Phenotyping roots in darkness: disturbance-free root imaging with near infrared illumination
Rongli Shi A * , Astrid Junker A * , Christiane Seiler A and Thomas Altmann A BA Department of Molecular Genetics, Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) Gatersleben, 06466 Seeland, Germany.
B Corresponding author. Email: altmann@ipk-gatersleben.de
Functional Plant Biology 45(4) 400-411 https://doi.org/10.1071/FP17262
Submitted: 31 January 2017 Accepted: 30 October 2017 Published: 5 January 2018
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Root systems architecture (RSA) and size properties are essential determinants of plant performance and need to be assessed in high-throughput plant phenotyping platforms. Thus, we tested a concept that involves near-infrared (NIR) imaging of roots growing along surfaces of transparent culture vessels using special long pass filters to block their exposure to visible light. Two setups were used to monitor growth of Arabidopsis, rapeseed, barley and maize roots upon exposure to white light, filter-transmitted radiation or darkness: root growth direction was analysed (1) through short-term cultivation on agar plates, and (2) using soil-filled transparent pots to monitor long-term responses. White light-triggered phototropic responses were detected for Arabidopsis in setup 1, and for rapeseed, barley and maize roots in setups 1 and 2, whereas light effects could be avoided by use of the NIR filter thus confirming its suitability to mimic darkness. NIR image-derived ‘root volume’ values correlated well with root dry weight. The root system fractions visible at the different pot sides and in different zones revealed species- and genotype-dependent variation of spatial root distribution and other RSA traits. Following this validated concept, root imaging setups may be integrated into shoot phenotyping facilities in order to enable root system analysis in the context of whole-plant performance investigations.
Additional keywords: NIR image, NIR pass filter, phototropism, transparent pot.
Introduction
Roots play an essential role for mechanical stabilisation of a plant as well as for plant nutrition with respect to water and nutrient uptake. In recent years, roots have gained more and more attention and – in addition to physiological functions such as nutrient uptake and transport – it became clear that especially root morphology is a key feature for future investigations. As part of the plant–soil interface, roots are exposed to various biotic and abiotic factors such as gravity, moisture and nutrient gradients, light (at the soil surface layer) and a multitude of micro-organisms that influence root growth behaviour and structure. In addition to the effects of endogenous processes, the spatial arrangement of root systems (referred to as root systems architecture, RSA) is adjusted in response to varying environmental conditions and their interaction with the endogenous factors. This plasticity enables plants to optimise the usage of the available soil volume, the supply of nutrient to the shoot and plant performance in general. Substantial diversity in RSA has been reported for several plant species including Arabidopsis (Pacheco-Villalobos and Hardtke 2012), rice (Uga et al. 2009) and maize (Cai et al. 2012). Root system traits of field-grown maize plants were evaluated using shovelomics and large variation was observed among genotypes across different years and environments (Trachsel et al. 2011). Phenotypic diversity of root traits in a collection of 180 rapeseed accessions and of 52 barley genotypes was also shown by a non-invasive, high-throughput phenotyping system called GrowScreen-PaGe (Gioia et al. 2017). This study also reported about genotype by nutrient condition interactions, as well as nutrient conditions affected RSA features such as seminal root length and branch root numbers. Changes in RSA can largely affect plant performance with respect to biomass formation and thus influence yield. Some recent reports have demonstrated the ability of alterations in root system architecture to improve plant performance under unfavourable or stress conditions, such as drought or nutrients deficiency (Gruber et al. 2013; Uga et al. 2013). It has also been reported that maize genotypes with few crown roots had greater nitrogen (N) acquisition from low N soils (Saengwilai et al. 2014). Root morphological traits such as root length, diameter, surface area and volume, presence of root hairs and length of root hairs contribute to inter- and intra-specific variation in P acquisition efficiency (Rao et al. 2016). The adaptive responses of root systems to soils with low fertility have been reviewed by Rao et al. (2016). Pestsova et al. (2016) describe the co-location of quantitative trait loci (QTL) for root traits and maize yield although their causal relationship will have to be investigated in more detail. Despite the recent progress, root architectural traits and their analysis in plant populations is still a relatively unexplored field for research and comprise a huge, hitherto largely unexploited potential for breeding towards yield and yield stability enhancement.
Phenotyping has become the major bottleneck for genetic analyses towards crop improvement (Fiorani and Schurr 2013), which applies in particular to root phenotyping. Traditional field root phenotyping approaches depended largely on very laborious and time-consuming destructive samplings (Adu et al. 2014), whereas recent developments in high-throughput plant phenotyping employing robotic-assisted imaging platforms and computer-assisted analysis tools focus to a large extent on aboveground phenotyping as the aerial part of plants is well accessible to imaging using optical approaches (Fahlgren et al. 2015). However, shoots and roots are highly dependent upon each other for growth and survival. It is essential to study the growth of both organs, which can exhibit contrasting diel growth patterns and sensitivity to environmental changes (Ruts et al. 2013). This explains the increasing need to develop high-throughput suitable methods for rapid and accurate quantification of RSA related traits.
Several developed high-throughput root phenotyping systems address RSA of roots grown in artificial substrates such as agar or other transparent materials, filter paper or in hydroponics (Nagel et al. 2009; Iyer-Pascuzzi et al. 2010; Downie et al. 2012; Gioia et al. 2017). Root trait expression in these systems may, however, deviate from that occurring under natural conditions, where it is influenced by processes involved in the interaction between soil and roots (White et al. 2013). Three dimensional (3D) imaging technics for non-invasive root phenotyping such as X-ray microtomography (Mairhofer et al. 2015) or magnetic resonance imaging (MRI, Schmittgen et al. 2015) are more close to the natural environment as they allow for the assessment of root traits within soil environments. However, these techniques mostly do not allow for high-throughput screenings due to long measurement times. As an alternative, two dimensional (2D) root imaging in rhizotrons enables the quantification of root growth along the interface between soil and a transparent cover. This method is amenable to high throughput and provides very comprehensive information on root system architecture with limitations on tall plants or late developmental stages (Nagel et al. 2012; Shrestha et al. 2014).
Although root growth under natural conditions is mainly restricted to the belowground area and thus occurs in darkness, photomorphogenic responses of roots are well known. Recently, several groups demonstrated that root development and response to hormones or abiotic stress are altered upon root illumination (Xu et al. 2013; Silva-Navas et al. 2015; Lee et al. 2017). Illumination of roots with white or blue light, in many plant species induces negative phototropism (Hubert and Funke 1937). Primary and lateral Arabidopsis roots, especially pronounced in the Arabidopsis starchless and gravitropically weakened pgm mutant, show opposite effects on root orientation with unilateral blue and red light inducing negative and positive phototropic responses, respectively (Ruppel et al. 2001; Kiss et al. 2002). These data indicate the necessity of protecting roots from light in order to ensure undisturbed root growth and development.
Here we present a root phenotyping approach based on NIR-imaging of roots in transparent pots which is amenable to high-throughput. Roots grown in transparent pots will be protected from visible light exposure by covers of special VIS blocking and NIR transparent filter material (longpass filter excluding all wavelengths shorter than 750 nm, similar to that reported by Wells et al. 2012). This filter ensures roots to be maintained in darkness throughout the whole growth and imaging processes. This has the advantage of avoiding any mechanical movements, either of culture vessels from light protecting holding devices to imaging stations or of light impermeable cover shields around the pots for the time of image acquisition. Roots growing along the side and the bottom of these pots can thus be imaged using NIR-sensitive digital cameras and suitable illumination.
