Association of aspartate aminotransferase in statin-induced rhabdomyolysis
Xu Cong Ruan 1 , Lian Leng Low 2 , Yu Heng Kwan 31 MD Candidate; Duke-NUS Medical School, Singapore
2 Associate Consultant, Department of Family Medicine and Continuing Care, Singapore General Hospital, Singapore
3 MD-PhD Candidate, Program in Health Services and Systems Research, Duke-NUS Medical School, Singapore
Correspondence to: Yu Heng Kwan, Duke-NUS Medical School, 8 College Road, 169857, Singapore. Email: yuheng@u.duke.nus.edu
Journal of Primary Health Care 9(4) 316-320 https://doi.org/10.1071/HC17051
Published: 12 December 2017
Journal Compilation © Royal New Zealand College of General Practitioners 2017.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: The risk of rhabdomyolysis in the general population is elevated by the increased prevalence of statin use. As the presentation of rhabdomyolysis is varied, there is a risk of delayed diagnosis leading to patient complications and increased healthcare costs. Creatine kinase (CK) alone is not sufficiently predictive for risk stratification. Beyond serum CK, other biomarkers such as transaminases may be used as surrogates to evaluate rhabdomyolysis severity and predict complication risks.
AIM: To assess if other biomarkers are associated with peak CK and severity of rhabdomyolysis to aid in clinical diagnosis of rhabdomyolysis.
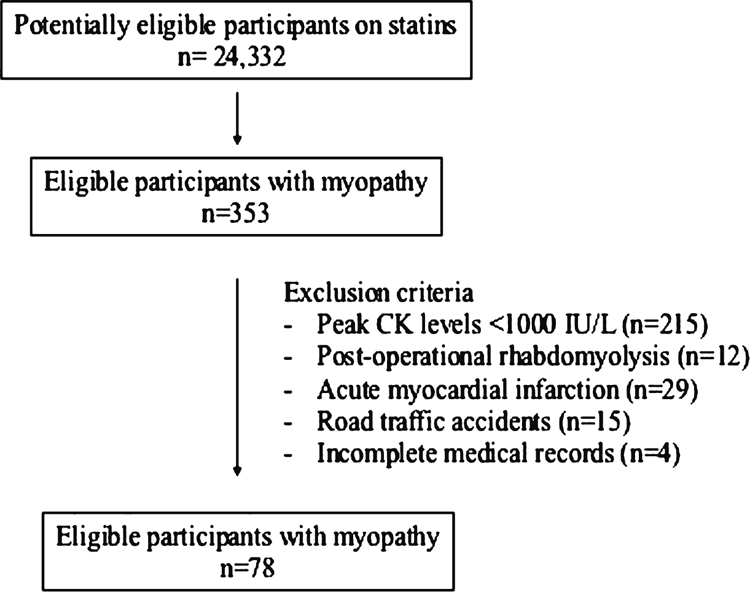
METHODS: A retrospective study was conducted at an acute care hospital from 2008 to 2011. Inclusion criteria were: (1) patients diagnosed with statin-induced rhabdomyolysis; and (2) peak CK levels of ≥1000 IU/L. Patients with post-operational rhabdomyolysis, acute myocardial infarction and who had suffered from road traffic accidents were excluded. A total of 24,332 patients were screened, and 78 patients fulfilled our inclusion criteria.
RESULTS: Aspartate aminotransferase (AST) was found to be positively associated with peak CK levels in the multivariable linear regression model after adjusting for alanine aminotransferase (ALT) levels (P = 0.002; β = 83.18). Aspartate aminotransferase was found to be associated with severity of rhabdomyolysis in the multivariable logistics regression model after adjusting for ALT levels (P = 0.015; OR = 1.01).
DISCUSSION: Aspartate transferase is associated with raised peak CK levels and severity of rhabdomyolysis. Clinicians may consider ordering AST to aid in the clinical diagnosis of rhabdomyolysis.
