Should we switch from bendrofluazide to chlorthalidone as the initial treatment for hypertension? A review of the available medication
Bruce Arroll 1 , Henry Wallace 11 Department of General Practice and Primary Health Care, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand
Correspondence to: Bruce Arroll, Department of General Practice and Primary Health Care, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand. Email: bruce.arroll@auckland.ac.nz
Journal of Primary Health Care 9(2) 105-113 https://doi.org/10.1071/HC16038
Published: 9 June 2017
Journal Compilation © Royal New Zealand College of General Practitioners 2017.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: Thiazide diuretics are commonly prescribed in the treatment of hypertension. However, thiazide diuretics may not all be equal in their ability to reduce cardiovascular disease outcomes.
AIM: To determine if bendroflumethiazide/bendrofluazide, the most commonly used diuretic for hypertension in New Zealand, is as effective as other diuretics in terms of cardiovascular disease outcomes.
METHODS: Using recent reviews of thiazide-like (chlorthalidone or indapamide) and thiazide-type diuretics (hydrochlorothiazide and bendrofluazide) and a separate search of bendrofluazide, data on cardiovascular disease outcomes was extracted.
RESULTS: Nineteen relevant papers with 21 comparisons were found. All thiazide-based diuretics have been reported in at least one trial showing them to be more effective than placebo for cardiovascular disease outcomes, with the exception of chlorothiazide. There were no comparisons of bendrofluazide alone with other medications, but there were two studies with either bendrofluazide or hydrochlorothiazide compared with β-blockers; however, the pooled relative risk (RR) was not significant (RR = 1.10 (95% CI, 0.84–1.43)). For chlorthalidone, there were four comparisons with other medications, and the summary RR was statistically significant for cardiovascular disease outcomes (RR = 0.91 (95% CI, 0.85–0.98)). Chlorthalidone was significantly more effective for some cardiovascular disease outcomes when compared with doxazosin, amlodipine and lisinopril.
CONCLUSIONS: All thiazide-based medicines available in New Zealand are effective in terms of cardiovascular disease outcomes compared with placebo when used for treating hypertension, with the exception of chlorothiazide. Of the diuretics available in New Zealand for hypertension, only chlorthalidone has been shown to be more effective than other blood pressure-lowering medicines. It may be time to change from using bendrofluazide and start using chlorthalidone as a treatment for hypertension.
KEYWORDS: Thiazides; diuretics; hypertension; cardiovascular diseases
| WHAT GAP THIS FILLS |
| What is already known: Thiazide-like diuretics (chlorthalidone or indapamide) and thiazide-type diuretics (hydrochlorothiazide and bendrofluazide) adequately lower blood pressure, but thiazide-like diuretics are associated with larger reductions in cardiovascular outcomes. |
| What this study adds: This is the first review to consider the effectiveness of the thiazide diuretics available in New Zealand. Of all the thiazide diuretics available in New Zealand for hypertension, only chlorthalidone has been shown to be more effective than other blood pressure-lowering medicines at reducing adverse cardiovascular outcomes. |
Introduction
Thiazide diuretics are recommended as first-line therapy for hypertension in New Zealand and are among the most commonly prescribed drugs worldwide.1,2 However, not all thiazide diuretics are equal in their ability to reduce cardiovascular disease outcomes.3,4 According to their molecular structure, thiazide diuretics can be divided in thiazide-type and thiazide-like diuretics.