The main objective of this work was to test and validate a root phenotyping concept suitable to be integrated into an existing high-throughput phenotyping platform hitherto only set up for shoot trait assessment (Junker et al. 2015). In this respect, the following questions were addressed for four representative model and crop plant species (Arabidopsis, rapeseed, barley and maize). (1) How much does light exposure affect the expression of root traits? (2) Can darkness be mimicked by shielding roots from light of wavelengths shorter than 750 nm as blocked by an optical filter? (3) Is NIR imaging of roots growing at the surfaces of soil-filled pots suitable to quantify relevant root traits? The results presented here contribute to building up combined high-throughput root and shoot phenotyping facilities, which will enhance our understanding of root systems architecture in the context of biomass and yield formation.
Materials and methods
Plant materials and growth conditions
For all experiments the following plant material has been used: rapeseed (Brassica napus L. cv. Reston), maize (Zea mays L. cv. B73), barley (Hordeum vulgare L. cv. Barke) and Arabidopsis thaliana Col-0. Maize genotypes (B73, N22, P148, PHT77 and S052) are derived from a maize diversity panel (Muraya et al. 2016) and represent accessions with a wide variation in root and shoot traits.
Short-term light exposure experiment
Rapeseed, barley and maize seeds were sterilised for 5 min in 70% ethanol and for 30 min in 3% sodium hypochlorite solution. Arabidopsis seeds were sterilised with ethanol 70% + TritonX-100 (0.05%) for 20 min and were washed with distilled water before sowing on agar. Rapeseed, barley and maize seeds were soaked in saturated CaSO4 for ~4 h before sowing on 1/2 MS, 1.5% (w/v) agar medium (pH 5.6 without sugar).
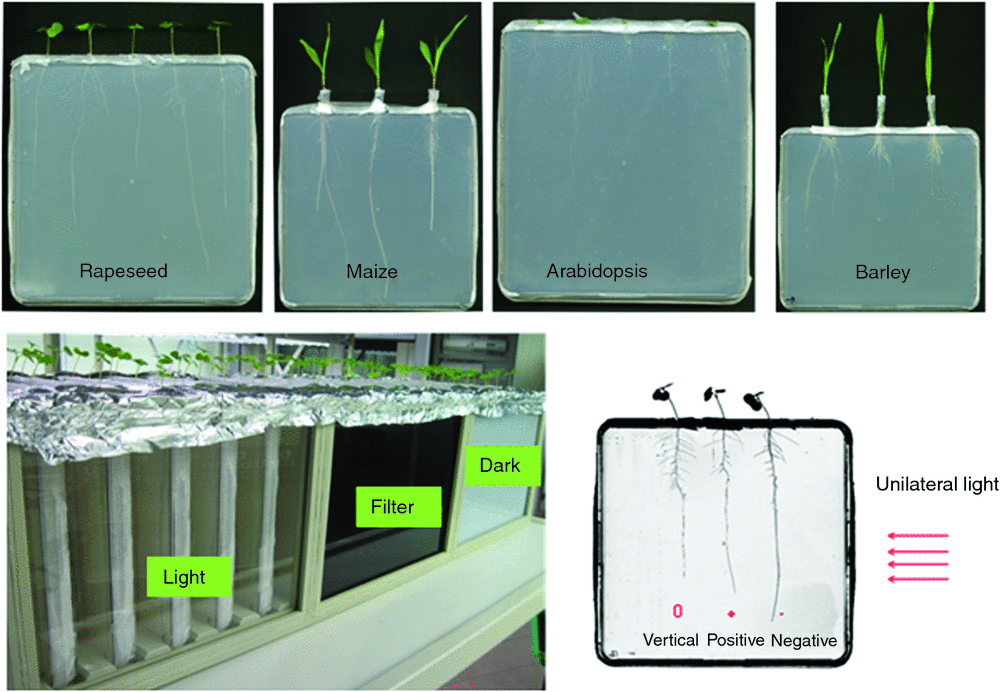
Melted agar medium (see above) was poured into large sterilised plates (245 × 245 × 18 mm) with holes on the top in order to allow plant shoots to grow outside the plates but roots on the medium inside (plates are placed in a vertical position for plant growth). Three holes were used for maize (9 mm diameter) and barley (7 mm diameter), five holes (3 mm diameter) for rapeseed and Arabidopsis. Arabidopsis and rapeseed were sown directly into the holes by placing the sterilised seeds onto the agar surface through the holes. Maize and barley seeds were sown either in Eppendorf tubes (1.5 mL) (maize) or 1 mL tips (barley), which were cut at the tip and inserted into the holes. After sowing, the holes/the top of Eppendorf tube were covered with Parafilm to keep the moisture. When seedlings grew bigger, the Parafilm was removed. All plates were placed vertically inside a custom-made container (Fig. 1) and kept in a phytochamber with the following conditions: 20°C/18°C, 16h/8 h day/night, LED light intensity (on the top) 120 µmol photons m–2 s–1 PAR. A fan at one side of the container provided ventilation and a homogeneous temperature distribution inside the container (to avoid temperature effects on root growth). The container was separated into three sections with 5 plate holders in each section. Each section corresponded to one of the three light treatments which used lateral light provided by halogen bulbs (Halogen Decostar 51 s Standard, OSRAM GmbH) to reach the root growth area inside the container through different cover materials: ‘Light’, lateral cover made of Plexiglas, for white, unfiltered light exposure; ‘Dark’, cover made of light impermeable polyvinyl chloride (PVC) plastic (thickness: 3 mm), no light exposure; ‘NIR pass filter’, (SOLARIS IR S306, longpass filter with transmission of light above 750nm, PSC A/S, Brønderslev, DK), >750nm light exposure (see Fig. S1, available as Supplementary Material to this paper). The transmission of different materials was measured by using strips cut from the PVC plate, NIR pass filter and the transparent pot with UVIKON (Goebel Instrumentelle Analytik GmbH). The results showed that the transparent pot allowed transmission of light of the whole wavelength range while the longpass filter only transmitted light of wavelengths above 750nm. The PVC plate showed no transmission at all (Fig. S2). Lateral light treatments were initiated at 5 days after sowing (DAS) for rapeseed, barley and maize, when the root length was ~5 cm, and at 14 DAS for Arabidopsis. Before the treatments roots grew in darkness. Just before initiation of treatments, all the plates were scanned in grayscale at 300 dots per inch resolution using an Epson Expression 10 000 XL scanner (Seiko Epson) and the tip position of the primary root, or the longest seminal root (barley) of each plant, respectively, was marked on the plate. The plates then were placed randomly into the three sections of the box. Unilateral light was supplied on the root growth area inside the container for 48 h. At harvest, all the plates were scanned again and orientation of the previously marked roots was recorded as vertical, positive and negative (Fig. 1). Roots were classified as ‘vertical’ when no phototropic effect could be observed (roots grew straight); as ‘positive’ when root grew towards the lateral light source (positive phototropism) and as ‘negative’ when root grew away from the lateral light source (negative phototropism).
