Faecal immunochemical tests for occult blood testing should not be used outside of bowel screening: an audit of a large general practice
Ui Ho Byun 1 5 , Neil Anderson 2 , Arlo Upton 3 , Paul Frankish 41 Department of Surgery, University of Auckland, 85 Park Road, Grafton, Auckland, New Zealand.
2 Coast to Coast Healthcare, 220 Rodney St, Wellsford, Auckland, New Zealand.
3 Southern Community Laboratories, 472 George Street, Dunedin, New Zealand.
4 Waitemata District Health Board, 124 Shakespeare Road, Takapuna, Auckland, New Zealand.
5 Corresponding author. Email: ubyu572@aucklanduni.ac.nz
Journal of Primary Health Care 11(3) 259-264 https://doi.org/10.1071/HC18068
Published: 3 September 2019
Journal Compilation © Royal New Zealand College of General Practitioners 2019. This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: The faecal immunochemical test (FIT) detects small quantities of human haemoglobin in faeces. This test has increasingly become the screening tool of choice in bowel cancer screening programmes worldwide, including New Zealand’s upcoming national screening programme.
AIM: This study audited the appropriate use of faecal immunochemical tests (FIT) in general practice as current recommendations discourage the use of FIT outside the National Bowel Screening Programme.
METHODS: Data on all FIT requested by a multiclinic general practice serving 16 000 patients from May 2017 to May 2018 were extracted from clinical records. Patient characteristics, results of tests, clinical rationale for the test, number of referrals and results and the completeness of clinical evaluation were recorded.
RESULTS: In all, 184 patients received an FIT, with 13 (7.1%) positive and 145 (78.8%) negative tests, and 26 (14.1%) tests declined by the laboratory. Nine patients (69.2%) with a positive FIT, 12 patients (8.1%) with a negative FIT and one patient (3.8%) with a declined test were referred to gastroenterology services. Seven colorectal cancers were detected, all in patients with a positive FIT who were aged between 67 and 91 years. FIT was requested most for changes in bowel habit (53%) and blood in stool (15%); 10% of tests were ordered for reassurance and 9% did not record an indication for the test. Two general practitioners (of 17 in the practice) accounted for over half of all tests requested.
CONCLUSIONS: Because FIT is only a screening tool for colorectal cancer, direct referral is recommended for symptomatic patients. Although cancers were detected only in patients with positive FITs, these patients would have qualified for direct referral for definitive investigation, and a referral was made concurrently. Awaiting test results may also delay necessary referrals and a negative FIT may produce false reassurance.
KEYwords: Faecal occult blood test (iFOBT); gastrointestinal; family practice.
| WHAT GAP THIS FILLS |
| What is already known: In the community setting, bowel cancer remains a diagnosis of clinical suspicion based on a comprehensive history and examination. The FIT, the screening tool used in New Zealand’s national bowel cancer screening rollout, remains as a test which can be ordered in the community. |
| What this research adds: This expanded audit reports on the use of FIT in the community and its ad-hoc use outside of a well-resourced screening programme; this raises the issue of variability of care including interpretation, false reassurance, and overuse of the test. As FIT is a non-diagnostic screening test, its utility lies in the average-risk population of some risk (increased age). Patients with high-risk features of bowel malignancy should be directly referred for diagnostic visualization. |
Introduction
This paper reports and expands on an audit recommended by the Best Practice Advocacy Centre (BPAC)1 to investigate the appropriate use of faecal immunochemical tests (FIT) in general practice. The launch of the Bowel Screening Pilot (BSP) in 2012 for people aged 50–74 years in the Waitemata District Health Board (vs 60–74 years in the national rollout)2 has affected the role of the FIT in Auckland and Northland.
Routine use of FIT was not recommended in Auckland and Northland because the National Bowel Cancer Screening Programme started to roll out in 2018. According to a late 2017 consultation document written by the medical director of Labtests and members of the National Bowel Cancer Working Group and Screening Programme, ‘FIT should not be available in the Auckland and Northland communities outside of the National Screening Programme’.3 Community laboratories aimed to discontinue FIT in these regions and to use the test solely for colorectal cancer screening in the National Programme. The consultation document provides the following advice regarding common clinical scenarios encountered by general practitioners.