Drugs with a similar pharmacologic action on the kidney that do not have the thiazide chemical structure, such as indapamide, chlorthalidone and metolazone, are termed ‘thiazide-like diuretics’.5 Thiazide-like diuretics have a longer elimination half-life than thiazide-type diuretics, and have been shown to exert additional pharmacological effects, which may differently affect cardiovascular risk.4
Thiazide-type diuretics include bendrofluazide, chlorothiazide and hydrochlorothiazide. Bendrofluazide is the most commonly used thiazide diuretic for hypertension in New Zealand, representing ~88% of the market (PHARMAC, pers. comm. to B. Arroll 2015). Chlorthalidone 7%, indapamide 5% and chlorothiazide 0.03% are the other thiazide diuretics available in New Zealand, but chlorothiazide is available only in a liquid form in the New Zealand market (PHARMAC, pers. comm. to B. Arroll 2015). Despite being a commonly prescribed medication, there is limited evidence on the ability of bendrofluazide to lower blood pressure. A Cochrane review found only one trial investigating blood pressure lowering by bendrofluazide, compared with eight trials for chlorthalidone, 40 trials for hydrochlorothiazide and 10 trials for indapamide.5
The most commonly used anti-hypertensive in the United States is hydrochlorothiazide, but it is only available in combination with an angiotensin-converting enzyme (ACE) or angiotensin receptor blocker (ARB) medication in New Zealand. There has been debate in the literature over the dose of hydrochlorothiazide, which seems to have a dose-dependent blood pressure-lowering effect that does not occur with other diuretics.5 As a result of its dose-dependent response, as well as its ‘paltry’ antihypertensive efficacy and poor adherence, there been a challenge to the use of hydrochlorothiazide in the USA and it has been suggested that chlorthalidone be used in its place.6 Additionally, according to current United Kingdom National Institute of Health and Clinical Excellence (NICE) guidelines, ‘If diuretic treatment is to be initiated or changed, offer a thiazide-like diuretic, such as chlorthalidone or indapamide, in preference to a conventional thiazide diuretic such as bendroflumethiazide or hydrochlorothiazide.’7 These international recommendations for the use of thiazide-like diuretics instead of thiazide-type diuretics raise the issue of how bendrofluazide, the New Zealand hydrochlorothiazide equivalent, compares with other diuretics such as chlorthalidone and indapamide. There is no single review considering the New Zealand context where only four types of thiazide diuretic are available. New Zealand has the second highest stroke rate in the developed world and we speculate that this may, in part, be due to using a relatively ineffective diuretic.8
Methods
We have taken two recent systematic reviews of thiazide-like and thiazide-type diuretics, published in 2015,3,4 and assumed that these reviews contain all relevant studies published before 2015. We then performed a follow-up systematic search for relevant papers published during the remainder of 2015. We have also conducted a literature search on bendrofluazide, which was not included in the searches of the 2015 reviews, for studies reporting cardiovascular disease or mortality outcomes.
The search was conducted on 26 November 2015 and covered the Cochrane Central Register of Controlled Trials [CENTRAL] (all years to date), MEDLINE (1950-present), Embase (1980-present), and PubMed (1966-present). The reference lists of included studies were also scanned for any additional and relevant studies. We wished to examine all randomised placebo controlled trials (RCT) using thiazide-like (chlorthalidone and indapamide) and thiazide-type (bendrofluazide, hydrochlorothiazide and chlorothiazide) diuretics as an intervention for hypertension, with results reported in terms of cardiovascular disease outcomes. We chose combined cardiovascular disease outcomes, rather than cardiovascular mortality, as we are aware that some research was underpowered to detect a difference in mortality and hence wished to use an outcome that was likely to be common to all studies.
The data from identified papers were extracted in duplicate by HW and BA, including the PICOs (participants, interventions, comparison and outcomes) information, as shown in Table 1. Risk of bias was assessed using the Cochrane Collaboration tool for assessing the risk of bias (Table 8.5.a in the Cochrane Handbook for Systematic Reviews of Interventions, http://handbook.cochrane.org/). Summary measures were the relative risk and confidence intervals and data were pooled where possible using the Cochrane RevMan software (version 5.3.5, The Cochrane Collaboration, Copenhagen, Denmark). All analyses, unless otherwise specified, used a random effects model as the most conservative option.

|
Results
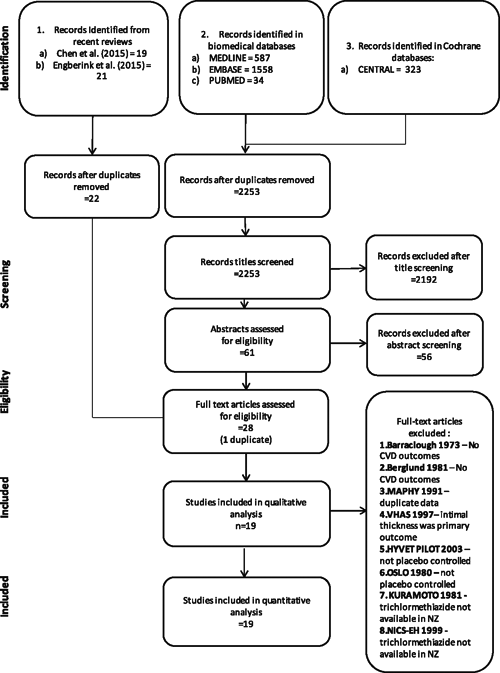
We found 19 papers with 21 comparisons that met our selection criteria (see flow chart Figure 1). All but one reported multiple cardiovascular outcomes, and for these studies, we have reported combined cardiovascular disease outcomes.9 For the remaining study, we have reported only cardiovascular disease mortality.
