Re-analysis of plant CO2 responses during the exponential growth phase: interactions with light, temperature, nutrients and water availability
Miko U. F. Kirschbaum A C and Suzanne M. Lambie BA Landcare Research, Private Bag 11 052, Palmerston North 4442, New Zealand.
B Landcare Research, Private Bag 3127, Hamilton 3240, New Zealand.
C Corresponding author. Email: kirschbaumm@landcareresearch.co.nz
Functional Plant Biology 42(10) 989-1000 https://doi.org/10.1071/FP15103
Submitted: 23 April 2015 Accepted: 1 July 2015 Published: 19 August 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
Many short-term experiments have been conducted under increasing CO2 but results have been varied and have not yet led to a conclusive quantitative understanding of the CO2 response of plant growth. This may have been partly due to a lack of explicit consideration of the positive feedback inherent in plant growth during periods of exponential growth. This feedback can increase an initial physiological enhancement of relative growth rate (RGR) into a much larger biomass enhancement. To overcome this problem, we re-analysed existing experimental data from 78 publications. We calculated the RGRs of C3 plants and their relative enhancement under elevated CO2 and derived response indices that were independent of the duration of experiments and the RGR at normal atmospheric CO2. The RGR of unstressed plants increased by 14 ± 2% under doubled CO2, with observed RGR enhancement linearly correlated with calculated photosynthetic enhancements (based on the Farquhar-von Caemmerer-Berry photosynthesis model), but at only half their numeric values. Calculated RGR enhancements did not change significantly for temperatures from 12 to 40°C, but were reduced under nutrient limitation, and were increased under water stress or low irradiance. We concluded that short-term experiments can offer simple and cost-effective insights into plant CO2 responses, provided they are analysed by calculating relative changes in RGR during the strictly exponential initial growth phase.
Additional keywords: nutrient limitation, photosynthesis, PAR, photosynthetically active radiation, relative growth rate, RGR, water limitation.
Introduction
The pre-industrial atmospheric carbon dioxide concentration [CO2] was ~280 μmol mol–1. It is currently increasing by ~2 μmol mol–1 year–1 and reached nearly 400 μmol mol–1 by 2013 (Hartmann et al. 2013). Plants grow by absorbing CO2 from the atmosphere, and current atmospheric concentrations are less than saturating for photosynthesis for most plants (e.g. Farquhar and von Caemmerer 1982). Plant growth is therefore likely to increase with increasing atmospheric [CO2] (Franks et al. 2013). The important question is by how much?
For modelling studies of plant productivity under climate change (e.g. Medlyn et al. 2011; Kirschbaum et al. 2012; Peters et al. 2013; Reyer 2015), it is possible to compare growth responses to changing temperatures or rainfall with observed growth in regions that currently experience different climatic patterns. This is more difficult for responses to [CO2] as available observations are largely restricted to a limited number of studies with experimentally altered CO2 concentrations (Hickler et al. 2015). Therefore, CO2 responses remain one of the most uncertain aspects of predicting future plant performance.
One can readily observe short-term photosynthetic responses to [CO2], and studies with C3 plants have demonstrated consistent and positive photosynthetic responses to increasing [CO2] (Drake et al. 1997; Long et al. 2006; Kirschbaum 2011). These responses are generally in line with our understanding of the fundamental biochemical processes that govern leaves’ responses to their external environment (Farquhar and von Caemmerer 1982; Franks et al. 2013). However, photosynthetic carbon gain interacts with other plant processes. The ultimate growth response to elevated [CO2], therefore, cannot simply be equated with the CO2 response of photosynthesis (e.g. Poorter et al. 2013).
To better understand the response of plant growth, many experiments have been conducted over relatively short time frames under growth-chamber or glasshouse conditions, using plants grown in pots or hydroponically (e.g. Kimball 1983; Poorter 1993; Pinkard et al. 2010). Other experiments have been conducted in the field using open-top chambers or free air CO2 enrichment (FACE) methods (Ainsworth and Long 2005; Norby and Zak 2011). The latter is generally considered to create the most realistic conditions as there are no artificial below- and aboveground barriers to modify the plants’ environment.
However, FACE experiments tend to be very resource intensive and costly (Pinkard et al. 2010), which allows only limited exploration of the response of different species or [CO2] × environment interactions. Responses also vary from year-to-year simply because of uncontrolled climatic variability between years (e.g. Annicchiarico 2002) or inevitably-fluctuating CO2 concentration in FACE studies (Bunce 2013). This makes it difficult to investigate CO2 responses systematically under a variety of co-limitations, or for a range of different plant species. Short-term experiments under controlled conditions therefore retain an important role in advancing the understanding of the interaction between plant responses to elevated [CO2] and other environmental factors.
Interpreting experimental findings
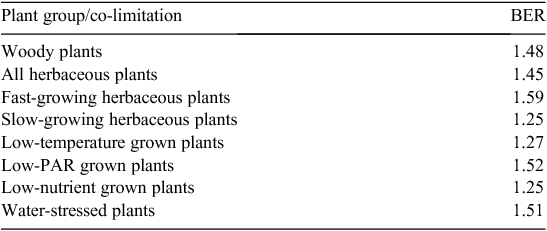
In its simplest from, short-duration experiments can be analysed by comparing the biomass of plants grown at elevated [CO2] for a given length of time with that of plants grown at control [CO2]. This ratio is termed the biomass enhancement ratio (BER). Poorter and Navas (2003) compiled results from a wide range of experiments and found that BERs averaged ~1.45 for herbaceous and 1.48 for woody plants (Table 1).
|
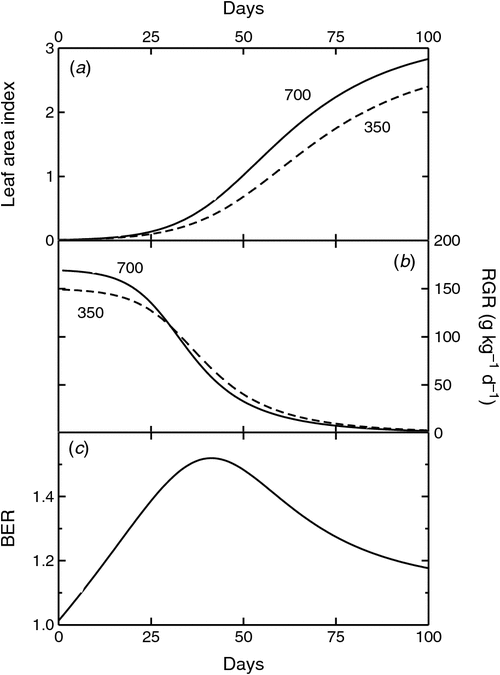
A different way to analyse CO2 responses of young plants is by calculating their relative growth rate (RGR). Plants typically grow exponentially for some time before growth falls below that predicted by on-going exponential growth. Plant growth during and after the exponential growth phase can be described by a simple growth model (Fig. 1; Kirschbaum 2011). This allows the analysis of the interaction between time, the RGR of plants grown under low [CO2] (RGR350) and the ‘relative’ enhancement of RGR by elevated [CO2] (RERC). The model by Kirschbaum (2011) assumes a constant ratio of plant size to leaf area index, a RERC of 10% (Poorter and Navas 2003), and an eventual cessation of exponential growth through increasing self-shading as is typically observed in longer-term experiments (e.g. Poorter et al. 1988; Körner 2006).