Long-term light response experiment
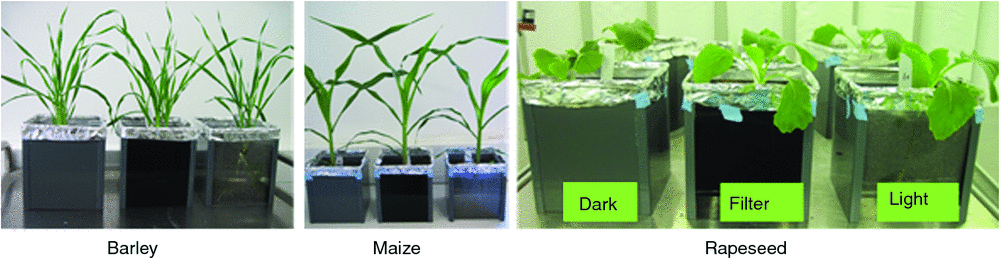
Five litre transparent pots (Fuerst) filled with mixture of substrate (self-made compost, IPK) and sand (1 : 1) were used for plant cultivation. Each pot was covered by a custom-made four-sided open top container. One side of the container was made up by either PVC plastic, plexiglass, or NIR pass filter material to create Dark, Light and NIR Filter treatments respectively (Fig. 2). The seeds were sown directly into the soil with the initiation of different treatments. In order to be able to visualise as many roots as possible on the pot surface, seeds were sown to the soil edges of the four sides of the pot (four seeds per pot, one at each side centre position). After germination, only one seedling was kept per pot. This side is referred to as side 1. The experiment was conducted with six replicates per plant species and treatment (for maize, a second experiment was performed with another six replicates). The plants were irrigated regularly according to the soil moisture status with small amounts of water to avoid water logging (~50 mL daily). The plants were grown in a phytochamber with the following conditions: 16/8 h day/night (D/N), 60% relative air humudity, light intensity of 240 µmol photons m–2 s–1 PAR (rapeseed and maize), 180 µmol photons m–2 s–1 PAR (barley) and the temperature of 18°C/16°C (D/N, rapeseed), 20°C/16°C (D/N, barley), 25°C/22°C (D/N, maize). The three (Dark, Light and NIR Filter) treatments were compared for barley, maize and rapeseed respectively.
After 3 weeks of plant growth, the transparent pots with the plants were taken out of the container and roots growing along the pot surface were imaged using a NIR sensitive camera (Manta, G-419, ALLIED). Pictures were taken from the bottom and the four sides of the pot through the NIR pass filter with halogen illumination. These NIR images were analysed using the SmartRoot software (Lobet et al. 2011) (Fig. S3a).
The plants were harvested after taking NIR images. Shoots and roots were separated and leaf number was counted, shoot length was measured using a ruler. Roots were carefully washed, scanned and analysed by WinRhizo Pro ver. 2013c (Regent Instruments) (Fig. S3b). The root traits including total root length (cm), root surface area (cm2) and root volume (cm3) were extracted from both root analysis software. The shoots and roots dry weights were recorded after drying in an oven at 70°C for 3 days.
Statistical analysis
The statistical analyses were performed using one-way ANOVA by SigmaPlot ver. 11.0 (Systat Software). The data of the maize long-term experiment were subjected to ANOVA using Genstat ver. 16.0 (the first experiment and the second experiment were considered as two blocks). Correlations were analysed using the Pearson product moment correlation. Chi-square tests were performed using Excel (Microsoft).
Results
Short-term light exposure experiment
In order to evaluate the short-term effect of light (full spectrum or >750 nm) on root growth, plants were grown in vitro, with shoots outside and roots inside the agar plates. After 2 days exposure to unilateral light, the orientation of primary roots was recorded and the phototropic effects of the Dark, Light and NIR Filter treatments were evaluated. We measured the root curvature/bending angles of all the plants (Fig.S4), but due to the very large scatter of the values we decided to follow the approach by Kutschera and Briggs (2012) to evaluate the phototropic effects as shown in Fig. 1. According to the root tip orientation after the 48 h light treatment towards or away from the light source, seedling roots were classified as positive (‘+’) or negative (‘–’) phototropic, respectively, and as neutral (‘0’) when vertical roots growth was observed. Compared with the Dark treatment (= control treatment, used as expected values in chi-square tests), the distribution of +/−/0 roots in plants grown under the Light treatment differed significantly for all plant species tested (Table 1) with higher number of ‘–’ roots and/or lower number of ‘+’ roots indicative of negative phototropic responses. Significant differences in the distribution of +/−/0 roots between Dark and NIR Filter treatments were observed only in Arabidopsis (but not in rapeseed, barley, or maize). Here, the filtered light (>750 nm) triggered positive phototropism with a higher number of ‘+’ roots and a lower number of ‘–’ roots. In maize, significant deviations from a 1 : 1 ratio of ‘+’: ‘–’ roots expected for undisturbed root growth were observed in all three treatments, always with ‘+’ < ‘–’. By tendency, however, also here the strongest and most significant difference was found for plants exposed to the lateral light.

|
Long-term light exposure experiment
In order to analyse more long-term effects of light on roots of soil-grown plants, maize, rapeseed and barley plants were cultivated in transparent pots for three weeks. Seeds were sown to the top edge of the soil close to the inner surface of the pot side wall (‘side 1’) and the various light treatments were carried out by covering this side with different materials for the Dark, Light and NIR Filter treatments (analogous to the short-term in-vitro experiment). The remaining sides of the pot and the bottom were kept in complete darkness. After imaging of all pot surfaces, roots were excavated and washed to assess also the entire root systems. In this setup, Arabidopsis was not tested due to the difficulties with respect to root excavation and thereby occurring loss of major fractions of the root system.
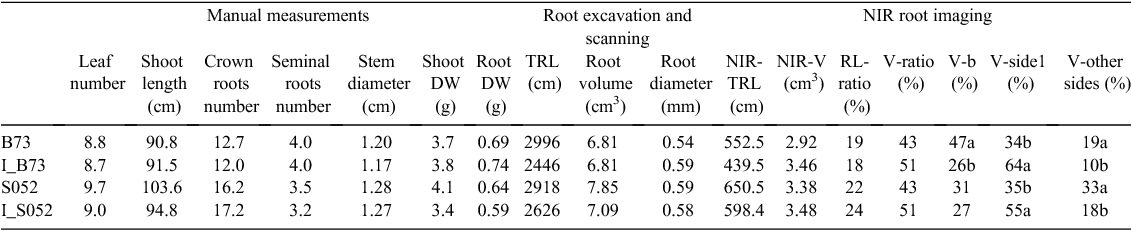
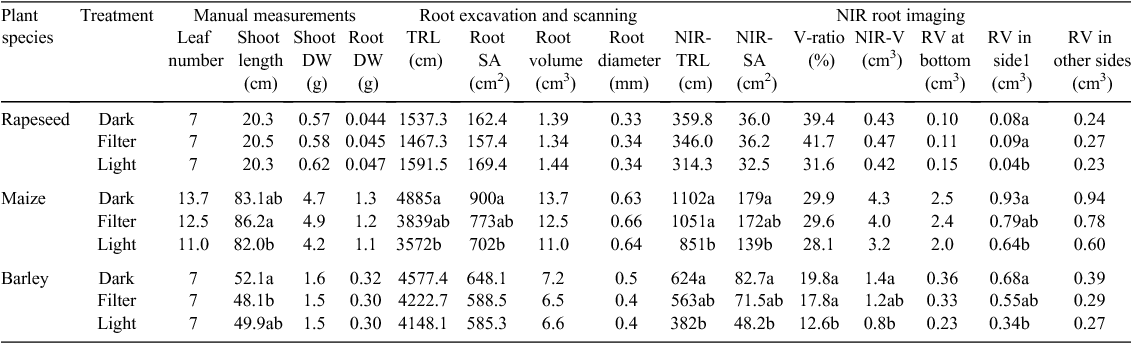
After 3 weeks of plant growth, root and shoot biomass- and architecture-related traits were assessed through NIR imaging of roots at the pot surfaces, scanning the entire root systems after excavation, and manual measurements. For the investigated plant species, none of the manually measured shoot parameters (leaf number, shoot length and shoot DW) showed significant differences between the Dark and Light treatments (Table 2). Similarly, no significant differences could be observed with regard to total root DW and scanning-derived architectural traits of the washed roots such as the total root length, root diameter, root surface area and root volume under the different treatments for rapeseed and barley. For maize, the total root length and root surface area was decreased in the Light vs the Dark treatment (Table 2). In contrast to architectural traits determined for the total root systems (washed and scanned), NIR-imaging derived root architectural traits detectable at the pot surfaces (including four sides and the bottom) such as the total root length, root surface area and root volume (NIR-TRL, NIR-SA, NIR-V) were found to be decreased significantly for barley and maize in the Light vs the ‘Dark’ treatment (Table 2). Similar tendencies of these parameters were detectable also for rapeseed, although not significant (Table 2). RV in side 1, in the pot surface which has been exposed to the three different treatments, showed significant differences between the Light and Dark treatments in all three species. We noted that none of the analysed root traits showed significant differences when the values of the NIR Filter treatment were compared with those of the dark treatment in any of the tested species.
