A repeat audit of primary care management of group A streptococcal pharyngitis in Northland, New Zealand 2016
Anil Shetty 1 , Clair Mills 1 , Kyle Eggleton 21 Public and Population Health, Northland District Health Board, Whangarei, New Zealand
2 Department of General Practice and Primary Health Care, The University of Auckland, Auckland, New Zealand
Correspondence to: Anil Shetty, Public and Population Health Unit, Northland District Health Board, PB 9742, Whangarei 0148, New Zealand. Email: anil.shetty@northlanddhb.org.nz
Journal of Primary Health Care 10(1) 18-24 https://doi.org/10.1071/HC17056
Published: 29 March 2018
Journal Compilation © Royal New Zealand College of General Practitioners 2018.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: One of the New Zealand Government’s Better Public Services targets was to reduce the rate of acute rheumatic fever (ARF) nationally by two-thirds by 2017. Māori children and young people are disproportionately affected by ARF in the Northland District Health Board region. General practice contributes to ARF prevention in detecting and appropriately treating group A streptococcal (GAS) pharyngitis. An audit in 2012 suggested improvements in adherence to national guidelines were needed.
AIM: The aim was to reassess general practice adherence to national guidelines for the management of GAS pharyngitis in Northland, New Zealand, following implementation of the national Rheumatic Fever Prevention Programme.
METHODS: Throat swab and dispensing data were obtained and analysed for children and young people aged 3–20 years who attended general practice in Northland between 1 April and 31 July 2016 and had laboratory-proven GAS pharyngitis.
RESULTS: Between 2012 and 2016, the number of throat swabs carried out in general practice more than doubled, and amoxicillin was more commonly prescribed. The proportion of GAS pharyngitis patients in general practice not receiving recommended antibiotics, or receiving an inadequate length of treatment or no prescription, has not reduced. There are significant differences in the management of care for Māori and non-Māori patients, with much higher risk of ARF for Māori.
Discussion: The management of GAS pharyngitis by general practice in Northland remains substandard. Implicit bias may contribute to inequity. Focused engagement with identified subgroups of general practices and practitioners who disproportionately contribute to non-guideline prescribing should be further investigated.
KEYWORDS: Pharyngitis; prevention and control; primary health care; rheumatic fever; school health services; Streptococcus pyogenes
| WHAT GAP THIS FILLS |
| What is already known: An audit in 2012 estimated that one-in-five children and young people with a laboratory-proven GAS-positive throat swab seen in general practice in Northland did not receive national guidelines recommended treatment, despite the high incidence of acute rheumatic fever (ARF) in our community. Changing general practice prescribing behaviour can be challenging. |
| What this study adds: Despite 4 years of implementation of the national Rheumatic Fever Prevention Programme and a decline in ARF cases in Northland, the quality of general practice management of this common condition remains suboptimal and inequitable. Given the failure of guidelines and other resources to address this, implicit bias could be a contributing factor. This issue needs to be further explored and addressed in general practice. |
Introduction
The New Zealand Ministry of Health set a national goal of a two-thirds reduction in the incidence of acute rheumatic fever (ARF) cases by 2017. In addition to national targets, regional targets for District Health Boards (DHBs) have also been set.1 Over 40,000 children and young people aged 3–20 years live in the Northland District Health Board (NDHB) region, 52% of whom are Māori.2 Rheumatic fever disproportionally affects Māori children, and Northland has one of the highest rates of ARF nationally. Ethnic inequality was demonstrated in a 2012 review of ARF cases in Northland.3 The review highlighted that 95% of cases were Māori children, with a mean age of 11.4 years. Māori children aged 5–15 years had an ARF incidence of 78/100,000 per year, compared to non-Māori of 4.6/100,000 per year.3
By mid-2016, in addition to the existing school-based rheumatic fever prevention programmes that were operating in Northland since 2012, an additional three programmes were introduced to enhance equitable access to throat swabbing services (covering a total of 9500 children attending decile one to three schools). These programmes are delivered by kaimahi (community health workers) with nurse oversight. They offer throat swabbing services to children with sore throats and free antibiotic treatment if Group A Streptococcus (GAS) positive.