|
Loehle (1995), Gifford et al. (1996), and Kirschbaum (2011), using models like the one described above, showed that the BER during the exponential growth phase can substantially exceed the enhancement calculated on the basis of the underlying RERC. BERs depend not only on a plant’s inherent responsiveness to elevated [CO2], but also on time (Fig. 1c) and on RGR350 (Fig. 2). Over the initial growth phase, after imposition of CO2 treatments, BERs increase with time because the positive feedback during exponential growth can amplify the initial CO2 response (Fig. 1c; Loehle 1995; Gifford et al. 1996; Körner 2006; Kirschbaum 2011). Put simply, on the first day of a CO2 treatment, one can compare the CO2 response of two plants of the same size and with the same leaf area. On day 2, however, the high-CO2 plant will benefit not only from higher CO2, but also from its slightly higher leaf area developed during the previous day’s high-CO2 exposure. This amplification is small at first, but increases over time to become quantitatively important (Fig. 1c).
|
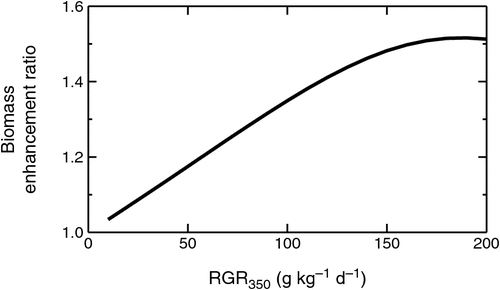
However, RGRs cannot remain at peak values for very long, but begin to decrease when other growth limitations such as pot volumes or physical space that leads to self-shading, provide additional over-riding growth constraints (Gifford et al. 1996; Fig. 1b). If these effects are directly linked to plant size, they will have a greater effect on high- than low-[CO2] grown plants (Fig. 1b) so that BERs decrease when these constraints take effect (Fig. 1c). The model also shows that for a given length of experiments, BERs correlate with RGR350 (Fig. 2). This is because with increasing RGR350, the positive feedback during exponential growth can more rapidly amplify the initial RERC signal into the numerically much greater BER.
Experimental observations summarised by Poorter and Navas (2003) indicated that BERs varied with changes in plant type or growing conditions, and faster-growing species had greater BERs than more-slowly growing plants (Table 1). This could mean that faster-growing species are inherently more responsive to elevated [CO2] than slower-growing species. In contrast, Poorter and Navas (2003) also showed that the ‘absolute’ enhancement of RGR increased linearly with plants’ RGR350. This meant that the ‘relative’ enhancement of relative growth rate by elevated [CO2] (RERC) remained constant and was therefore independent of the RGR. If fast- and slow-growing plants had the same RERC, it implies that there are no fundamental physiological differences in their responses to elevated [CO2]. Patterns emerging from the analyses of BERs can thus lead to different conclusions from those derived from analyses of RERCs.
Table 1 also shows that BERs of water-stressed plants and plants grown under low photosynthetically active radiation (PAR) were the same as those of plants grown without limitations (Poorter and Navas 2003). This conflicts with the theoretical understanding of the photosynthetic CO2 response of plants that predicts greater CO2 responsiveness for water-limited than non-limited plants (e.g. McMurtrie et al. 2008). When plants are grown with non-limiting water supply, any CO2 response must be based on the enhancement of photosynthesis by increasing CO2. Under water limitation, however, increasing [CO2] also leads to partial stomatal closure (e.g. Morison 1985; Franks et al. 2013), which enhances plant water-use efficiency. As water-use efficiency is increased by increases in photosynthesis and decreases in stomatal conductance, it is numerically greater than the photosynthetic enhancement alone. This leads to the expectation of relatively greater CO2 responses under water-limited than well-watered conditions (e.g. McMurtrie et al. 2008). However, the BER data in Table 1 imply that water-limited and well-watered plants had the same CO2 responsiveness.
For plants grown under PAR-limited conditions, RERC might also be higher because plants operate closer to their light-compensation point where the ratio of photosynthesis to respiration is closer to one (e.g. Sims and Pearcy 1994). Any enhancement of photosynthesis could thus lead to greater relative growth enhancements under low PAR than for plants grown under high PAR where the photosynthesis to respiration ratio is already higher. Under PAR-limited conditions, plants are also more likely to be limited by carbohydrate availability than under high PAR. When plants have excess carbohydrate resources, photosynthesis may be downregulated which could reduce any beneficial effect of elevated [CO2]. This is more likely to occur in high-PAR grown plants than in those grown under low PAR, leading to the expectation of greater CO2 responses in low-PAR grown plants. However, this theoretical expectation also conflicts with the observation of similar BERs for plants grown under low and high PAR (Table 1).
However, explicit consideration of the positive feedback during exponential growth could reconcile these apparently conflicting findings. Plants growing under water-stressed or low-PAR conditions must have had lower RGR350 than non-stressed plants. If their RERCs had been the same as those of non-stressed plants, it should have led to lower BERs (cf. Fig. 2). Instead, their BERs remained similar to that of unstressed plants (Table 1), which could only be possible if the RERC of water- and PAR-limited plants had actually been greater than that of unstressed plants. At a direct physiological level, stressed plants could thus have responded more strongly to elevated [CO2] (as expressed in higher RERC) than unstressed plants, but because of the stressed plants’ lower RGR350 that would not have led to enhanced BERs.
Nutrient-limited plants must have also had lower RGR350 than plants grown with adequate nutrition. Their observed lower BERs (Table 1), therefore, could have also been due to their lower RGR350 rather than indicate a lower inherent CO2 responsiveness of nutrient-limited plants. Put differently, even if RERC had remained unaffected by nutrient limitations, the BERs of nutrient-limited plants could have still been reduced through their lowered RGR350, leaving it uncertain whether CO2 responsiveness interacts with nutrient limitations.
Photosynthetic theory also clearly shows a strong [CO2] × temperature interaction, with much greater CO2 responsiveness at higher temperatures (e.g. Farquhar and von Caemmerer 1982; Medlyn et al. 2002). This has led to the expectation of similar interactions between temperature and plant-growth responses to elevated [CO2] (e.g. Long 1991; Kirschbaum 1994). A critical analysis of experimental observations is needed to ascertain whether that expectation based on photosynthetic observations is consistently expressed in plant growth responses.
In the present work, we have re-analysed available reports of CO2 responses during plants’ exponential growth phase in combination with temperature, and PAR, nutrient or water limitations. We analysed each reported response pattern with respect to calculated RGR350 and the relative enhancement of respective RGRs under elevated CO2 (RERC). Through these analyses, we tried to obtain insights into the physiological relationships between the CO2 responses of plants and these different co-limiting factors. This included only C3 plants as the photosynthetic CO2 response is very different for C3 and C4 plants (e.g. Ainsworth and Long 2005) and therefore likely to interact differently with other growth-limiting factors.
Materials and methods
We obtained data from 78 experiments published in the literature. From each experiment, we extracted information on experimental conditions, such as mean daytime temperatures, high and low [CO2], and the imposition of any PAR, nutrient or water limitations. We used observations of total plant dry weights, or the nearest equivalents (such as aboveground biomass when total biomass was not given), reading values from available graphs or tables and fitting a simple growth model to each dataset to derive RERCs under the experimental conditions. The principal criterion for inclusion in this analysis was for growth to be exponential for at least the early part of respective experimental periods so that relative growth rates could be calculated. All data presented below were derived from these experimental data obtained from the literature. Details of the data sources have been given in the Supplementary Material, which is available online.
In the model, the growth increment of biomass dB/dt was calculated at daily intervals as:

where B is biomass, t is time, and k1 and k2 are parameters fitted to respective datasets. In this expression, the first exponential term described the exponential growth phase and the second exponential term the down-turn in growth towards the end of the exponential growth phase.
