, Tofte AN, Rockwell FE, Holbrook NM (2013) Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant, Cell & Environment 36, 1938–1949.
Wiegand KM (1906) Pressure and flow of sap in the maple. American Naturalist 40, 409–453.
| Pressure and flow of sap in the maple.Crossref | GoogleScholarGoogle Scholar |
Zwieniecki MA, Holbrook NM (2009) Confronting Maxwell’s demon: biophysics of xylem embolism repair. Trends in Plant Science 14, 530–534.
| Confronting Maxwell’s demon: biophysics of xylem embolism repair.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1Wqs7%2FF&md5=9cfa377c35e131ed0efddef7d74e6062CAS |