|
The root phenotyping set-up employed here, in which transparent pots were used, combined with NIR-imaging of all four sides and the bottom of the pot, allowed for evaluations of root partitioning among the different pot sides, which is related to root architectural properties (e.g. the distinction between shallow and deep rooting genotypes). Respective NIR images were acquired at the end of the long-term experiment of 3-week-old maize, rapeseed and barley root systems. The trait ‘root volume’ was used in all further analyses, as it combines root length and root diameter information. Species-specific distribution patterns were found when comparing the root partitioning across the different pot sides and bottom (Table 2). The rapeseed root system grown in darkness was found to be evenly represented at side 1, 2 + 3 + 4 and the bottom (20, 60, 20% respectively) whereas a bigger proportion of the maize root system (>50%) accumulated and was visible at the bottom indicating a fast vertical penetration of the available rooting space. In the case of barley, the major part of the root system (~48%) was visible at side 1 and only ~28% at sides 2 + 3 + 4 and ~25% at the bottom. In the NIR Filter treatments the root partitioning patterns were similar to those of the dark treatments for maize, rapeseed and barley. In contrast, exposure to the full light spectrum substantially decreased the root accumulation on side 1 for all the plant species. A significant reduction of more than 50% in rapeseed and barley, and of approximately 30% in maize root volume visible at side 1 was found in the light treatment (Table 2).
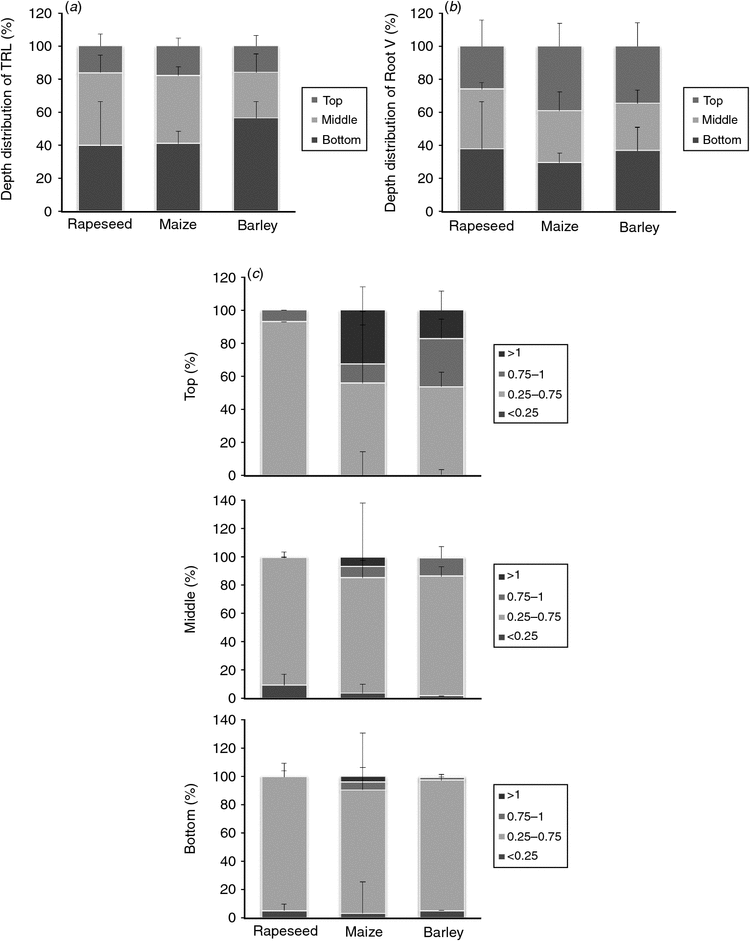
To extract even more root architectural traits, the NIR-root images from side1 of the treatment ‘Filter’ were divided into three sections, top, middle, bottom and the analysis of the total root length (TRL) and root volume (RV) for each image enabled to draw conclusions about the depth distributions of rapeseed, maize and barley root systems (Fig. 3a, b). Similar depth distributions have been found for maize and rapeseed with ~40% of the total root length represented in each bottom and middle section and ~20% in the upper top section of the rooting zone (Fig. 3a). Compared with maize and rapeseed, the barley root systems tended to accumulate more of the total root length in the bottom section (50%), whereas the fraction of the middle and upper sections were found to be decreased or similar respectively (Fig. 3a). The analogous analysis for the depth distribution of the root volume did not reveal obvious differences in the three image sections for all three species. Furthermore, the fractions of four root diameter classes (<0.25, 0.25–0.75, 0.75–1, >1 mm) were calculated for each zone based on the root length assigned to each diameter class (Fig. 3c). This analysis revealed (as expected) that maize roots have a larger diameter than rapeseed and barley, mainly in the top and middle sections. All rapeseed roots and the majority of barley roots have a diameter <1 mm. For all three plant species the majority of roots were assigned to diameter classes <0.75 mm and are prevalent in the middle and bottom sections (Fig. 3c).