The inclusion of this second exponential term allowed the incorporation of data obtained beyond the initial exponential growth phase, although data that deviated too strongly from exponential growth were excluded from the analysis. The asymptotic term k2 was constrained to be the same for plants exposed to different [CO2], but was allowed to differ between plants exposed to different co-limiting factors. This implicitly assumed that growth after the exponential phase was reduced through factors that could be linked to plant biomass per se with no difference between high- and low-[CO2] grown plants. It meant that such size limitations affected high-[CO2] grown plants slightly earlier than low-[CO2] grown plants (cf. Poorter et al. 1988).
Initial biomass at time 0 was set to the same value for plants grown under different conditions and different [CO2]. This was generally a fitted value based on observations reported for older seedlings unless the actual biomass of plants at the start of respective treatments was reported. Goodness of fit was assessed by residual sums of squares of log-transformed biomass data. For each data pair at high and low [CO2], we thus obtained two k1 values, k1,low and k1,high, which represented the fitted values at low and high [CO2]. The observed relative CO2 enhancement of RGR, cenh, is the physiological relevant measure of the growth enhancement under specific experimental conditions. It was calculated as:

Many experiments used 350 for low, and 700 μmol mol–1 for high [CO2], but other experiments used a variety of concentrations. To improve comparability of the findings from different studies, all data were normalised by calculating the relative enhancement of relative growth rate by [CO2], RERC, at standardised high and low [CO2] of 350 and 700 μmol mol–1 based on the theoretical CO2 response of C3 plants:

where E350, 700 is the theoretical enhancement of photosynthesis calculated under 350 and 700 μmol mol–1, respectively, and Elow,high is the calculated enhancement under the actual low and high [CO2] used in respective experiments.
The CO2 enhancement of photosynthesis, Elow,high, was calculated as:

where chigh and clow are photosynthetic responses at high and low [CO2] respectively.
The theoretical photosynthetic CO2 response, cp, for RuBP-regeneration limited photosynthesis was calculated following Kirschbaum (1994) as:

where ci, is the intercellular [CO2] and Γ* is the CO2 compensation point in the absence of non-photorespiratory respiration.
The ci was taken to be two-thirds of ambient [CO2] and assumed to remain constant under changing [CO2] (Ball et al. 1987). Γ* was calculated following Bernacchi et al. (2001), as:

where T is the estimated mean daytime temperature.
Differences in CO2 stimulation under different co-limitations (low PAR, nutrient or water stress), ΔRERC, were simply calculated as:

where RERCs and RERCo are the respective CO2 stimulations under stressed and optimal conditions.
The extent of growth-limitation by the co-limitations, glim, was calculated as:

where k1,low(s) and k1,low(o) are the calculated RGR350 for stressed and optimal growth conditions, respectively. The closer this ratio is to 0, the stronger is the environmental limitation, while the ratio becomes 1 for optimal growth.
Initial parameter fitting was done using a customised Excel spreadsheet, using Excel’s ‘Solver’ add-in to find parameter sets with minimised residual sums of squares. A copy of the spreadsheet is available from the authors upon request. Subsequent statistical analyses were undertaken using Genstat 12 (VSN International, Hemel Hempstead, UK). Regression analysis was used to assess correlations between RERC and photosynthetic CO2 enhancement, and between ΔRERC and various co-limiting factors. Two-sample, two-tailed, t-tests were used to assess for differences between woody and herbaceous plants. Differences were considered to be significant if P < 0.05.
Illustrating the parameterisation routine
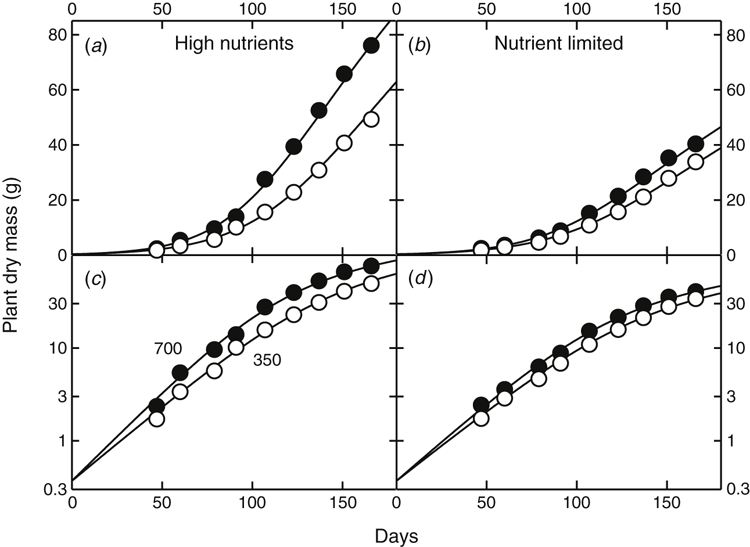
Use of the fitting and analysis routine is demonstrated in Fig. 3 with a dataset (for well-watered plants) obtained from Townend (1995). Over the initial growth phase to about day 70, data followed an exponential growth pattern, shown by the near-linear relationships on the logarithmic plots in Fig. 3c, d.
|
Growth then increasingly fell below exponential growth after day 70, with data points after this time falling below an implicit straight line fitted to the early points that indicated exponential growth. The second exponential term in Eqn 1 allowed the reduced growth to be described adequately as well. Townend (1995) also reported some data beyond day 180, but observations at those later stages deviated so strongly from exponential growth that those data were excluded from the analysis.
The key derived parameters for the present work were the initial slopes of the fitted curves over the exponential growth phase (equivalent to k1). However, it was often difficult to confidently describe parameters for the exponential phase alone because most experiments provided few data points over that period, and the duration of the exponential growth period was also often uncertain. These problems were lessened by use of Eqn 1 that allowed data from the early post-exponential phase to be included as well.
The initial slopes of the curves fitted to these data gave the RGRs under different conditions. The relative difference between RGRs at high and low [CO2] (ΔRERC) was taken as the appropriate measure of the plants’ CO2 response as it was independent of the duration of experiments and thus unconfounded by time or other factors. This CO2 response could then be related to interactions with other co-limiting factors. In this particular example (Fig. 3), increasing [CO2] increased RGR by 19% (RERC = 0.19) for plants with adequate nutrition, and by 11% for nutrient-limited plants.
Results
Growth enhancements at different [CO2]
Different experiments used different combinations of low and high [CO2], with only ~12% of experiments using the ‘standard’ combination of 350 and 700 μmol mol–1. This provided an opportunity to assess the response of the relative growth enhancements of RGR, cenh, to differences in the combination of low and high [CO2]. RERC differs from cenh through its normalisation to a standard combination of low and high [CO2].
Photosynthetic enhancements were calculated from mean daytime temperature and the experimental low and high [CO2] but used no actual measurements from those experiments whereas cenh was calculated from observed growth measurements. Although individual cenh observations showed considerable scatter, mean cenh, calculated over defined ranges of photosynthetic enhancement, were tightly and linearly correlated (r2 = 0.94, P < 0.001) with photosynthetic enhancements (Fig. 4). We also compared the response of woody and herbaceous plants, but found no significant difference in the deviation of individual data from the line of best fit between woody and herbaceous plants (P = 0.070).
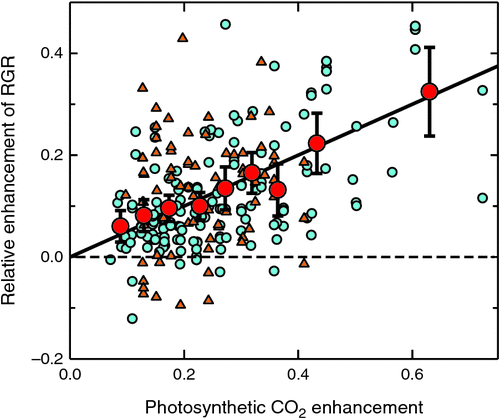
|
This linear correlation between cenh and calculated photosynthetic enhancement (in the averaged data) was surprisingly tight across a wide range of calculated photosynthetic CO2 enhancements, with no indication for cenh to have deviated from a linear relationship over the whole range of experimental conditions. However, the observed cenh was only about half (0.495) of the calculated photosynthetic enhancement (Fig. 4), indicating that some other plant factors (i.e. changes in specific leaf area) or negative feedback factors (e.g. in response to excess carbohydrate) caused the ultimate growth response to be only half of the initial photosynthetic enhancement.