Patients with high-risk features of bowel cancer should be investigated directly either with referral to a gastroenterologist or with colonoscopy. FIT is not a definitive test, so a negative result does not rule out colorectal pathology; a meta-analysis showed a sensitivity of 79% and specificity of 94% for colorectal cancer detection.4 Furthermore, a single FIT is only approximately 20–30% sensitive for advanced adenoma,5 and a well-resourced bowel cancer screening programme is required for serial testing of participants with negative tests.6 Diagnostic value is not added by performing an FIT before referral because test results may cause inappropriate reassurance or patient anxiety. The recommendation does not suggest that the FIT is not useful, but that testing should take place in the context of a well-designed screening programme that is suitably resourced to further investigate individuals with positive test results.
If iron deficiency is found and obvious causes are excluded, males and postmenopausal females should be referred for consideration of gastroscopy and colonoscopy. Premenopausal women with a personal history of neoplastic polyps, inflammatory bowel disease or significant family history should also be referred.
The FIT is a test that is now recommended only within the National Bowel Screening Programme to screen the average-risk population instead of investigating people with symptoms or risk factors for bowel cancer or other pathology. The latter group should be considered for referral instead to an endoscopist or a gastroenterologist.
This paper audits the use of FIT in a multiclinic general practice, focusing on test results, reasons for requesting FIT, number of referrals made and completeness of clinical documentation.
Methods
All FITs performed from May 2017 to May 2018 at Coast-to-Coast Healthcare, comprising five clinics in the Greater Auckland Waitakere District Health Board region, were extracted from computerised clinical records using Medtech’s (Viaduct Harbour, Auckland, NZ) Evolution query builder. For each FIT, the National Health Index code (NHI) of the tested person, their age and sex, the FIT result (positive or negative), reason recorded for ordering a FIT, risk status for bowel cancer, whether a referral was made and the result of colonoscopy were extracted.
Moderate or high-risk status was defined as patients with a personal history of bowel cancer, irritable bowel disease or colorectal polyps, family history of inherited colorectal cancer and a first-degree relative diagnosed with colorectal cancer when aged <55 years. This was noted if mentioned in clinical documentation.
Statistical analysis was descriptive. Due to some clinicians ordering more than one FIT at a time concurrently, most analyses were done on a per-patient basis.
Because this study was a minimal-risk observational audit with no identifiable patient features or conflicts of interest, no ethics approval was advised.7
Results
Study group
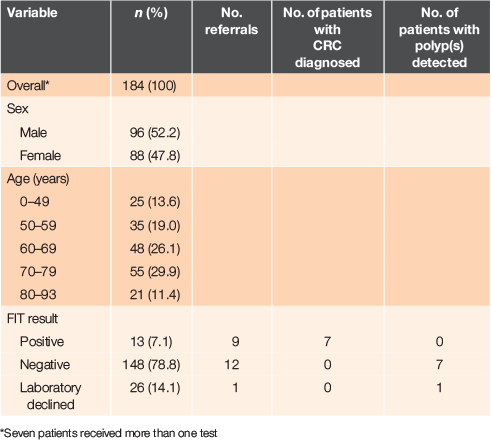
This audit was performed in a multiclinic general practice that serves 16,000 patients in the Greater Auckland region, centred in Wellsford. In all, 184 patients received an FIT in the 1-year study period. Ninety-six patients (52%) were male. Patient ages ranged from 6 months to 93 years, with a median age of 67 years. Overall, there were 193 tests (1.05 FIT per patient). More details are provided in Table 1.
|
Test results
There were 13 (7.1%) patients with positive results, 26 (14.1%) whose tests were declined by the laboratory, and 148 (78.8%) with negative results, as summarised in Table 1. Excluding the declined tests, 8.1% of FITs were positive and 91.9% were negative.
For seven patients, FIT was performed more than once during the audit period. Five patients received two tests and two patients received three tests. One patient tested positive once and the subsequent test was negative 3 weeks later. For two other patients the tests were repeated for reassurance. For the four remaining patients, the reason for repeating was not documented.
Of the 26 patients with tests declined by the laboratory, the reasons for not testing were either due to not fulfilling an age criterion (≥50 years) and where concurrent microbial stool investigations had been requested. Of these patients, only one, a 53-year-old male with bowel habit change, received a referral for colonoscopy, which found diverticulosis and a 1-mm low-grade polyp.
There were 13 patients with positive results. The ages of these patients ranged from 35 to 91 years (median = 67 years). Of these 13 patients, nine (69%) received referral for colonoscopy. The patients who were not referred included one patient with a recent colonoscopy, one with a repeat negative FIT and one with haemorrhoids on a per rectum (PR) examination without any red flags. A 35-year-old man was not followed-up with his positive result for unknown reasons: no clinical notes could be found.