|
Relations between the visible root volume (NIR imaging) and total root volume and DW
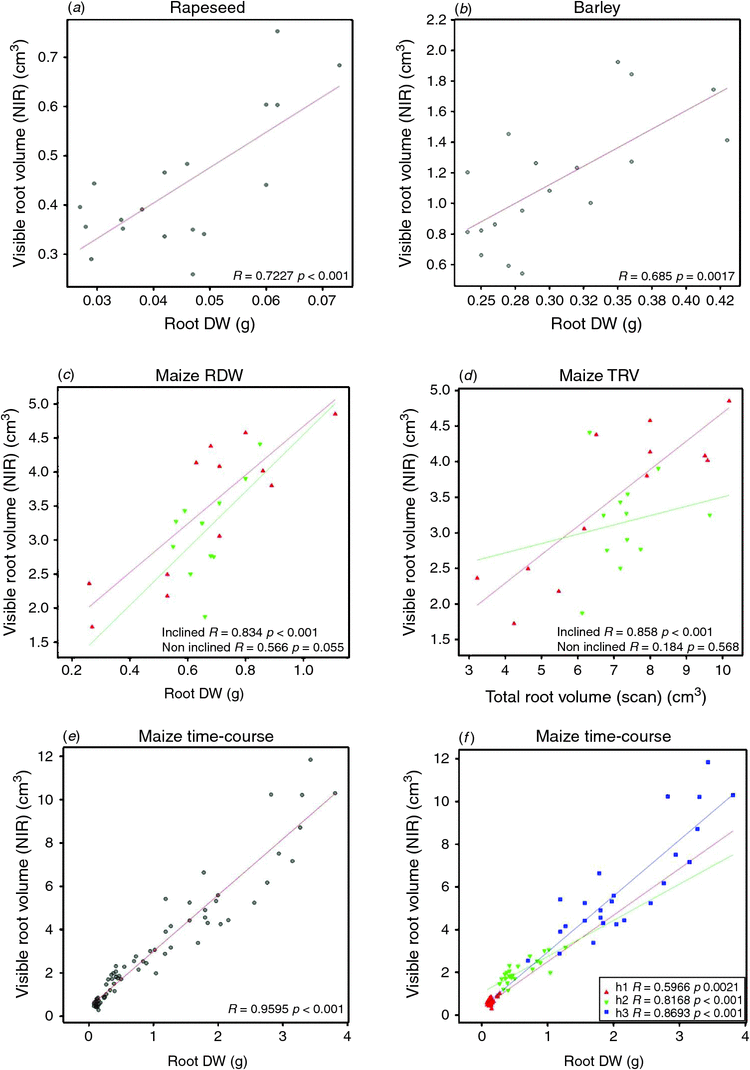
To further assess the significance of root trait quantification using the presented root phenotyping approach, the NIR imaging derived root volume trait (visible roots) was compared with the root volume extracted from scanner images acquired after root washing and excavation (total roots). The ratio of visible to total roots was calculated and in average ~39.4, 29.9 and 19.8% of the total roots were detected by NIR-imaging of all sides and bottom of the pots for rapeseed, maize and barley respectively (Table 2). The correlations between NIR-root volume and scanned root volume for rapeseed, maize, barley were r = 0.656, P < 0.01, r = 0.85, P < 0.01, r = 0.743, P < 0.01 respectively.
Significant positive correlations were observed between scanned root volume and root DW for all the tested plant species (r = 0.728, P < 0.01, r = 0.91, P < 0.01, r = 0.685, P < 0.05 for rapeseed, maize barley respectively). It is also important to assess to which extent the NIR imaging-derived visible root volume is representative of root biomass. Therefore, the visible root volume was correlated with the manually recorded root DW. The results revealed significant positive correlations for rapeseed (r = 0.72, P < 0.01) and barley (r = 0.69, P < 0.01) (Fig. 4a, b). For maize, correlations between image-derived root volume and root DW were particularly high when the pots were inclined (~30°) during plant growth. In inclined pots, the visible root volume of plants of two maize genotypes at side 1 was found to be significantly increased from 34 to 64% (B73) and from 35 to 55% (S052) as compared with non-inclined pots, whereas the visible portion at the other sides of the pots were significantly decreased from 19 to 10% (B73) and from 33 to 18% (S052) (Table 3). Stronger and more significant correlations were found for NIR imaging-derived root volume vs. scanned root volume or DW for inclined pots as for non-inclined pots (r = 0.83, P = 0.01 vs r = 0.57, P = 0.05 and r = 0.86, P < 0.001 vs r = 0.18, P = 0.586 respectively, Fig. 4c, d). These results indicate that the NIR imaging-derived visible root volume is a representative proxy for root biomass in all three plant species tested.
Developmental changes in NIR-image derived root trait expression
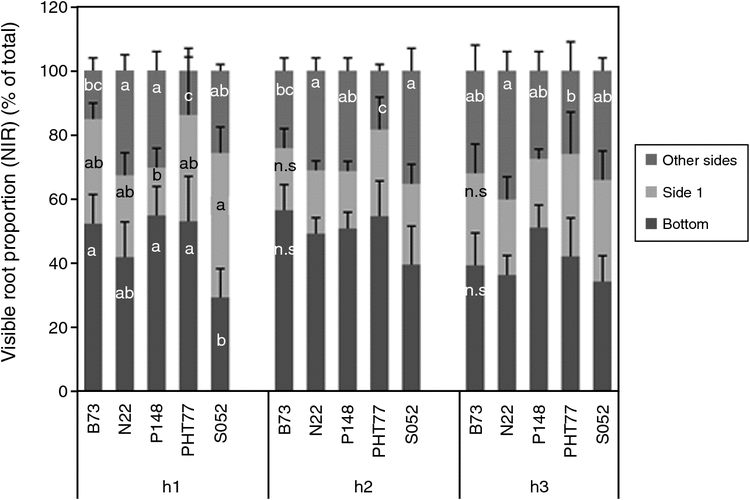
In order to assess the influence of the developmental stage on NIR imaging derived parameters, the representation of the maize root volume at the surfaces of the transparent pots was tested for five maize genotypes (five replicates each) and three developmental stages (2, 3 and 4 weeks after sowing referred to as H1, H2 and H3 respectively). Fig. S6 shows root- and shoot-related biomass parameters for each genotype and harvesting time point. The B73 and N22 plants were found to have the highest/lowest root and shoot DWs, respectively, over all three developmental stages (Fig. S5a, b). The NIR-imaging derived root volume behaved accordingly (Fig. S5c). A significant positive correlation between NIR-imaging derived root volume and root DW was found for all three harvesting time points (rH1: 0.59, rH2: 0.82, rH3: 0.87 rH1–3: 0.96, Fig. 3e, f). The comparison of NIR-imaging derived root volume (visible) with the scanning derived (total) root volume revealed genotype-dependent difference especially at H1 (Fig. S5d). The root volume proportion visible in NIR images compared with the total root volume did not change substantially over the three harvest time points (H1: 32%, H2: 33% and H3: 29%, Fig. S5d) although large genotypic variations were observed, especially for N22 at H1; these are probably the cause of the lower correlation with root DW at H1 compared with H2 and H3 (Fig. 4e, f). Differences in root partitioning give indications for genotype-dependent diversity of root growth properties. As shown in Fig. 5, the five genotypes differed in the proportions of roots detectable at the four sides and the bottom of the pots especially at the early harvest time-point (H1). Plants of the genotypes B73, P148 and PHT77 rapidly reached the bottom and accumulated there, whereas S052 roots were visible to a greater extent at the sides of the pot. At H3, no significant differences were detectable anymore comparing root accumulation/growth at bottom and at side1, whereas significant differences were still detected for the root fractions present at the other sides exhibiting a similar tendency for genotype-specific root partitioning as in H1 (PHT77 had the lowest value, N22 had the highest value). In general, the fraction of roots visible at the bottom and side1 accounted for ~70% of the total visible roots (of all genotypes).
Discussion
Roots are highly responsive to environmental signals encountered in the rhizosphere. Modifications of the root system architecture can contribute to the improvement of desirable agronomic traits such as yield, drought tolerance, and resistance to nutrient deficiencies (Uga et al. 2013; Saengwilai et al. 2014; Pestsova et al. 2016). In order to better understand the influence of root growth and architecture on plant performance, it is necessary to develop appropriate root phenotyping methods to determine important belowground features of plants. It is therefore highly desirable to assess root trait expression in parallel with properties of the shoot system. However, implementation of root phenotyping has been lagging behind the assessment of the aerial parts of plants, especially in platforms designed for cultivation of plants in soil substrates in pots. The main goal of the presented work therefore was to test components and procedures of a root phenotyping concept that is compatible with existing high-throughput (shoot) phenotyping platforms and that can be used to upgrade them for simultaneous shoot and root trait monitoring.