We then investigated the interactions between CO2 enhancement and other physiological variables after normalising observed CO2 enhancements to a standard combination of low and high [CO2], designated as RERC.
Interactions with temperature
Fig. 5a shows RERCs observed at different temperatures in individual studies. There was no indication of any systematic differences between woody and herbaceous plants (P = 0.058). Fig. 5b analyses these data further by calculating means and confidence intervals over 5°C temperature intervals. The overall mean RERC was 0.14 ± 0.02, with no significant change over temperatures from 12 to 40°C. It is readily apparent that the observed data did not conform to the theoretical relationship, with observations exceeding the expected CO2 enhancement at cold temperatures and falling below it at higher temperatures.
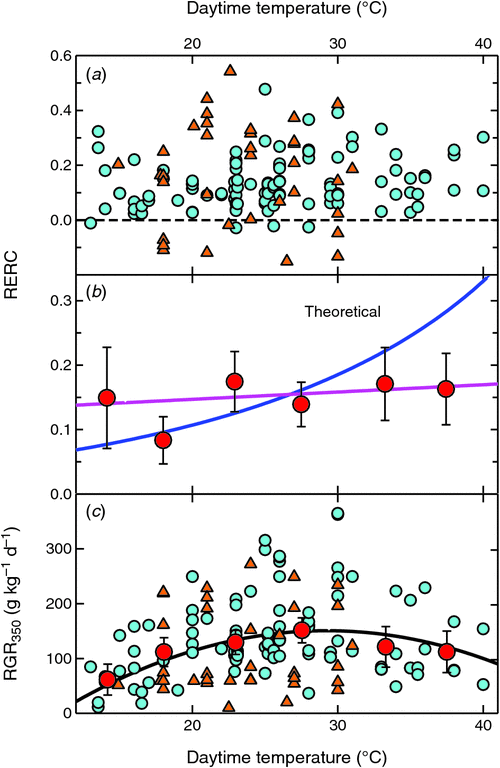
|
Observed RGR350 had an optimum between 25 and 30°C (Fig. 5c), but the below-optimum reductions in RGR350 were not extreme at either high or low temperatures. The observations thus covered a range of physiologically meaningful conditions. It discounted the possibility of high-temperature grown plants having failed to display the expected CO2 responsiveness because of growth under biologically damaging conditions. This was further confirmed by plotting RERC against RGR350 (Fig. 6). RERC was independent of RGR350, with no statistically significant correlation between them. The key observation here is that there was no positive correlation between RGR350 and RERC. Had plants with low RGR350 also shown low RERCs, it might have indicated that plants experiencing physiologically extreme conditions might not have shown a normal CO2 response. However, the absence of a positive correlation meant that it was unlikely for physiological dysfunction at extreme temperatures to have accounted for the lack of stronger CO2 enhancement.
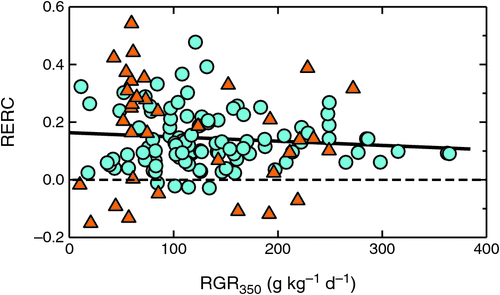
|
Interactions with PAR
Fig. 7 shows RERC differences (ΔRERC) between plants grown at limiting and adequate PAR. Values on the x-axis show the extent to which RGR350 was reduced under low PAR, and values on the y-axis show ΔRERC between plants grown under low and high PAR in the same experiment. If CO2 responsiveness had been independent of the extent of PAR limitation, the data should have simply scattered around the x-axis without any trends with changing PAR limitations.
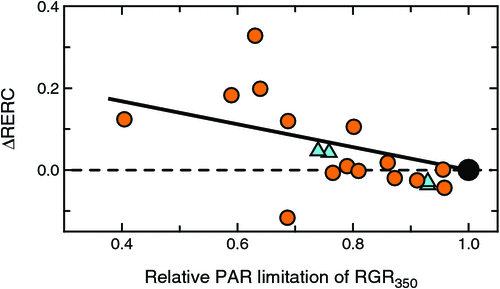
|
However, plants grown under severely limiting PAR displayed stronger CO2 responsiveness than that observed under higher PAR levels. The difference in responsiveness was only slight for minor PAR limitations, with glim > 0.8, but for lower ratios, the effect became stronger and clearly apparent. For glim < 0.8, eight out of 10 observations showed higher CO2 responsiveness under limiting than adequate PAR (Fig. 7).
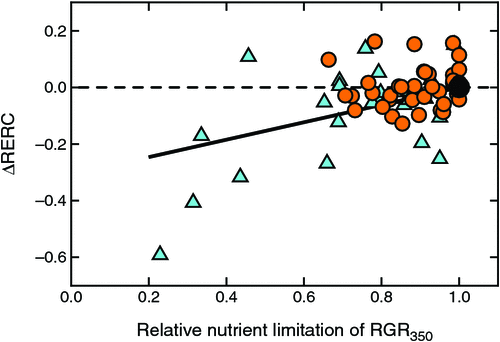
Interactions with fertility
There was little fertility effect on ΔRERC for relative nutrient limitations >0.6, but the most severely stressed plants showed markedly reduced CO2 responsiveness (Fig. 8). Three of the five experiments with glim < 0.5 even had calculated negative RERCs (data not shown), which meant that their growth was actually reduced by exposure to elevated [CO2].
|
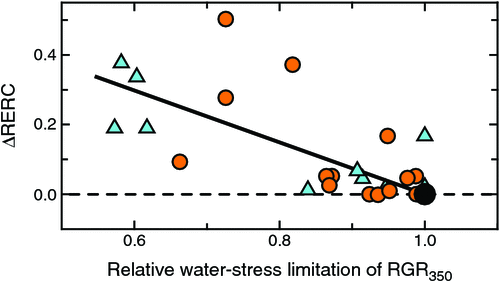
Interactions with water stress
ΔRERC increased strongly under water stress, especially with glim < 0.8 (Fig. 9). The extent of ΔRERC was also quite pronounced. Under well watered conditions, RERC was only ~0.14 (cf. Fig. 5b), yet that was increased up to 3-fold when plants were water stressed (with glim <0.6).
|
Discussion
The present work focussed on analysing plant responses to elevated [CO2] over the initial phase of exponential growth, but how relevant is the response over that short growth phase? Although the growth response of young plants is of some importance in itself, its greater relevance lies in providing an indication of likely plant responses during their longer and more relevant later linear growth phase. As a first approximation, one can quantitatively equate the relative enhancement of RGR (RERC) with an expected relative growth enhancement of linear growth. If that extrapolation can be made, these short-term experiments can provide valuable insights into the interactions between CO2 responsiveness and other growth-limiting factors.
Although conditions in FACE experiments are such that plant responses can manifest themselves in the most natural way without artificial confounding factors (e.g. Ainsworth and Long 2005; Norby and Zak 2011), their costs and logistic challenges limit the extent to which interactions with other growth-limiting factors can be investigated (e.g. Pinkard et al. 2010). It is simply not feasible to conduct enough FACE experiments to gain sufficient insights either into species differences, or into all the possible interactions between plants’ CO2 responses and other growth-limiting factors.