KEYWORDS: Rhabdomyolysis; statins; biomarkers
| WHAT GAP THIS FILLS |
| What is already known: Statin use is prevalent in an ageing population. Routine liver function tests are necessary to monitor liver function. |
| What this study adds: Aspartate aminotransferase (AST) is associated in statin-induced rhabdomyolysis patients. AST may be useful to look out for rhabdomyolysis. |
Introduction
Rhabdomyolysis is precipitated by skeletal muscle breakdown leading to the release of creatine kinase (CK) into the bloodstream.1 In addition to CK elevation, the breakdown of skeletal muscles also leads to leakage of intracellular contents such as potassium, aldolase, phosphate, myoglobin, lactate dehydrogenase (LDH) and aspartate aminotransferase (AST).1 The presentation of rhabdomyolysis is varied, with some individuals developing myalgia and muscle weakness while others are asymptomatic.2 If untreated, patients may progress to acute kidney injury (AKI), compartment syndrome and eventual demise.1,2
The risk of rhabdomyolysis in the general population is further elevated as more patients are placed on statins to control cardiovascular disease risk factors.3 Statin-induced rhabdomyolysis, resulting in hospital admission, is a concern and adds to healthcare costs.4
Given the potential for poor outcomes with delayed treatment in patients with rhabdomyolysis,1 it is important for clinicians to stratify patients based on their risk of complications. Biomarkers, such as CK, that directly correlate with the degree of muscle injury, have been studied.1,2 McMahon et al. showed that patients with high serum CK (>40,000 IU/L) are at high risk for AKI, and early hydration is vital to prevent complications.5 However, CK levels alone are not sufficiently predictive to stratify risk.5
Beyond serum CK, other routine biomarkers, such as electrolytes, transaminases and urine myoglobulin may be performed in patients with rhabdomyolysis. Potentially, these markers may be used as surrogates to evaluate the severity of rhabdomyolysis and predict the risk of complications. Our study objective was to find out which routinely performed biomarkers are associated with elevated serum CK, thereby facilitating clinicians in achieving an earlier diagnosis of rhabdomyolysis, stratifying patients with higher risk of complications and instituting early treatment of these patients to prevent disease progression.1 Therefore, we aimed to find out which routinely performed biomarkers are associated with peak serum CK and severity of rhabdomyolysis. This knowledge will allow increased vigilance in anticipating rhabdomyolysis in statin users.
Methods
Study design
A retrospective study was conducted at an acute care hospital in Singapore. Based on the hospital’s medical records, hospitalized patients from June 2008 to May 2011 were screened for incidences of statin-induced rhabdomyolysis. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for a primary diagnosis of other disorders of muscle, ligament and fascia (728.89, including rhabdomyolysis) was used to identify potential cases of rhabdomyolysis and myopathy. The study was approved by Institutional Review Board with a waiver for patient consent.
Study group
The inclusion criteria were: (1) patients diagnosed clinically with statin-induced rhabdomyolysis; and (2) peak CK levels of ≥1000 IU/L.2 Patients with post-operational rhabdomyolysis, acute myocardial infarction and who suffered from road traffic accidents were excluded. Figure 1 shows a flow diagram to illustrate patient selection into this study.6
Data collection
Data were retrieved from the hospital’s electronic clinical data monitoring system and patients’ case-notes. Information extracted included demographics data and laboratory test results including peak CK levels, urine myoglobulin, electrolytes, liver, renal and lipid panels during hospitalization.
Statistical analysis
Univariable linear regression analysis was performed to identify variables associated with an increase in peak CK in our study population. Variables found to have a P value of < 0.1 were subsequently included in a multivariable linear regression model. Variables with P values < 0.05 in the final model were considered statistically significant.
We defined severe rhabdomyolysis as peak CK ≥5000 IU/L, while mild rhabdomyolysis was defined as peak CK of 1000–5000 IU/L.7 To assess factors associated with severity of rhabdomyolysis, univariable logistics regression analysis was performed. Variables found to have a P value of <0.1 were subsequently included in the multivariable logistics regression model. Variables with P values <0.05 in the final model were considered statistically significant. We conducted further sensitivity analysis using both forward and backward stepwise regressions to assess if the estimates differed from the multivariable linear regression.