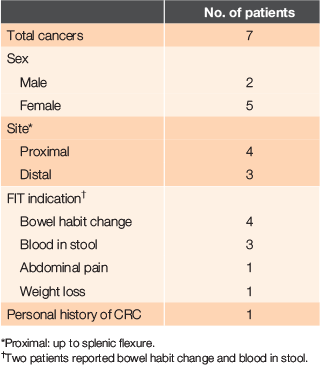
Of the nine patients with a positive FIT, seven (aged 67–91 years) were diagnosed with colorectal cancer (for more details, see Table 2), including a patient with recurrent colorectal cancer. Four general practitioners (GPs) were involved in the care of these patients. Of the other two patients with a positive FIT, one had diverticulitis and the other had anal stenosis.
|
Of the 148 patients with a negative test result, 12 (8.3%) were referred. Seven patients had one to three adenomas detected on endoscopy (none was advanced adenoma), one patient had diverticulosis, one had haemorrhoids and one had no abnormalities. Two patients underwent computed tomography (CT) scans (normal) and were subsequently discharged. No cancers were found in patients with a negative FIT.
Rationale for requesting FIT
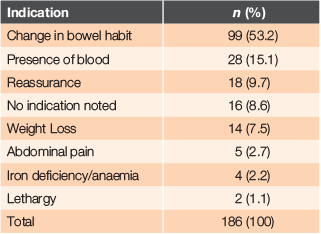
The clinical indication for ordering FIT was recorded. In all cases except two, there was one documented reason for requesting an FIT. Most patients (53%) had a change in bowel habit recorded and 15% of patients reported rectal bleeding. Other indications are outlined in Table 3.
|
Clinician characteristics
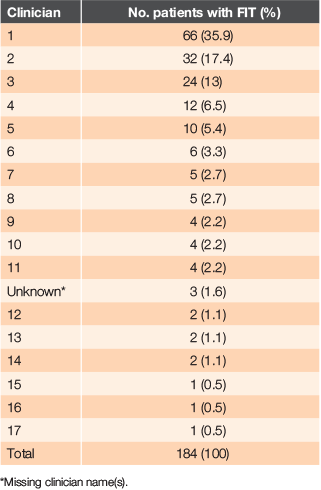
Seventeen 17 GPs ordered at least one FIT in a 12-month period. One GP ordered for 66 patients (35.9%), followed by another GP ordering for 32 patients (17.4%); over half of all FITs were ordered by these two clinicians (see Table 4).
|
FIT use in higher-risk patients
Nineteen patients (10.3%) were at moderate or high risk for colorectal cancer, with six patients having a history of previous colorectal cancer. In this subgroup, two tests (10.5%) were positive, three (15.8%) rejected and 14 (73.7%) were negative. Three patients were referred.
Other findings
One patient (negative FIT) had a history of Lynch syndrome.
Although our audit did not systematically extract data regarding family history, many records were missing information regarding first-degree family history of bowel cancer or other malignancies. Histories rarely mentioned exclusion of hereditary conditions, such as Lynch syndrome or familial adenomatous polyposis.
Discussion
Bowel cancer is a difficult diagnosis to make early in its natural history due to its non-specific nature of history and clinical findings.8 The diagnosis requires clinicians to have an index of suspicion for further investigation. GPs in New Zealand are encouraged to review the succinct referral criteria for colonoscopy and CT colonography available from the Ministry of Health, and to triage patients into 2- and 6-week categories. There are also criteria for declining to accept referrals. High-risk (2-week category) features include known or suspected colorectal cancer on PR examination or on imaging, PR bleeds with iron-deficiency anaemia with benign causes ruled out, and over 6 weeks of altered bowel habit with unexplained rectal bleeding when aged >50 years.9 The gold standard of diagnosis remains as direct visualisation, usually with colonoscopy.
According to the 2015 National Institute for Heath and Care Excellence (NICE) colorectal cancer referral pathway,10 FIT can be offered to patients without rectal bleeding with unexplained symptoms that could be suggestive of colorectal cancer and to patients who meet no other referral criteria. Although this implies the community use of FIT is justifiable, the other referral criteria included almost all patients in this audit.
In this large general practice, 184 patients had FIT performed in the audited year and seven colorectal cancers were detected. All tests returned positive were among patients found to have colorectal cancer. However, seven patients were found to have neoplastic polyps with a negative test. Despite all cancers arising from patients with positive results, their symptoms warranted referral outside the screening programme and were made concurrently to the test. Because the FIT is a screening test, its utility is in the population at some risk (increased age) and currently asymptomatic. When patients are symptomatic, they should be considered for diagnostic testing.