Short-term and low-cost experiments in growth chambers and glasshouses can thus cost effectively add valuable insights to the overall understanding of the interactions between the key interacting factors. In the analysis of such experiments it is critically important, however, to be mindful of the positive feedback on growth during the exponential growth phase (Loehle 1995; Gifford et al. 1996; Körner 2006; Kirschbaum 2011). Biomass enhancement ratios (BERs) are inappropriate for expressing research results as the observed relative CO2 enhancement is confounded by time so that a constant enhancement of RGR turns into an increasing BER with the length of experiments (Gifford et al. 1996; Fig. 1c). It is similarly confounded with the RGR of plants under normal [CO2], RGR350 (Fig. 2). Instead, a more generic measure of plant growth response to elevated [CO2] is the relative enhancement of RGR by elevated [CO2] (RERC). If needed, BERs can be easily calculated for specified combinations of RERC, RGR350 and time.
Quantifying differences in RERC made it possible to study interactions between the CO2 response of young plants and temperature, PAR, fertility, and water stress without being confounded by the length of experiments or RGR350. In plants grown under optimal conditions, RERC was independent of plants’ RGR350 (Fig. 6), which is consistent with the findings by Poorter and Navas (2003), who had primarily compared species with different inherent growth potential. Fig. 6 showed that the same pattern also holds when differences in RGR350 were further increased by differences in experimental temperatures. As a first conclusion, fast- and slow-growing plants thus had the same RERC (Fig. 6), indicating a similar photosynthetic response and similar interaction with feedback processes. The reported differences in BERs between fast- and slow-growing plants reported in Table 1 resulted simply from differential amplification of the same underlying CO2 response during the exponential growth phase (cf. Fig. 2).
Response to [CO2]
Fig. 4 shows that it was possible to predict the growth response of plants to elevated [CO2] directly from the calculated enhancement of photosynthesis. Photosynthetic theory can be used to calculate the enhancement of photosynthesis for any combination of temperature and low and high [CO2] (e.g. Farquhar and von Caemmerer 1982; Kirschbaum 1994). This could be linked to the experimentally observed relative growth enhancement through the empirical constant 0.495 obtained from the slope of the straight line in Fig. 4. This tight correlation between calculated photosynthetic enhancement and observed growth enhancement thus provided an extremely useful approach for predicting the growth response of plants during their exponential growth phase.
The slope of 0.495 accounted for any changes in carbon use efficiency, specific leaf area, leaf mass fraction, or any possible reduction in photosynthesis in response to carbohydrate saturation under specific circumstances (Poorter and Navas 2003; Ainsworth and Long 2005). The correlation between photosynthesis and growth appeared to hold over a wide range of [CO2], with no indication of saturation at high [CO2] beyond that represented in the relevant photosynthetic equations. This provided an objective and quantitative way of accounting for the various feedbacks and secondary physiological and morphological responses that together determined the CO2 response of plants.
At the same time, while there was a tight linear correlation between the average calculated photosynthetic enhancements and the observed growth enhancements, individual observations scattered widely around the mean response. Much of that scatter was simple random variation, given that it was often difficult to deduce the relevant parameters from sparse or noisy datasets. Additional deviation would have been related to temperature, which differed from the expected dependence (Fig. 5 and see below).
Differences between woody and herbaceous species, however, did not appear to add to the variability (Figs 4–6). However, there could have been other systematic differences in the response of different plants, such as between N-fixing and non-fixing plants, or other unspecified differences between species. Future data analyses to calculate differences in RERC could provide an effective way to search for further differences between species or functional groups.
Interactions with temperature
We found that on average, RERC was increased by 14 ± 2% when [CO2] was doubled, with the relative CO2 responsiveness of plants being almost completely independent of temperature (Fig. 5). This contrasts with the findings by Poorter and Navas (2003; Table 1), who reported that BERs were lower for low-temperature grown plants. However, low temperatures are usually associated with reduced RGRs (Fig. 5c). The low BERs of low temperature-grown plants may have been a consequence of their lower RGRs that transformed invariant RERCs to lower BERs (Fig. 2).
However, it is difficult to reconcile the observed temperature-invariant RERC with the well-established interaction between temperature and photosynthetic CO2 responses (e.g. Farquhar and von Caemmerer 1982). Photosynthesis in C3 plants responds to [CO2] primarily because oxygen and CO2 compete for the primary binding site on Rubisco, with increasing temperature favouring oxygenations. Hence, with increasing temperature, photosynthesis becomes increasingly limited by [CO2] at normal atmospheric concentrations (e.g. Farquhar and von Caemmerer 1982; Kirschbaum 1994; Medlyn et al. 2002). On the basis of that strong and universally observed photosynthetic relationship, a similar interaction was assumed to exist between temperature and the growth response to elevated [CO2] (e.g. Idso et al. 1987; Long 1991; Rawson 1992; Kirschbaum 1994; Polley 2002).
However, studies of growth responses to [CO2] under contrasting temperatures have provided ambiguous results. The early work reported by Idso et al. (1987), working with four different species, reported a strong [CO2] × temperature interaction, but subsequent experiments produced more variable results. Rawson (1992) compared the strength of the [CO2] × temperature interaction observed in various studies and concluded that, while there was greater CO2 responsiveness at higher temperatures, it was only weakly and inconsistently expressed. Similarly, Morison and Lawlor (1999) summarised data from 106 different observations, covering 18 different species, and found only a weak [CO2] × temperature interaction. A recent meta-analysis of experiments with increased temperatures and [CO2] also found little support for a [CO2] × temperature interaction in whole-plant growth responses (Wang et al. 2012).
These earlier studies, together with the very weak [CO2] × temperature interaction observed here, indicate that the [CO2] × temperature interaction that is clearly apparent in photosynthetic responses does not carry over into whole-plant growth responses. However, there are no obvious reasons for the absence of greater growth enhancements at higher temperatures. It could have been possible that plants experiencing extremely high or low temperatures might not have functioned normally and therefore been unable to utilise the extra carbohydrate made available through elevated [CO2]. However, there were only moderate reductions in RGR350 at even extreme temperatures (Fig. 5c), and there was no indication of reduced CO2 responsiveness for the slowest-growing plants (Fig. 6). Thus, there was no evidence of physiological dysfunction to have prevented plants from utilising an enhanced carbohydrate supply.
An uncoupling of photosynthesis and growth is often associated with the assumption that plants might be sink- rather than source-limited (e.g. Millard et al. 2007; Kirschbaum 2011). If plants already have excess carbohydrate resources under normal [CO2], it would be unlikely for their growth to be enhanced by elevated [CO2], even if short-term photosynthetic rates could be increased. However, plants are more likely to be sink-limited under cooler conditions (Poorter et al. 2013) when maintenance respiration and growth processes might be slowed by low temperatures. This could lead to sink limitation that could hypothetically prevent a CO2 response. In contrast, higher temperatures should stimulate respiration and growth processes (e.g. Atkin and Tjoelker 2003; Way and Yamori 2014), deplete carbohydrate reserves, and shift plants from sink to source limitation. High temperatures should create conditions favourable for full utilisation of increased photosynthetic carbon gain, and carbohydrate feedbacks should have further enhanced the [CO2] × temperature interaction, rather than negated it. These considerations thus can provide no explanation for the absence of the expected [CO2] × temperature interaction.
It is difficult to think of other possible explanations for this intriguing absence of the expected response pattern. However, whatever the cause for the absence of a [CO2] × temperature interaction, it has profound implications for the modelling of plant responses to changes in future [CO2].