Results
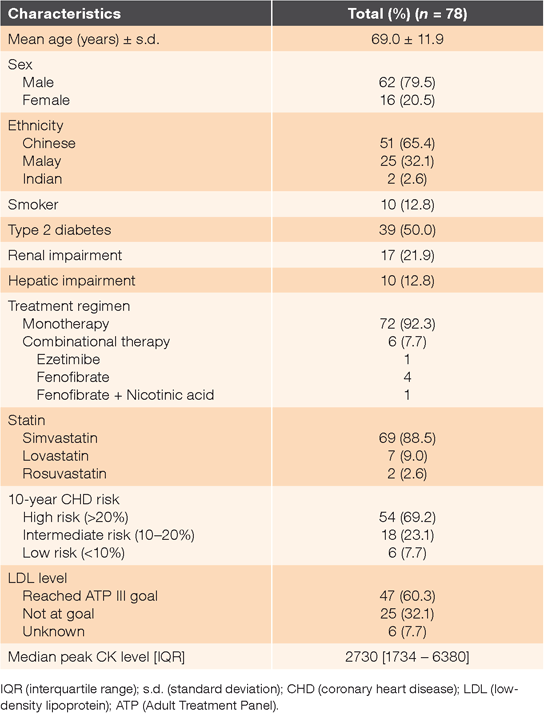
A total of 24,332 patients were screened and 78 patients fulfilled our inclusion criteria. The mean age of the study group was 69.0 years (standard deviation = 11.9 years) (Table 1). Most patients were male (79.5%) and Chinese (65.4%), 50% were diabetic and 21.9% had a history of renal impairment before hospitalization. Most patients (69.2%) were at high risk of coronary heart disease (CHD). Of the patients with statin-induced rhabdomyolysis, 60.3% were at the Third Report of the National Cholesterol Education Program-Adult Treatment Panel (NCEP-ATP III) low-density lipoprotein (LDL) goal.
|
Variables associated with an increase in peak CK
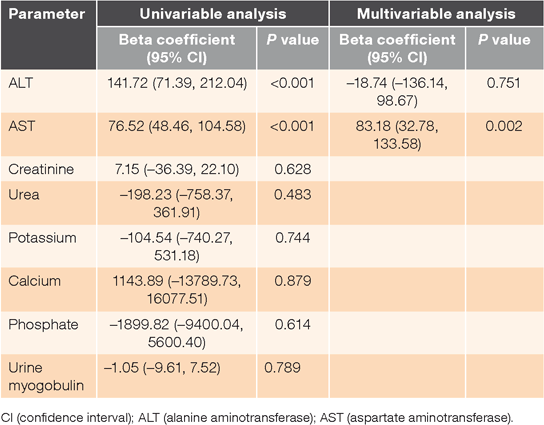
From the univariable linear regression model, alanine aminotransferase (ALT) (P < 0.001; β = 141.72) and AST (P < 0.001; β = 76.52) were selected for the multivariable linear regression model (Table 2). Creatinine, urea, potassium, calcium, phosphate and urine myoglobulin were not considered for the multivariable model as their univariable P values were >0.1. Only AST was found to be positively associated with peak CK levels in the multivariable linear regression model after adjusting for ALT levels (P = 0.002; β = 83.18) (Table 2). The results were similar when the data were analysed with both forward and backward stepwise regression.
|
Variables associated with severity of rhabdomyolysis
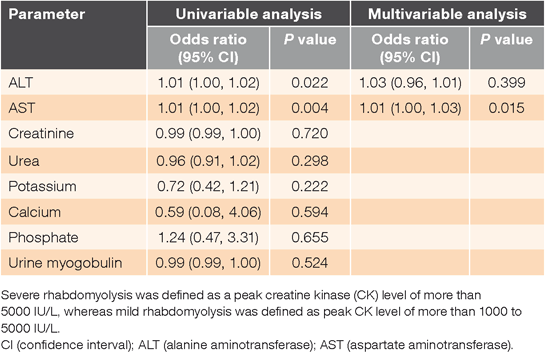
Similar results were found when we binarised the peak CK values to define severity of rhabdomyolysis. From the univariable logistics regression model, ALT (P = 0.022; OR = 1.01) and AST (P = 0.004; OR = 1.01) were selected for the multivariable logistics regression model (Table 3). Creatinine, urea, potassium, calcium, phosphate and urine myoglobulin were not considered for the multivariable model as their univariable P-values were >0.1. Only AST was found to be positively associated with severity of rhabdomyolysis in the multivariable logistics regression model after adjusting for ALT levels (P = 0.015; OR = 1.01) (Table 3). The results were similar when the data were analysed with both forward and backward stepwise regression.