|
There were three papers reporting bendrofluazide9–11 outcomes, two papers using chlorothiazide,12,13 five papers reporting five outcomes for chlorthalidone,14–18 10 papers using hydrochlorothiazide9,11,19–26 and one paper reporting on indapamide27 (Table 1). Two of these studies used bendrofluazide or hydrochlorothiazide.9,11 The hypertension in the very elderly trial (HYVET) pilot study and the OSLO study (treatment of mild hypertension: A five year controlled drug trial –The Oslo study) were eliminated due to lack of a placebo control group.28,29 There was at least one study for all diuretics (bendrofluazide, chlorthalidone, hydrochlorothiazide and indapamide) reporting the medication to be more effective than placebo in terms of reducing cardiovascular events, other than chlorothiazide.
Chlorothiazide
The pooled data for chlorothiazide versus placebo was not statistically significant (RR = 0.82 (95% CI, 0.66–1.01)). There were no studies involving chlorothiazide versus other medications.
Chlorthalidone
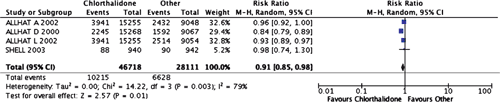
The only diuretic available in New Zealand to show a statistically significant benefit compared with multiple medications was chlorthalidone. There were two studies comparing chlorthalidone with placebo and the summary relative risk was statistically significant (RR = 0.68 (95% CI, 0.58 – 0.80)).16,17 Compared with chlorthalidone, doxazosin was associated with more combined cardiovascular disease adverse outcomes (RR = 1.25 (95% CI, 1.17–1.33)), as well as congestive heart failure (RR = 2.04 (95% CI, 1.79 – 2.32)) and stroke (RR = 1.19 (95% CI, 1.01 – 1.40)).15 Similarly, compared with chlorthalidone, lisinopril was associated with more combined cardiovascular disease adverse outcomes (RR = 1.10 (95% CI, 1.05 – 1.16)), stroke (RR = 1.15 (95% CI, 1.02 – 1.30)) and heart failure (RR = 1.19 (95% CI, 1.07 – 1.31)).15 Also, compared with chlorthalidone, amlodipine had a higher incidence of heart failure (RR = 1.38 (95% CI, 1.25 – 1.52)) and combined cardiovascular disease outcomes (RR = 1.04 (95% CI, 0.99 – 1.09)).15 Overall, for chlorthalidone, there were four comparisons versus other medications, and the summary relative risk was statistically significant for cardiovascular disease outcomes (RR = 0.91 (95% CI, 0.85 – 0.98; Figure 2)). There was no comparison where doxazosin, amlodipine and lisinopril were more effective than chlorthalidone.
|
Indapamide
There was only one study involving indapamide.27 It was found to be significantly better than placebo for combined cardiovascular disease outcomes (RR = 0.71 (95% CI, 0.574 – 0.872)).27 There were no studies comparing indapamide with other medications.
Bendrofluazide/Hydrochlorothiazide
The two low-dose hydrochlorothiazide studies identified used hydrochlorothiazide concurrently with amiloride23 or triamterene19 and hence are not a pure comparison. Both were effective when compared with placebo in terms of reducing cardiovascular disease outcomes and the pooled relative risk (RR = 0.68 (95% CI 0.57 – 0.81)) was also significant. Two other studies compared bendrofluazide or hydrochlorothiazide and neither was more effective than β-blocker(s) with the pooled relative risk (RR = 1.10 (95% CI, 0.84 – 1.43)). There were two papers reporting that a high dose of hydrochlorothiazide (100 mg per day) was significantly more effective than placebo (VA I and VA II) (Veterans Administration Cooperative Study Group on Antihypertensive Agents). As the data came from the same study, we used a fixed-effects analysis and the pooled relative risk (RR = 0.3 (95% CI, 0.19 – 0.45)). When hydrochlorothiazide was compared with other medications, the pooled relative risk (RR = 0.99 (95% CI, 0.86 – 1.15)) was not significant. There were no comparisons of bendroflumethiazide/bendrofluazide alone with other medications.