One of the key components of the root phenotyping approach is a NIR longpass filter that protects roots from visible light exposure during plant growth but allows root imaging in the NIR spectral range. Similar filter material has been used for root phenotyping of plants grown in agar-solidified synthetic media in vertical Petri dishes (Wells et al. 2012), but it has not been extended for use in soil-filled pots and it has been unclear whether darkness could be mimicked by shielding roots from light of wavelengths shorter than 750 nm.
In the present study we conducted two series of experiments to evaluate if the NIR pass filter is able to mimic darkness and to assess whether NIR light passed through the filter would cause any phototropic response in roots. In accordance with other reports of roots phototropic responses (e.g. Hubert and Funke 1937; Ruppel et al. 2001; Kiss et al. 2003a), we found a significant white light-induced difference in the orientation of rapeseed, maize, barley and Arabidopsis roots when grown on agar and exposed to short-term (48 h) unilateral illumination. The ratio of negatively and positively oriented maize roots deviated significantly from 1 : 1 under all treatments (including darkness) and thus was (at least in part) independent of the light conditions. It has to be attributed to other, yet unknown factors/influences, which remain to be investigated. While no significant phototropic responses to the light transmitted by the NIR pass filter were observed for rapeseed, maize and barley, Arabidopsis roots showed a very clear positive response. As mentioned by Kutschera and Briggs (2012) and Liscum et al. (2014), only the phytochromes and phototropins are relevant to root phototropism and these photoreceptors perceive light in the range of ~330–800 nm. Phytochrome A- and B-dependent positive root phototropism triggered by red light but not by far red (700–750 nm) has been reported for Arabidopsis (Kiss et al. 2003a). Light of wavelengths shorter than 750 nm are almost completely blocked by the filter, but phytochrome-mediated responses to light of different wavelengths are complex and include reactions to continuous far red (Li et al. 2011). In contrast to Arabidopsis, rice has been reported not to show positive root phototropism (Wang et al. 2007). Kiss et al. (2003b) have proposed that the relative strength of tropic responses in roots is gravitropism > negative phototropism > positive phototropism. The red light-triggered response resulting in positive phototropism might thus be too weak to elicit any obvious curvature in plant species with relatively large/thick roots.
In the long-term experiment conducted with rapeseed, barley and maize, the substrate in transparent pots was illuminated from one side. No differences with respect to shoot and root DWs were detected among the treatments (light/NIR pass filter/darkness). However root partitioning in the pot was found to be changed significantly showing a decrease in the fraction of the root volume visible at the white-light illuminated pot side (side one) compared with the darkness and NIR pass filter treatments (Table 2). This is in agreement with other studies showing alterations in root architecture in the light (Xu et al. 2013; Silva-Navas et al. 2015). These findings underline the necessity to maintain roots in darkness during growth and development in order to avoid disturbance of the system through unnatural light exposure. Through the use of the NIR pass filter, significant differences in both shoot and root-related parameters were avoided in comparison to the dark situations. This led us to conclude that use of the NIR pass filter does not influence the orientation of root growth for most plant species, except for Arabidopsis roots, which may even be attracted to the filter surface due to positive phototropic responses, potentially leading to an enhanced fraction of visible roots. The obtained results thus confirm the suitability of this filter material for appropriate long-term light protection during root growth thus allowing undisturbed root phenotyping.
The root phenotyping concept presented here enables the extraction of a set of relevant root parameters including root biomass related traits as well as root architectural information. Compared with a rhizotron system, in which the root system is visible along one large imaging surface, root growth in transparent pots can be monitored at all four sides of the pot and at its bottom side (five imaging surfaces). Due to the rapid growth of roots and the limited dimensions of the pots, the complete root system (except parts hidden in the soil substrate) is visible at one pot side only in very early developmental stages. During growth progression, roots soon reach the bottom of the pot (and the other sides) with major parts of the primary root(s) visible near the surface of side one (where the seed was sown) and lateral roots bending into the soil or around the pot edges. Therefore, the presented setup with the restricted sizes of pots usually used for plant cultivation in the glasshouse might have some limitations with respect to the quantification of root architectural traits (such as root angles and root length density) compared with rhizotrons. Nevertheless, broader classifications according to relevant features of root systems such as wide or deep rooting can be achieved by comparing the relative root proportions at the different sides and the bottom of the pot. Rapeseed, for example, revealed a wider, more shallow root system than maize. Rapeseed roots allocated evenly to the sides and the bottom of the pot, whereas nearly half of the maize roots rapidly accumulated at the bottom. Genotypic differences of root partitioning in maize were also observed in the presented setup, especially in the early growth stage (Fig. 5). Some maize genotypes such as PHT77, which has previously been reported to develop particularly long roots (Kumar et al. 2012; Pace et al. 2015) are prone to grow deep roots, whereas others tend to grow a wider root system (e.g. S052). This indicates the suitability of the setup for detection of intraspecific differences in root system architecture which however decreased at later growth stages due to rapid accumulation of roots (especially at the bottom of the pot) and difficulties to detect individual roots when grown to high densities. Therefore, limitations of the proposed setup with respect to proper root trait quantification in different plant developmental phases have to be taken into account. Further RSA traits such as the depth distributions of total root length, root volume and root diameter classes could be monitored in the NIR images (Fig. 3) by separate evaluation of the upper, middle and bottom zones.
Root biomass-related traits can be assessed in the transparent pot system achieving similar results as in rhizotrons. In the present study, ~39.4, 29.9 and 19.8% of the total root volume of rapeseed, maize and barley, respectively, could be monitored using the NIR root imaging. These results are similar to previous studies that reported percentages of visible roots in rhizotrons of ~42, 17 and 33% of the total root length for rapeseed, maize and barley respectively (Nagel et al. 2012). Although only a fraction of the total root system is visible in both non-destructive root phenotyping approaches (either rhizotron or transparent pot), it is important to note that the visible root volumes of all species tested revealed significant positive correlations to the manually measured root DWs (Fig. 4). The correlation coefficients (0.72, 0.57 and 0.69 for rapeseed, maize and barley respectively) determined in the transparent-pot-system were found to be somewhat lower than those reported for rhizotrons (0.98, 0.59 and 0.96 for rapeseed, maize and barley respectively) (Nagel et al. 2012) but the ranking of the species was similar. The observed differences can be attributed to the different setups of the systems with the transparent pots offering less imaging area for root growth compared with rhizotrons. Furthermore, for optimal detection of roots, rhizotrons are positioned in an inclined way thereby driving more roots towards growth along the transparent surface thus being detectable by imaging. As mentioned by Nagel et al. (2012), the percentage of visible barley roots was increased from ~14 to 33% when the inclination angle increased from 0° to 43° in a rhizotron system. Similar improvements of the NIR visible root fraction were achieved here for maize by inclination of the transparent pots where the fraction of the root system visible at side 1 was increased from 34 to 64% (for B73) and from 35 to 55% (for S052) (Table 3). This led to a very substantial increase of the correlation coefficients between NIR image derived (‘visible’) root volume and total root DW from r = 0.57 to r = 0.83 (converging to a similar correlation found for maize roots in rhizotrons).
The advantages of the NIR root imaging concept presented here are also related to the ease in practical handling of the transparent culture vessels compared with rhizotrons. Furthermore, the use of the filter material allows for a higher throughput since it avoids time needed for mechanical removal of a light cover plate from the rhizotrons for imaging and potential effects of visible light on root growth during the imaging process. The different components of the presented root imaging concept are suitable for integration into existing high throughput shoot phenotyping systems set up at IPK (Junker et al. 2015) or elsewhere, thereby supporting automation and upscaling of root phenotyping and enabling the integrated analyses of shoot and root growth and developmental dynamics. However, we would suggest using sufficient replication when applying this method for high-throughput root phenotyping. Furthermore (semi-)automatic root image analysis software will be necessary to assist the extraction of various different root traits.