|
Discussion
This is the first research report examining biomarkers associated with the peak CK values and severity of rhabdomyolysis in patients with statin-induced rhabdomyolysis, while adjusting with a comprehensive set of variables. We found AST to be associated with peak CK levels. This is congruent with the understanding that rhabdomyolysis leads to the leakage of intracellular contents from skeletal muscles, which precipitates AST elevation.1,2 AST is an indirect surrogate for the level of muscle injury.8 ALT, in contrast, is mainly released from liver cells, and is therefore less influenced by changes in muscle metabolism.7
Raurich et al. reported that the mortality rate of patients with rhabdomyolysis was higher in patients with elevated AST.9 We postulate that AST levels may aid in the prediction of complication rates among rhabdomyolysis patients and could be used as a stratification tool in addition to peak CK.
There are several limitations in our study. Study participants were recruited from tertiary care, so our findings may not be generalisable to patients with myalgia or myositis who were followed up in primary care. In addition, given that this was a retrospective study, the lack of aldolase level measurements among our patient cohort meant we were unable to incorporate it as a study variable. This is a common issue faced by other studies relying on retrospective data.
Conclusion
Elevated AST is associated with raised peak CK levels and severity of rhabdomyolysis. Clinicians may consider ordering an AST test to aid in the clinical diagnosis of rhabdomyolysis and allow for the early initiation of intravenous hydration for hospitalized rhabdomyolysis patients.
COMPETING INTERESTS
None.
ACKNOWLEDGEMENTS
We would like to thank Changi General Hospital, Singapore, for providing us with the support and resources to make this research project possible.
References
[1] Kruger D, Han J. Assessing acquired rhabdomyolysis in adults. JAAPA 2017; 30 20–6.| Assessing acquired rhabdomyolysis in adults.Crossref | GoogleScholarGoogle Scholar |
[2] Cervellin G, Comelli I, Benatti M, et al. Non-traumatic rhabdomyolysis: background, laboratory features, and acute clinical management. Clin Biochem 2017; 50 656–62.
| Non-traumatic rhabdomyolysis: background, laboratory features, and acute clinical management.Crossref | GoogleScholarGoogle Scholar |
[3] Mosshammer D, Schaeffeler E, Schwab M, Mörike K. Mechanisms and assessment of statin-related muscular adverse effects. Br J Clin Pharmacol 2014; 78 454–66.
| Mechanisms and assessment of statin-related muscular adverse effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVWltbjN&md5=995aa6618aa3f185ff193fa88858d6baCAS |
[4] Amend KL, Landon J, Thyagarajan V, et al. Incidence of hospitalized rhabdomyolysis with statin and fibrate use in an insured US population. Ann Pharmacother 2011; 45 1230–9.
| Incidence of hospitalized rhabdomyolysis with statin and fibrate use in an insured US population.Crossref | GoogleScholarGoogle Scholar |
[5] McMahon GM, Zeng X, Waikar SS, et al. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med 2013; 173 1821–8.
| 1:CAS:528:DC%2BC3sXhvVCmu7fI&md5=96a8274dee45265b5e411a913d969bb4CAS |
[6] Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015; 351 h5527
[7] Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care 2016; 20 135
| Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice.Crossref | GoogleScholarGoogle Scholar |
[8] Banfi G, Colombini A, Lombardi G, et al. Metabolic markers in sports medicine. Adv Clin Chem 2012; 56 1–54.
| Metabolic markers in sports medicine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmvVejs7o%3D&md5=25538abe9c6b8a669253b2a97aa9941bCAS |
[9] Raurich JM, Llompart-Pou JA, Rodriguez-Yago M, et al. Role of elevated aminotransferases in ICU patients with rhabdomyolysis. Am Surg 2015; 81 1209–15.