Risk of bias
The risk of bias (using the Cochrane Collaboration system) was low for the significant chlorthalidone studies; that is ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial), main SHEP (Systolic Hypertension in the Elderly Program) and the indapamide study (HYVET). For the bendrofluazide versus placebo study, there was a high risk of bias, as the medication and study personnel were not blinded. The two chlorothiazide studies were at low risk of bias.
Discussion
Other than chlorothiazide, there was at least one study for each of the thiazide diuretics used to treat hypertension in New Zealand (bendrofluazide, chlorthalidone, indapamide, chlorothiazide) reporting the medication to be more effective than placebo in terms of reducing cardiovascular events.
Of those four thiazide diuretics, the only one that was significantly more effective than other medications was chlorthalidone. It was more effective than lisinopril, amlodipine and doxazosin for several cardiovascular disease outcomes. Furthermore, there were no analyses where doxazosin, amlodipine or lisinopril was more effective than chlorthalidone.
Bendrofluazide has not been compared against other anti-hypertensive medications, but was superior to placebo in one study (MRC I)10 for combined cardiovascular disease events. It was effective for stroke but not coronary heart events. The study was unblinded and hence at high risk of bias. In both the SHEP pilot and main SHEP trial, using chlorthalidone was significantly more effective than placebo. On balance, we feel that there is more evidence for using chlorthalidone than bendrofluazide, and that New Zealand-based clinicians should consider this evidence when choosing a thiazide diuretic for treating hypertension.
In terms of side-effect profiles, a recent review was unable to draw conclusions about the prevalence of thiazide-induced hyponatremia with respect to individual thiazide medications.30 Another review reported that when compared to other antihypertensive therapy, both thiazide-like and thiazide-type diuretics showed a similar number of adverse events in patients with comparable reductions in blood pressure.4
Strengths and limitations
While hydrochlorothiazide is the most commonly prescribed thiazide diuretic worldwide, we have not focused on it, as it is not available in New Zealand as a stand-alone medicine. We concur with other critics that the usual dose of 25–50 mg is not supported by evidence and that if it is used alone, then doses of 100 mg need to be considered.6 We considered combined cardiovascular disease outcomes as our primary outcome as this was likely to be reported in most papers. There was only one paper that did not report this, but reported cardiovascular disease mortality as the outcome.9 We acknowledge that using the cardiovascular disease composite outcomes could have led to overstatement of the benefits of the drugs in each of the studies we used, and that the use of non-uniform composite outcomes could also bias results towards studies with a broad criteria of a cardiovascular disease outcome.
Other literature
A recent review of thiazide diuretics concluded that ‘preferential use of thiazide-like diuretics over thiazide-type diuretics may result in greater cardiovascular disease benefits in hypertensive patients’ and suggested that ‘the use of thiazide diuretics in hypertensive patients results in a reduction in the risk of cardiovascular disease outcomes’. Moreover, thiazide-like diuretics have a greater protective effect against cardiovascular disease outcomes than thiazide-type diuretics, especially with regard to heart failure, suggesting that preferential use of thiazide-like diuretics over thiazide-type diuretics may result in greater cardiovascular benefits in hypertensive patients.3 Another review reported that thiazide-like diuretics resulted in a 12% additional risk reduction when compared with thiazide-type diuretics for cardiovascular disease events, and an additional 21% risk reduction for heart failure.4 There has been debate over the blood pressure-lowering ability of these medications, but our view is that cardiovascular disease outcomes are the gold standard when looking at effectiveness.6,31 Blood pressure lowering is a surrogate outcome.
There is also a suggestion that some of chlorthalidone’s effectiveness is due to its effect on other processes and longer duration of action.32 Additionally, the fact that there was only a 2-mmHg difference between chlorthalidone and lisinopril in the ALLHAT trial is because the former had more patients reach the target blood pressure. An editorial on the ALLHAT trial also suggested there were issues of a certain number of patients getting drug classes from other treatment arms, and that an on-treatment analysis would be welcome, although Fagard conceded that the intention-to-treat analysis was the most conservative and appropriate.33 Another editorial accompanying the Rik et al. (2015)4 review favoured either indapamide or chlorthalidone. While it concluded that indapamide had some better features (e.g. cost, availability and formulation), none of these are issues in New Zealand. Considering the available evidence, our view is that chlorthalidone is the more effective medication for hypertension in terms of cardiovascular disease outcomes.