Of patients who received FIT, 10% requested the test for reassurance. It may be difficult to manage patients’ expectations during consultations, but appropriate advice, including the non-diagnostic nature of the test, waiting to be enrolled in the national programme and perhaps information on the appropriate use of public resources with respect to false positive FIT results, could be explained.
There was evidence of a few clinicians using the FIT as a first-line investigation for any bowel-related pathology, whether infective or more sinister in nature. Our audit identified a few GPs who had a greater tendency towards ordering FIT, with one GP ordering 35% of all tests. It may be that these GPs are seeing a different population (e.g. older or more anxious) than their colleagues. Shortfalls in clinical documentation was also a finding in this audit. In 9% of patients there was no clear indication noted for ordering the FIT. Although not quantitatively measured, many consultations did not specify family history of cancer or other relevant diseases. Because patients with a family history of genetic syndromes, such as Lynch syndrome, should be under surveillance colonoscopy and not FIT, documentation around this is critical.
The provision of FIT in the community outside a screening programme is a contentious issue. Individuals and primary care practitioners are understandably concerned about colorectal cancer, a common cancer in New Zealand and a common cause of cancer-related morbidity and mortality. GPs have used FIT in their practice because no screening programme has been available. However, ad hoc screening comes with its own problems, including variable indications for testing, interpretation of results and potentially false reassurance of a negative result in a patient with worrying symptoms, and testing being repeated more often than necessary. This audit has highlighted that testing in primary care outside a screening programme is somewhat practitioner dependent, and that some of the tests were not clinically indicated. The audit has also highlighted that FIT is an effective screening test for detecting colorectal cancer.
This audit was performed in a large primary health organisation consisting of six clinics with 16 000 enrolled patients. However, we make no claim of generalisability of results from this single centre to other practices. This is also a cross-sectional study; despite only 8.3% of patients with negative tests being referred and having found no cancers, this study does not take into account the dynamic nature of patient management.
Conclusion
Direct referral for colonoscopy for patients suspected of having colorectal malignancy is preferred to FIT, which may not add diagnostic value. Accordingly, the current recommendation is to not perform FIT in patients with symptoms suggestive of colorectal cancer, but to refer them for further diagnostic investigations.
A minority of GPs had used FIT first line for bowel-related presentations. GPs should reflect on their use of this screening test and, as for any health resource, evaluate its clinical utility. Will the test result change management?
The audit has highlighted room for improvement in documentation, especially with regard to family history in the consultation notes for better risk stratification.
The FIT is now not recommended to be used in the community outside the National Bowel Screening Programme, which is currently being rolled out. Clinicians should maintain their level of suspicion to make referrals where necessary.
Competing interests
The authors declare no competing interests.
Funding statement
This research did not receive any specific funding.
References
[1] Best Practice Advocacy Centre. Appropriate use of the faecal occult blood test for colorectal cancer. 2012. [Cited 2018 June 17]. Available from: https://bpac.org.nz/BT/2012/June/06_faecal_occult.aspx[2] Ministry of Health. About the National Bowel Screening Programme. 2018. [Cited 2018 June 17]. Available from: https://www.timetoscreen.nz/bowel-screening/about-the-national-bowel-screening-programme/
[3] Labtests. Consultation document: faecal occult blood testing in the Auckland and Northland regions. 2017. [Cited 2018 June 17]. Available from: https://www.labtests.co.nz/images/News/E_Updates/171127_Consultation_FOB_testing.pdf
[4] Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014; 160 171–81.
| Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 24658694PubMed |
[5] Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterol 2017; 152 1217–1237.e3.
| Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on colorectal cancer.Crossref | GoogleScholarGoogle Scholar |
[6] Guidance for Best Practice Management in the National Bowel Screening Programme. Consultation document. Wellington: Ministry of Health; 2018.
[7] National Ethics Advisory Committee. Ethical guidelines for observational studies: observational research, audits and related activities. Revised edition. Wellington: Ministry of Health; 2012.
[8] Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer 2005; 93 399–405.
| Clinical features of colorectal cancer before diagnosis: a population-based case-control study.Crossref | GoogleScholarGoogle Scholar | 16106247PubMed |
[9] Ministry of Health. Referral criteria for direct access outpatient colonoscopy or computed tomography colonography. 2019. [Cited 2019 April 23]. Available from: https://www.health.govt.nz/publication/referral-criteria-direct-access-outpatient-colonoscopy-or-computed-tomography-colonography
[10] National Institute for Heath and Care Excellence (NICE). Suspected cancer: recognition and referral. 2015. [Cited 2018 June 17]. Available from: https://www.nice.org.uk/guidance/ng12