Interactions with PAR limitations
The analysis showed that low PAR-grown plants were more responsive to elevated [CO2] than plants grown under high PAR (Fig. 7). Plant growth could be more responsive to [CO2] under PAR limitation because plants operate closer to their light-compensation point, and because PAR-limited plants are likely to be carbohydrate limited so that enhanced photosynthesis would convey greater advantages than for plants grown under higher PAR. In contrast, an analysis based on BERs had shown low- and high PAR-grown plants to be similarly responsive to elevated [CO2] (Table 1; Poorter and Navas 2003). So, as for other interactions presented here, it is likely that the actual greater RERC under low PAR was masked by the reduction of BERs through lower RGR350 in the plants grown under limiting PAR (cf. Fig. 2).
Greater CO2 responsiveness of PAR-limited plants might be a factor contributing to the increasing density of rainforest lianas that has been observed over recent decades (e.g. Phillips et al. 2002; van der Heijden et al. 2013). Lianas typically start their growth under PAR-limited conditions in the forest understorey. Greater CO2 responsiveness would thus allow them to take greater advantage of increasing [CO2] than the over-storey trees with which they compete. This could give lianas a competitive boost that might contribute to their increasing abundance.
Interactions with nutrient limitations
CO2 responsiveness was reduced under nutrient-limited conditions, but the trend was weak and only readily apparent in studies with severe nutrient limitations (Fig. 8). However, it was difficult to analyse CO2 responses under nutrient-limited conditions with the approach adopted here because different nutrient levels were commonly set by applying different amounts of fertiliser at the start of experiments. For the youngest plants, those amounts could have been adequate for growth (cf. Ingestad and Lund 1986; Poorter et al. 2012) so that initial RGRs did not differ markedly between high- and low fertility-grown plants, which led to the majority of observations being bunched together with relative growth limitations ≥0.8 and similar CO2 responsiveness. More substantial growth responses often developed at later growth stages, but those downturns had little effect on the inferred initial relative growth rates. The present findings therefore relied on only a very small number of observations where the fertility constraints were imposed in such a way that RGRs were affected from the earliest growth stages.
However, although our study provides a less certain answer as to how far nutrient limitations curtailed CO2 responses at the plant-physiological level, it is likely that under long-term conditions in the field, feedbacks through whole-system nutrient availability strongly interact with the CO2 response of plant growth. Plants and soils have confined C : N ranges, and any increased carbon gain through elevated [CO2] is ultimately curtailed by the need to also obtain nitrogen so that C:N ratios can be maintained within their allowable ranges. This should reduce the CO2 responsiveness under nutrient-limitations compared with that of well fertilised plants. This feedback effect has been observed experimentally (e.g. Norby et al. 2010; McCarthy et al. 2010) and in modelling studies (e.g. Comins and McMurtrie 1993; Kirschbaum et al. 1998). While these constraints have been investigated primarily with respect to C : N ratios, similar, although more complex, relationships also hold for phosphorus and sulfur (Kirschbaum et al. 1998).
Interactions with water-stress limitations
CO2 responsiveness strongly increased in water-stressed plants (Fig. 9). This response was consistent and apparent in all plants with a water-stress limitation of 0.9 or lower. The response was also numerically strong, giving an approximate 3-fold increase in CO2 responsiveness from unstressed to the most severely stressed plants. When plants are water-stressed, growth limitations shift from photosynthesis that shows only moderate CO2 sensitivity to limitation by water-use efficiency, for which greater CO2 responsiveness can be expected (e.g. McMurtrie et al. 2008). However, just as for fertility experiments, water stress in most experiments was imposed in such a way that it only marginally affected early plant growth so that the analysis had to rely on few usable observations, but those data showed strongly increased CO2 responsiveness under water limitation.
On the other hand, the pattern of increasing CO2 responsiveness under water-limited conditions is not consistently observed in FACE experiments (Kimball et al. 2002; Nowak et al. 2004; McCarthy et al. 2010), not even in desert environments, where one might expect a strong and consistently enhanced CO2 response (e.g. Newingham et al. 2013). It is thus not certain to what extent the findings from the short-term studies reported here are consistently expressed under field conditions, or, if they are not, what higher-order interactions prevent their expression under field situations.
General points
Despite much research over many years, there is still uncertainty about the growth response of plants to elevated [CO2] (e.g. Reddy et al. 2010; Wang et al. 2012). This is partly due to the complex interactions between the photosynthetic CO2 response and the numerous other factors that together control plant growth. Plant growth not only provides the ultimate sink for photosynthetically fixed carbon, but can also exert a strong feedback control on photosynthetic carbon gain to ensure a balance between photosynthetic carbon gain and its use in growth (e.g. Paul and Foyer 2001). The photosynthetic response to changing [CO2] can therefore only be fully understood if growth processes are simultaneously considered as well.
Understanding these interactions is challenging and demands experimental work in which different co-limitations can be varied together with changes in [CO2]. Short-term experiments conducted in growth chambers or glasshouses provide a cost-effective and feasible approach to generating the data for a better understanding of these important interactions. However, studies during the plants’ exponential growth phase need to be analysed in such a way to generate generic and unconfounded growth indices that are comparable between different growth conditions and between different experiments. The present work used a novel approach by calculating the relative enhancement of plants’ relative growth rate during the initial growth phase. As a first approximation, that relative enhancement of RGR can also be used for predicting growth responses during the plants’ subsequent linear growth phase.
Overall, the present work illustrated that useful information can be obtained from short-term growth-chamber or glasshouse-based studies. However, the key is that the analysis must separate the inherent CO2 response of plants from its amplification during the exponential growth phase. This made it possible to obtain the range of observations needed for better quantification of the CO2 response of plants, and disentangle the direct CO2 response from its various important co-limiting factors.
Acknowledgements
We thank Roger Gifford, Graham Farquhar, Peter Millard and David Whitehead for many useful comments and suggestions on the manuscript, and Anne Austin for scientific editing. Particular thanks to Hendrik Poorter, whose probing questions and in-depth discussions greatly helped to refine the ideas presented here and ensured the thorough testing of the underlying assumptions.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–372.| What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2.Crossref | GoogleScholarGoogle Scholar | 15720649PubMed |
Annicchiarico P (2002) ‘Genotype × environment interactions: challenges and opportunities for plant breeding and cultivar recommendations. FAO Plant Production and Protection Paper 174.’ (FAO: Rome)
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8, 343–351.
| Thermal acclimation and the dynamic response of plant respiration to temperature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXls1CjtLk%3D&md5=9f883caac5f5f6b40fa19682d0f826a1CAS | 12878019PubMed |
Ball JT, Woodrow IE, Berry JA (1987) A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In ‘Progress in photosynthesis research’. (Ed J Biggins) pp. 221–224. (Martin-Nijhoff Publishers: Dordrecht, The Netherlands)
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell & Environment 24, 253–259.
| Improved temperature response functions for models of Rubisco-limited photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsFGrt7k%3D&md5=a77db99a8d8f6d75b163db60d871247aCAS |
Bunce JA (2013) Effects of pulses of elevated carbon dioxide concentration on stomatal conductance and photosynthesis in wheat and rice. Physiologia Plantarum 149, 214–221.
| Effects of pulses of elevated carbon dioxide concentration on stomatal conductance and photosynthesis in wheat and rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslCqtLbJ&md5=6b29a1e73a7bde62136cd7a749485246CAS | 23368841PubMed |
Comins HN, McMurtrie RE (1993) Long-term response of nutrient-limited forests to CO2 enrichment - equilibrium behavior of plant–soil models. Ecological Applications 3, 666–681.
| Long-term response of nutrient-limited forests to CO2 enrichment - equilibrium behavior of plant–soil models.Crossref | GoogleScholarGoogle Scholar |
Drake BG, Gonzalez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology 48, 609–639.