In summary, we have shown that all components of the presented approach are well suitable for root NIR imaging, and that NIR imaging derived root traits represent good proxies for the real values of the entire root system. This validates the presented root phenotyping concept for the representative quantification of root traits. The work presented here will enable the built-up of combined high-throughput root and shoot phenotyping facilities, which will enhance our understanding of root systems architecture in the context of plant growth and development, yield formation and responses to environmental stresses.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Dr Rhonda Meyer for help in statistical analyses and Gunda Wehrstedt, Iris Fischer, Marion Michaelis for excellent technical assistance. This work was performed within the German Plant-Phenotyping Network (DPPN) which is funded by the German Federal Ministry of Education and Research (BMBF) (project identification number: 031A053).
References
Adu MO, Chatot A, Wiesel L, Bennett MJ, Broadley MR, White PJ, Dupuy LX (2014) A scanner system for high-resolution quantification of variation in root growth dynamics of Brassica rapa genotypes. Journal of Experimental Botany 65, 2039–2048.| A scanner system for high-resolution quantification of variation in root growth dynamics of Brassica rapa genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVGktbo%3D&md5=c8b9ba06563b2659148603d2570e11c1CAS |
Cai HG, Chen FJ, Mi GH, Zhang F, Mauren HP, Liu W, Reif JC, Yuan L (2012) Mapping QTLs for root system architecture of maize (Zea mays L.) in the field at different developmental stages. Theoretical and Applied Genetics 125, 1313–1324.
| Mapping QTLs for root system architecture of maize (Zea mays L.) in the field at different developmental stages.Crossref | GoogleScholarGoogle Scholar |
Downie H, Holden N, Otten W, Spiers AJ, Valentine TA, Dupuy LX (2012) Transparent soil for imaging the rhizosphere. PLoS One 7, e44276
| Transparent soil for imaging the rhizosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhtl2jtrzK&md5=e88d3ef3bf6148935f628b03d5d9ffe3CAS |
Fahlgren N, Gehan M, Baxter I (2015) Lights, camera, action, high-throughput plant phenotyping is ready for a close-up. Current Opinion in Plant Biology 24, 93–99.
| Lights, camera, action, high-throughput plant phenotyping is ready for a close-up.Crossref | GoogleScholarGoogle Scholar |
Fiorani F, Schurr U (2013) Future scenarios for plant phenotyping. Annual Review of Plant Biology 64, 267–291.
| Future scenarios for plant phenotyping.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXosFSktLw%3D&md5=1d37eec8f41c1b6d89c9f1fdef18d0fbCAS |
Gioia T, Galinski A, Lenz H, Müller C, Lentz J, Heinz K, Briese C, Putz A, Fiorani F, Watt M, Schurr U, Nagel KA (2017) GrowScreen-PaGe, a non-invasive, high-throughput phenotyping system based on germination paper to quantify crop phenotypic diversity and plasticity of root traits under varying nutrient supply. Functional Plant Biology 44, 76–93.
| GrowScreen-PaGe, a non-invasive, high-throughput phenotyping system based on germination paper to quantify crop phenotypic diversity and plasticity of root traits under varying nutrient supply.Crossref | GoogleScholarGoogle Scholar |
Gruber BD, Giehl RFH, Friedel S, von Wiren N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiology 163, 161–179.
| Plasticity of the Arabidopsis root system under nutrient deficiencies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVygsbjM&md5=9c8aed3035d99b34a1e86d24e6b04e9bCAS |
Hubert B, Funke GL (1937) The phototropism of terrestrial roots. Biologisch Jaarboek 4, 286–315.
Iyer-Pascuzzi AS, Symonova O, Mileyko Y, Hao Y, Belcher H, Harer J, Weitz JS, Benfey P (2010) Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiology 152, 1148–1157.
| Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsF2ls78%3D&md5=b9e7367dc86ea84bb82c9e66e97717b7CAS |
Junker A, Muraya MM, Weigelt-Fischer K, Arana-Ceballos F, Klukas C, Melchinger AE, Meyer RC, Riewe D, Altmann T (2015) Optimizing experimental procedures for quantitative evaluation of crop plant performance in high throughput phenotyping systems. Frontiers in Plant Science 5, 770
| Optimizing experimental procedures for quantitative evaluation of crop plant performance in high throughput phenotyping systems.Crossref | GoogleScholarGoogle Scholar |
Kiss JZ, Miller KM, Ogden LA, Roth KK (2002) Phototropism and gravitropism in lateral roots of Arabidopsis. Plant & Cell Physiology 43, 35–43.
| Phototropism and gravitropism in lateral roots of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtVyqtrk%3D&md5=db776e1ba641db4e6e9376a1dc9d43f6CAS |
Kiss JZ, Mullen JL, Correll MJ, Hangarter RP (2003a) Phytochromes A and B mediate red-light-induced positive phototropism in roots. Plant Physiology 131, 1411–1417.
| Phytochromes A and B mediate red-light-induced positive phototropism in roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXisFelsLw%3D&md5=3105c4bb3f2b365ba2dfaa23495ede3fCAS |
Kiss JZ, Correll MJ, Mullen JL, Hangarter RP, Edelmann RE (2003b) Root phototropism, how light and gravity interact in shaping plant form. Gravitational and Space Biology Bulletin 16, 55–60.
Kumar B, Abdel-Ghani AH, Reyes-Matamoros J, Hochholdinger F, Lübberstedt T (2012) Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines. Plant Breeding 131, 465–478.
| Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines.Crossref | GoogleScholarGoogle Scholar |
Kutschera U, Briggs WR (2012) Root phototropism, from dogma to the mechanism of blue light perception. Planta 235, 443–452.
| Root phototropism, from dogma to the mechanism of blue light perception.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XivFKqtL8%3D&md5=318267447a68c16aa9247830a369317fCAS |
Lee HJ, Park YJ, Ha JH, Baldwin IT, Park CM (2017) Multiple routes of light signaling during root photomorphogenesis. Trends in Plant Science 22, 803–812.
| Multiple routes of light signaling during root photomorphogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhtFSmt7vF&md5=1f8c800b37ca90a05afac24a3a57d1a3CAS |
Li J, Li G, Wang H, Deng XW (2011) Phytochrome signaling mechanisms. In ‘The Arabidopsis book’. (American Society of Plant Biologists: Washington, DC, USA).