Implications for research
We were surprised at the low number of trials for bendrofluazide, chlorthalidone and indapamide in comparison with hydrochlorothiazide. A definitive trial of chlorthalidone versus hydrochlorothiazide and bendrofluazide would be informative, but would require a large sample size of the order of the ALLHAT trail, which included 33,357 participants. None of these medications are covered by a patent, so there is not likely to be an industry-funded trial.
Implications for practice
Our view is that there is more evidence supporting chlorthalidone as the first choice of diuretic compared with bendrofluazide or indapamide. All three are fully funded in New Zealand. We feel it is time for clinicians to consider switching from bendrofluazide to chlorthalidone.
COMPETING INTERESTS
None.
FUNDING
Henry Wallace received a Summer Research Scholarship from the Royal New Zealand College of General Practitioners (RNZCGP). The RNZCGP is not involved in any other aspect of the project, such as the design of the project’s protocol and analysis plan, the collection, analyses or decision to publish.
References
[1] Britten N. Time to talk about prescriptions. Prescriber. 1999; 10 13–4.[2] Best Practice Advocacy Centre New Zealand Which antihypertensive? Best Practice Journal. 2010; 31 14–32.
[3] Chen P, Chaugai S, Zhao F, et al. Cardioprotective effect of thiazide-like diuretics: a meta-analysis. Am J Hypertens. 2015; 28 1453–63.
| Cardioprotective effect of thiazide-like diuretics: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[4] Olde Engberink RH, Frenkel WJ, van den Bogaard B, et al. Effects of thiazide-type and thiazide-like diuretics on cardiovascular events and mortality systematic review and meta-analysis. Hypertension. 2015; 65 1033–40.
| Effects of thiazide-type and thiazide-like diuretics on cardiovascular events and mortality systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosFGgt7g%3D&md5=d2093ef618cd2f3005626b9214d7f6aaCAS |
[5] Musini VM, Nazer M, Bassett K, et al. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst Rev. 2014; 29 CD003824
[6] Messerli FH, Bangalore S. Half a century of hydrochlorothiazide: facts, fads, fiction, and follies. Am J Med. 2011; 124 896–9.
| Half a century of hydrochlorothiazide: facts, fads, fiction, and follies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1GgsbbM&md5=e8f2e2d08242069cc663ae5bdeef412aCAS |
[7] Wilson JM, Jungner G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968.
[8] Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009; 8 355–69.
| Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[9] Wikstrand J, Warnold I, Olsson G, et al. Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study. JAMA. 1988; 259 1976–82.
| Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c7lsFygsg%3D%3D&md5=79e4c0384ca2e4837fa7747ab10b7aeaCAS |
[10] MRC Working Party MRC trial of treatment of mild hypertension: principal results. Medical Research Council Working Party. Br Med J (Clin Res Ed). 1985; 291 97–104.
| MRC trial of treatment of mild hypertension: principal results. Medical Research Council Working Party.Crossref | GoogleScholarGoogle Scholar |
[11] Wilhelmsen L, Berglund G, Elmfeldt D, et al. Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. J Hypertens. 1987; 5 561–72.
| Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c7hs1KnsA%3D%3D&md5=48004dc397dbd87a456d1e5d9fc35898CAS |
[12] Reader R. The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980; 1 1261–7.
[13] Smith WM. Treatment of mild hypertension: results of a ten-year intervention trial. Circ Res. 1977; 40 I98–105.
| 1:STN:280:DyaE2s7mtVCktw%3D%3D&md5=6751763bb9d1120ab7f8c4eecc582918CAS |
[14] Furberg CD, Wright JT, Davis BR, et al. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002; 288 2981–97.
| Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhslWr&md5=6747a22e5a0a08d4b39f2c529e4b0961CAS |
[15] Furberg CD, Wright JT, Davis BR, et al. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to prevent Heart Attack Trial (ALLHAT). JAMA. 2000; 283 1967–75.
| Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to prevent Heart Attack Trial (ALLHAT).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtVOjt74%3D&md5=18cecfa698071ca9bba3a6b00e621f48CAS |
[16] Perry HM, Smith WM, McDonald RH, et al. Morbidity and mortality in the Systolic Hypertension in the Elderly Program (SHEP) pilot study. Stroke. 1989; 20 4–13.
| Morbidity and mortality in the Systolic Hypertension in the Elderly Program (SHEP) pilot study.Crossref | GoogleScholarGoogle Scholar |
[17] Ernst ME. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991; 265 3255–64.
| Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group.Crossref | GoogleScholarGoogle Scholar |
[18] Malacco E, Mancia G, Rappelli A, et al. Treatment of isolated systolic hypertension: the SHELL study results. Blood Press. 2003; 12 160–7.
| Treatment of isolated systolic hypertension: the SHELL study results.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt1Wnurk%3D&md5=8660c4c9b0ba1588fd33528456563be9CAS |
[19] Amery A, Birkenhager W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet. 1985; 325 1349–54.
| Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial.Crossref | GoogleScholarGoogle Scholar |
[20] Borhani NO, Mercuri M, Borhani P, et al. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS): a randomized controlled trial. JAMA. 1996; 276 785–91.
| Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS): a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlvFOls7c%3D&md5=7499839d5feb1a7060bc7561fee7dab9CAS |
[21] Brown MJ, Palmer C, Castaigne A, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet. 2000; 356 366–72.
| Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXls1Clu7c%3D&md5=feb08c4c5d5380f700cb17464210af49CAS |
[22] Jamerson K, Weber M, Bakris G, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008; 359 2417–28.
| Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVCrurjJ&md5=fbae404e0f669017915df53403121feeCAS |
[23] Party MW. Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ. 1992; 304 405–12.
| Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party.Crossref | GoogleScholarGoogle Scholar |
[24] Veterans Administration Cooperative Study Group Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967; 202 1028–34.
| Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg.Crossref | GoogleScholarGoogle Scholar |
[25] Veterans Administration Cooperative Study Group Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970; 213 1143–52.
| Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg.Crossref | GoogleScholarGoogle Scholar |
[26] Wing LM, Reid C, Ryan P, et al. A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003; 348 583–92.
| A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for hypertension in the elderly.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtVyqt74%3D&md5=16a278e223b67ff383dc9979b57620baCAS |
[27] Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008; 358 1887–98.
| Treatment of hypertension in patients 80 years of age or older.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlt1Ggsbk%3D&md5=cb01541b50c6ee55b11f769f334e4d90CAS |
[28] Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens. 2003; 21 2409–17.
| Results of the pilot study for the Hypertension in the Very Elderly Trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsVGhsb8%3D&md5=b203b51dcd85755c6672241bd1c45b35CAS |
[29] Helgeland A. Treatment of mild hypertension: a five year controlled drug trial. The Oslo study. Am J Med. 1980; 69 725–32.
| Treatment of mild hypertension: a five year controlled drug trial. The Oslo study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M%2FlvFKiug%3D%3D&md5=f607d18fe77491595a4414f4212509c1CAS |
[30] Barber J, McKeever TM, McDowell SE, et al. A systematic review and meta-analysis of thiazide-induced hyponatraemia: time to reconsider electrolyte monitoring regimens after thiazide initiation? Br J Clin Pharmacol. 2015; 79 566–77.
| A systematic review and meta-analysis of thiazide-induced hyponatraemia: time to reconsider electrolyte monitoring regimens after thiazide initiation?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXkvFCktb0%3D&md5=a4d107475ed9400be89d726790fe5c1bCAS |
[31] Zanchetti A, Agabiti Rosei E, Dal Palu C, et al. The Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of long-term randomized treatment with either verapamil or chlorthalidone on carotid intima-media thickness. J Hypertens. 1998; 16 1667–76.
| The Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of long-term randomized treatment with either verapamil or chlorthalidone on carotid intima-media thickness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXms1Sh&md5=adb56148ca291ffb878b61b7509570ddCAS |
[32] Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012; 59 1110–7.
| Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XntVSgs7o%3D&md5=89ccc6f22cdf9ec077283d3f8d2194fbCAS |
[33] Fagard RH. The ALLHAT trial: strengths and limitations. J Hypertens. 2003; 21 229–32.
| The ALLHAT trial: strengths and limitations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptFKrtg%3D%3D&md5=5e10635f09f8244104777d6d3106b44fCAS |