| More efficient plants: a consequence of rising atmospheric CO2?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjs1eltbY%3D&md5=81b501107a90ca3af2438f6991205568CAS |
Farquhar GD, von Caemmerer S 1982. Modelling of photosynthetic response to environmental conditions. In ‘Physiological plant ecology II. Water relations and carbon assimilation. Encyclopedia of plant physiology. New series Vol. 12B’. (Eds OL Lange, PS Nobel, CB Osmond, H Ziegler) pp. 549–588. (Springer-Verlag: Berlin)
Franks PJ, Adams MA, Amthor JS, Barbour MM, Berry JA, Ellsworth DS, Farquhar GD, Ghannoum O, Lloyd J, McDowell N, Norby RJ, Tissue DT, von Caemmerer S (2013) Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century. New Phytologist 197, 1077–1094.
| Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVOjtbc%3D&md5=c7801b33c01337184a9ecb8b5fbcc7c9CAS | 23346950PubMed |
Gifford RM, Barrett DJ, Lutze JL, Samarakoon AB (1996) Agriculture and global change: scaling direct carbon dioxide impacts and feedbacks through time. In ‘Global change and terrestrial ecosystems’. (Eds B Walker, W Steffen), pp. 229–259. (Cambridge University Press: Cambridge, UK)
Hartmann DL, Klein Tank AMG, Rusticucci M, Alexander LV, Brönnimann S, Charabi Y, Dentener FJ, Dlugokencky EJ, Easterling DR, Kaplan A, Soden BJ, Thorne PW, Wild M, Zhai PM (2013) Observations: atmosphere and surface. In ‘Climate change 2013. The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex, PM Midgley) pp. 159–254. (Cambridge University Press: Cambridge, UK)
Hickler T, Rammig A, Werner C (2015) Modelling CO2 impacts on forest productivity. Current Forestry Reports 1, 69–80.
| Modelling CO2 impacts on forest productivity.Crossref | GoogleScholarGoogle Scholar |
Idso SB, Kimball BA, Anderson MG, Mauney JR (1987) Effects of atmospheric CO2 enrichment on plant-growth – the interactive role of air temperature. Agriculture, Ecosystems & Environment 20, 1–10.
| Effects of atmospheric CO2 enrichment on plant-growth – the interactive role of air temperature.Crossref | GoogleScholarGoogle Scholar |
Ingestad T, Lund A-B (1986) Theory and techniques for steady state mineral nutrition and growth of plants. Scandinavian Journal of Forest Research 1, 439–453.
| Theory and techniques for steady state mineral nutrition and growth of plants.Crossref | GoogleScholarGoogle Scholar |
Kimball BA (1983) Carbon dioxide and agricultural yield – an assemblage and analysis of 430 prior observations. Agronomy Journal 75, 779–788.
| Carbon dioxide and agricultural yield – an assemblage and analysis of 430 prior observations.Crossref | GoogleScholarGoogle Scholar |
Kimball BA, Kobayashi K, Bindi M (2002) Responses of agricultural crops to free-air CO2 enrichment. Advances in Agronomy 77, 293–368.
| Responses of agricultural crops to free-air CO2 enrichment.Crossref | GoogleScholarGoogle Scholar |
Kirschbaum MUF (1994) The sensitivity of C3 photosynthesis to increasing CO2 concentration. A theoretical analysis of its dependence on temperature and background CO2 concentration. Plant, Cell & Environment 17, 747–754.
| The sensitivity of C3 photosynthesis to increasing CO2 concentration. A theoretical analysis of its dependence on temperature and background CO2 concentration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXlslGns70%3D&md5=7ebbf457415b0ffee6ec7d9ddfa7df1eCAS |
Kirschbaum MUF (2011) Does enhanced photosynthesis enhance growth? Lessons learnt from CO2 enrichments studies. Plant Physiology 155, 117–124.
| Does enhanced photosynthesis enhance growth? Lessons learnt from CO2 enrichments studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFagsb8%3D&md5=aac263262e53a425f5977aeb2560c790CAS |
Kirschbaum MUF, Medlyn BE, King DA, Pongracic S, Murty D, Keith H, Khanna PK, Snowdon P, Raison JR (1998) Modelling forest-growth response to increasing CO2 concentration in relation to various factors affecting nutrient supply. Global Change Biology 4, 23–41.
| Modelling forest-growth response to increasing CO2 concentration in relation to various factors affecting nutrient supply.Crossref | GoogleScholarGoogle Scholar |
Kirschbaum MUF, Watt MS, Tait A, Ausseil A-GE (2012) Future wood productivity of Pinus radiata in New Zealand under expected climatic changes. Global Change Biology 18, 1342–1356.
| Future wood productivity of Pinus radiata in New Zealand under expected climatic changes.Crossref | GoogleScholarGoogle Scholar |
Körner C (2006) Plant CO2 responses: an issue of definition, time and resource supply. New Phytologist 172, 393–411.
| Plant CO2 responses: an issue of definition, time and resource supply.Crossref | GoogleScholarGoogle Scholar | 17083672PubMed |
Loehle C (1995) Anomalous responses of plants to CO2 enrichment. Oikos 73, 181–187.
| Anomalous responses of plants to CO2 enrichment.Crossref | GoogleScholarGoogle Scholar |
Long SP (1991) Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant, Cell & Environment 14, 729–739.
| Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhsVyns7s%3D&md5=b240011b9a2f37daa5b5a34d097c0a31CAS |
Long SP, Zhu X-G, Naidu SL, Ort DR (2006) Can improvements in photosynthesis increase crop yields? Plant, Cell & Environment 29, 315–330.
| Can improvements in photosynthesis increase crop yields?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xktlyltrw%3D&md5=6afa5628a843228382a604400ba781e7CAS |
McCarthy HR, Oren R, Johnsen KH, Gallet-Budynek A, Pritchard SG, Cook CW, LaDeau SL, Jackson RB, Finzi AC (2010) Re-assessment of plant carbon dynamics at the Duke free-air CO2 enrichment site: interactions of atmospheric [CO2] with nitrogen and water availability over stand development. New Phytologist 185, 514–528.
| Re-assessment of plant carbon dynamics at the Duke free-air CO2 enrichment site: interactions of atmospheric [CO2] with nitrogen and water availability over stand development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVyrsLs%3D&md5=dde4120abaef36914d834b89275aa852CAS | 19895671PubMed |
McMurtrie RE, Norby RJ, Medlyn BE, Dewar RC, Pepper DA, Reich PB, Barton CVM (2008) Why is plant-growth response to elevated CO2 amplified when water is limiting, but reduced when nitrogen is limiting? A growth-optimisation hypothesis. Functional Plant Biology 35, 521–534.
| Why is plant-growth response to elevated CO2 amplified when water is limiting, but reduced when nitrogen is limiting? A growth-optimisation hypothesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpt1emtbY%3D&md5=2b2edf8b7538ab14e96974b9471bc5bbCAS |
Medlyn BE, Dreyer E, Ellsworth DE, Forstreuter M, Harley PC, Kirschbaum MUF, LeRoux X, Loustau D, Montpied P, Strassemeyer J, Walcroft A, Wang K, Loustau D (2002) Temperature response of parameters of a biochemically-based model of photosynthesis. II. A review of experimental data. Plant, Cell & Environment 25, 1167–1179.
| Temperature response of parameters of a biochemically-based model of photosynthesis. II. A review of experimental data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvVWitL0%3D&md5=7ceaf02ceff7664ed7b835b39f8e82e6CAS |
Medlyn BE, Duursma RA, Zeppel MJB (2011) Forest productivity under climate change: a checklist for evaluating model studies. Wiley Interdisciplinary Reviews: Climate Change 2, 332–355.