Liscum E, Askinosie SK, Leuchtman DL, Morrow J, Willenburg KT, Coats DR (2014) Phototropism: growing towards an understanding of plant movement. The Plant Cell 26, 38–55.
| Phototropism: growing towards an understanding of plant movement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXks1Sgtb8%3D&md5=430cd979c6d0242eb34bd458d2b1dde8CAS |
Lobet G, Pages L, Draye X (2011) A novel image analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiology 157, 29–39.
| A novel image analysis toolbox enabling quantitative analysis of root system architecture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Sit77P&md5=02c0ce015aff0db10c034265a812493bCAS |
Mairhofer S, Sturrock C, Wells DM, Bennett MJ, Moonery SJ, Pridmore T (2015) On the evaluation of methods for the recovery of plant root systems from X-ray computed tomography images. Functional Plant Biology 42, 460–470.
| On the evaluation of methods for the recovery of plant root systems from X-ray computed tomography images.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXms1OjsLw%3D&md5=e3cd6f6362551428d0c9f7cca05f589bCAS |
Muraya MM, Chu J, Zhao Y, Junker A, Klukas C, Reif JC, Altmann T (2016) Genetic variation of growth dynamics in maize (Zea mays L.) revealed through automated non-invasive phenotyping. The Plant Journal 89, 366–380.
| Genetic variation of growth dynamics in maize (Zea mays L.) revealed through automated non-invasive phenotyping.Crossref | GoogleScholarGoogle Scholar |
Nagel KA, Kastenholz B, Jahnke S, van Dusschoten D, Aach T, Mühlich M, Truhn D, Scharr H, Terjung S, Walter A, Schurr U (2009) Temperature responses of roots, impact on growth, root system architecture and implications for phenotyping. Functional Plant Biology 36, 947–959.
| Temperature responses of roots, impact on growth, root system architecture and implications for phenotyping.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlOgs7vM&md5=e11622e5a5708baf3ecc3b21c086e1deCAS |
Nagel KA, Putz A, Gilmer F, Heinz K, Fischbach A, Pfeifer J, Faget M, Blossfeld S, Ernst M, Dimaki C, Kastenholz B, Kleinert AK, Galinski A, Scharr H, Fiorani F, Schurr U (2012) GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Functional Plant Biology 39, 891–904.
| GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons.Crossref | GoogleScholarGoogle Scholar |
Pace J, Gardner C, Romay C, Ganapathysubramanian B, Lübberstedt T (2015) Genome-wide association analysis of seedling root development in maize (Zea mays L.). BMC Genomics 16, 47
| Genome-wide association analysis of seedling root development in maize (Zea mays L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXkt1Wns78%3D&md5=6de82675ddf2b71114170b5005419c76CAS |
Pacheco-Villalobos D, Hardtke CS (2012) Natural genetic variation of root system architecture from Arabidopsis to Brachypodium, towards adaptive value. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367, 1552–1558.
| Natural genetic variation of root system architecture from Arabidopsis to Brachypodium, towards adaptive value.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xos1Oqs74%3D&md5=5b45e3224ad49e83a45234a5156f59f0CAS |
Pestsova E, Lichtblau D, Wever C, Presterl T, Bolduan T, Ouzunova M, Westhoff P (2016) QTL mapping of seedling root traits associated with nitrogen and water use efficiency in maize. Euphytica 209, 585–602.
| QTL mapping of seedling root traits associated with nitrogen and water use efficiency in maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhvFSiug%3D%3D&md5=743a0b0ad7cb3de71d5d398f4f35146dCAS |
Rao IM, Miles JW, Beebe SE, Horst WJ (2016) Root adaptations to soils with low fertility and aluminium toxicity. Annals of Botany 118, 593–605.
| Root adaptations to soils with low fertility and aluminium toxicity.Crossref | GoogleScholarGoogle Scholar |
Ruppel NJ, Hangarter RP, Kiss JZ (2001) Red-light-induced positive phototropism in Arabidopsis roots. Planta 212, 424–430.
| Red-light-induced positive phototropism in Arabidopsis roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtV2kt78%3D&md5=16aae60b74df0575890e1f6a41b36647CAS |
Ruts T, Matsubara S, Walter A (2013) Synchronous high-resolution phenotyping of leaf and root growth in Nicotiana tabacum over 24-h periods with GROWMAP-plant. Plant Methods 9, 2
| Synchronous high-resolution phenotyping of leaf and root growth in Nicotiana tabacum over 24-h periods with GROWMAP-plant.Crossref | GoogleScholarGoogle Scholar |
Saengwilai P, Tian X, Lynch JP (2014) Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiology 166, 581–589.
| Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFKit7rF&md5=9c62321700e2ffe5ccb678f85001ba54CAS |
Schmittgen S, Metzner R, Van Dusschoten D, Jansen M, Fiorani F, Jahnke S, Rascher U, Schurr U (2015) Magnetic resonance imaging of sugar beet taproots in soil reveals growth reduction and morphological changes during foliar Cercospora beticola infestation. Journal of Experimental Botany 66, 5543–5553.
| Magnetic resonance imaging of sugar beet taproots in soil reveals growth reduction and morphological changes during foliar Cercospora beticola infestation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitVOitbbE&md5=6efb5ec12f7024d93ef57c7f1d60367bCAS |
Shrestha R, Al-Shugeairy Z, Al-Ogaidi F, Munasinghe M, Radermacher M, Vandenhirtz J, Price AH (2014) Comparing simple root phenotyping methods on a core set of rice genotypes. Plant Biology 16, 632–642.
| Comparing simple root phenotyping methods on a core set of rice genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmtV2msrY%3D&md5=96871ff2303d90f7ffc29245429f6a83CAS |
Silva-Navas J, Moreno-Risueno MA, Manzano C, Pallero-Baena M, Navarro-Neila S, Tellez-Robledo B, Garcia-Mina J, Baigorri R, Gallego J, del Pozo JC (2015) D-Root, a system for cultivating plants with the roots in darkness or under different light conditions. The Plant Journal 84, 244–255.
| D-Root, a system for cultivating plants with the roots in darkness or under different light conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFygtL3O&md5=579da6925bd3df4c0fa5d2ff664d0262CAS |
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant and Soil 341, 75–87.
| Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjt1Wjsrc%3D&md5=0e42dd86d59caa3c57f5abef42de836fCAS |
Uga Y, Ebana K, Abe J, Morita S, Okuno K, Yano M (2009) Variation in root morphology and anatomy among accessions of cultivated rice (Oryza sativa L.) with different genetic backgrounds. Breeding Science 59, 87–93.
| Variation in root morphology and anatomy among accessions of cultivated rice (Oryza sativa L.) with different genetic backgrounds.Crossref | GoogleScholarGoogle Scholar |
Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, Inoue H, Takehisa H, Motoyama R, Nagamura Y, Wu J, Matsumoto T, Takai T, Okuno K, Yano M (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics 45, 1097–1102.
| Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1WgsrnM&md5=b88478ee447eccd55130d1b472ac11a6CAS |
Wang YX, Wang Z, Suo B, Gu YJ, Wang HH, Chen YH, Dai YX (2007) Discussion on photoreceptor for negative phototropism in rice roots. Rice Science 14, 315–318.
| Discussion on photoreceptor for negative phototropism in rice roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvVegug%3D%3D&md5=bc0ba86f501c142e404f41456e10a899CAS |
Wells DM, French AP, Naeem A, Ishaq O, Traini R, Hijazi H, Bennett MJ, Pridmore TP (2012) Recovering the dynamics of root growth and development using novel image acquisition and analysis methods. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367, 1517–1524.
| Recovering the dynamics of root growth and development using novel image acquisition and analysis methods.Crossref | GoogleScholarGoogle Scholar |
White PJ, George T, Gregory PJ, Bengough AG, Hallett PD, Mckenzie M (2013) Matching roots to their environment. Annals of Botany 112, 207–222.
| Matching roots to their environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFSiur7P&md5=392383aff4a41a9e44817807253f5c72CAS |
Xu W, Ding G, Yokawa K, Baluska F, Li Q, Liu Y, Shi W, Liang J, Zhang J (2013) An improved agar-plate method for studying root growth and response of Arabidopsis thaliana. Scientific Reports 3, 1273
| An improved agar-plate method for studying root growth and response of Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
* These authors contributed to the paper equally.