| Forest productivity under climate change: a checklist for evaluating model studies.Crossref | GoogleScholarGoogle Scholar |
Millard P, Sommerkorn M, Grelet GA (2007) Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytologist 175, 11–28.
| Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1Cksrw%3D&md5=950a04353dc01b948e2a072dcabc73dfCAS | 17547663PubMed |
Morison JIL (1985) Sensitivity of stomata and water-use efficiency to high CO2. Plant, Cell and Environment 8, 467–474.
| Sensitivity of stomata and water-use efficiency to high CO2.Crossref | GoogleScholarGoogle Scholar |
Morison JIL, Lawlor DW (1999) Interactions between increasing CO2 concentration and temperature on plant growth. Plant, Cell & Environment 22, 659–682.
| Interactions between increasing CO2 concentration and temperature on plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXksVartLc%3D&md5=87153b6c1d0f15ba5ff96edf915b9062CAS |
Newingham BA, Vanier CH, Charlet TN, Ogle K, Smith SD, Nowak RS (2013) No cumulative effect of 10 years of elevated [CO2] on perennial plant biomass components in the Mojave Desert. Global Change Biology 19, 2168–2181.
| No cumulative effect of 10 years of elevated [CO2] on perennial plant biomass components in the Mojave Desert.Crossref | GoogleScholarGoogle Scholar | 23505209PubMed |
Norby RJ, Zak DR (2011) Ecological lessons from free-air CO2 enrichment (FACE) experiments. Annual Review of Ecology Evolution and Systematics 42, 181–203.
| Ecological lessons from free-air CO2 enrichment (FACE) experiments.Crossref | GoogleScholarGoogle Scholar |
Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proceedings of the National Academy of Sciences of the United States of America 107, 19368–19373.
| CO2 enhancement of forest productivity constrained by limited nitrogen availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVGlsrfP&md5=b9a692aacec03598c09b1ad22d389e90CAS | 20974944PubMed |
Nowak RS, Ellsworth DS, Smith SD (2004) Functional responses of plants to elevated atmospheric CO2 – do photosynthetic and productivity data from FACE experiments support early predictions? New Phytologist 162, 253–280.
| Functional responses of plants to elevated atmospheric CO2 – do photosynthetic and productivity data from FACE experiments support early predictions?Crossref | GoogleScholarGoogle Scholar |
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. Journal of Experimental Botany 52, 1383–1400.
| Sink regulation of photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvVCmtLo%3D&md5=c7c9ed6ad489f4e5d3e5c8a24496eb82CAS | 11457898PubMed |
Peters EB, Wythers KR, Zhang SX, Bradford JB, Reich PB (2013) Potential climate change impacts on temperate forest ecosystem processes. Canadian Journal of Forest Research 43, 939–950.
| Potential climate change impacts on temperate forest ecosystem processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsV2msLfE&md5=d4154a9276c069f5a08b688f7e0fbda8CAS |
Phillips OL, Martinez RV, Arroyo L, Baker TR, Killeen T, Lewis SL, Malhi Y, Mendoza AM, Neill D, Vargas PN, Alexiades M, Cerón C, Di Fiore A, Erwin T, Jardim A, Palacios W, Saldias M, Vinceti B (2002) Increasing dominance of large lianas in Amazonian forests. Nature 418, 770–774.
| Increasing dominance of large lianas in Amazonian forests.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmtV2gu7g%3D&md5=7780bc1dbdd96b259182cae18c976eb9CAS | 12181565PubMed |
Pinkard EA, Beadle CL, Mendham DS, Carter J, Glen M (2010) Determining photosynthetic responses of forest species to elevated [CO2]: alternatives to FACE. Forest Ecology and Management 260, 1251–1261.
| Determining photosynthetic responses of forest species to elevated [CO2]: alternatives to FACE.Crossref | GoogleScholarGoogle Scholar |
Polley HW (2002) Implications of atmospheric and climatic change for crop yield and water use efficiency. Crop Science 42, 131–140.
| Implications of atmospheric and climatic change for crop yield and water use efficiency.Crossref | GoogleScholarGoogle Scholar | 11756263PubMed |
Poorter H (1993) Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 104–105, 77–97.
| Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Navas M-L (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytologist 157, 175–198.
| Plant growth and competition at elevated CO2: on winners, losers and functional groups.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Pot CS, Lambers H (1988) The effect of an elevated atmospheric CO2 concentration on growth, photosynthesis and respiration of Plantago major. Physiologia Plantarum 73, 553–559.
| The effect of an elevated atmospheric CO2 concentration on growth, photosynthesis and respiration of Plantago major.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXmtVyntbk%3D&md5=3202992886d0ac8cc6d54418cc0967a4CAS |
Poorter H, Fiorani F, Stitt M, Schurr U, Finck A, Gibon Y, Usadel B, Munns R, Atkin OA, Tardieu F, Pons TL (2012) The art of growing plants for experimental purposes; a practical guide for the plant biologist. Functional Plant Biology 39, 821–838.
| The art of growing plants for experimental purposes; a practical guide for the plant biologist.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Anten NPR, Marcelis LF (2013) Physiological mechanisms in plant growth models: do we need a supra‐cellular systems biology approach? Plant, Cell & Environment 36, 1673–1690.
| Physiological mechanisms in plant growth models: do we need a supra‐cellular systems biology approach?Crossref | GoogleScholarGoogle Scholar |
Rawson HM (1992) Plant responses to temperature under conditions of elevated CO2. Australian Journal of Botany 40, 473–490.
| Plant responses to temperature under conditions of elevated CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXpvVSjsg%3D%3D&md5=04bd3a65f8c990ee24a243f0957aabcaCAS |
Reddy AR, Rasineni GK, Raghavendra AS (2010) The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Current Science 99, 46–57.
Reyer C (2015) Forest productivity under environmental change – a review of stand-scale modeling studies. Current Forestry Reports 1, 53–68.
| Forest productivity under environmental change – a review of stand-scale modeling studies.Crossref | GoogleScholarGoogle Scholar |
Sims DA, Pearcy RW (1994) Scaling sun and shade photosynthetic acclimation of Alocasia macrorrhiza to whole-plant performance. 1. Carbon balance and allocation at different daily photon flux densities. Plant, Cell & Environment 17, 881–887.
| Scaling sun and shade photosynthetic acclimation of Alocasia macrorrhiza to whole-plant performance. 1. Carbon balance and allocation at different daily photon flux densities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmvFOgtLg%3D&md5=85b9aea939c3ac13da7d6c3e897176d8CAS |
Townend J (1995) Effects of elevated CO2, water and nutrients on Picea sitchensis (Bong.) Carr. seedlings. New Phytologist 130, 193–206.
| Effects of elevated CO2, water and nutrients on Picea sitchensis (Bong.) Carr. seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXnt1CjtLw%3D&md5=3c6f85429af917d08f7b9d5c50155934CAS |
van der Heijden GM, Schnitzer SA, Powers JS, Phillips OL (2013) Liana impacts on carbon cycling, storage and sequestration in tropical forests. Biotropica 45, 682–692.
| Liana impacts on carbon cycling, storage and sequestration in tropical forests.Crossref | GoogleScholarGoogle Scholar |
Wang D, Heckathorn SZ, Wang X, Philpott SM (2012) A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169, 1–13.
| A meta-analysis of plant physiological and growth responses to temperature and elevated CO2.Crossref | GoogleScholarGoogle Scholar | 22037993PubMed |
Way DA, Yamori W (2014) Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynthesis Research 119, 89–100.
| Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFKgtb3L&md5=530f645e1ad2947cb06075a46db7000aCAS | 23812760PubMed |