The annual number and incidence of ARF has declined substantially since 2012, from 15–20 cases per year to less than five per year in recent years. Four cases of ARF were notified in school-aged children in Northland during 2016.4
In our 2012 audit, we estimated that one-in-five children and young people with GAS-positive pharyngitis seen in Northland general practice did not receive recommended treatment5,6 despite the high incidence of ARF in Northland. This was 18% lower than in the school programmes, where more than 98% of children received guideline-recommended treatments (P < 0.001).7 Quarterly reporting from the school programmes shows this quality has been maintained, so school programmes have not been included in this second audit.
Timely access to appropriate treatment for GAS pharyngitis is important. National guidelines, such as the 2014 New Zealand National Heart Foundation Guidelines5 and the 2012 New Zealand Primary Care Handbook guidelines for rheumatic fever prevention,6 suggest treatment with once daily amoxicillin for 10 days as soon as possible. The rationale for the use of amoxicillin is to improve adherence.5,6,8 In New Zealand, most children and young people access general practice for their health needs. Consequently, general practice plays a critical function in treating GAS pharyngitis and preventing ARF.
The aim of this audit was to re-assess adherence by prescribers in general practices in Northland to national GAS pharyngitis guidelines, following 4 years of implementation of the national Rheumatic Fever Prevention Programme (RFPP)9 and specific activities implemented after the 2012 audit. These include general practitioner (GP) continuing medical education (CME) sessions, dissemination of the updated 2014 national sore throat guidelines and Ministry of Health RFPP resources,10 and a focus on increased nurse management of sore throat in general practices, using standing orders and dispensing antibiotics via Medical Practitioner Supply Order (MPSO) to high-risk children.
The objective of this study was to establish the proportion of Māori and non-Māori children and young people aged 3–20 years who received guideline-recommended management for confirmed GAS-positive pharyngitis in general practice in Northland, and compare this with the results from the previous 2012 audit.
Methods
We used the same methods as in the 2012 audit. Data on throat swabs taken from children and young people aged 3–20 years were accessed from Northland’s laboratories (NDHB hospital laboratories (DHBL) and Northland Pathology Laboratory (NPL)) for 1 April to 31 July 2016, as in the 2012 audit. Throat swabs from inpatients processed in DHBL were excluded from the analysis. Dispensed medication data were accessed from the New Zealand Health Information Service (NZHIS) Pharmaceutical Claims Data Mart (PHARMS) for the study timeframe. This dataset includes dispensing claims from pharmacists across New Zealand. To allow for delays in prescriptions being filled, the timeframe was extended for an additional 9 days. Also included in the PHARMS dataset are patient identifiers (national health index numbers (NHIs)), dispensing date, therapeutic class and name of medicine, daily dose and days supplied.11 Inclusion criteria were children and young people aged 3–20 years with a positive GAS throat swab during the study timeframe. Swab results were matched, in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA), by NHI to dispensed medication data.
The national guidelines recommend treatment with amoxicillin, penicillin V, benzathine penicillin G, erythromycin ethyl succinate or roxithromycin; amoxicillin/clavulanic acid and clindamycin were also included as these can be used for treatment of recurrent GAS.5 Similar to the previous audit, we assumed that antibiotic prescriptions were made at an appropriate dose for the child’s weight. Statistical analysis (chi-square tests) was completed using Stata version 11 (StataCorp, College Station, TX, USA).
The study was reviewed and approved by the NDHB locality assessment and the Northland Primary Health Organisation (PHO) clinical governance group.
Results
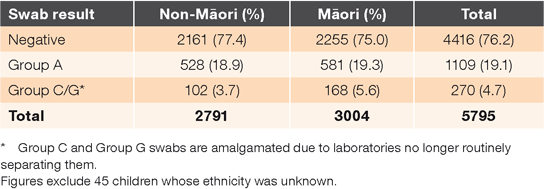
In total, Northland general practices collected 5795 throat swabs during the audit period (Table 1) for children and young people aged 3–20 years (participants). This was more than double the number of swabs taken in the same period in 2012 (2365 swabs). Nearly half (46.0%) were from males (compared with 44.4% in 2012). Approximately half (3004; 51.8%) were from Māori participants and 2791 (48.2%) from non-Māori participants, compared with 46.9% and 53.1% respectively in the 2012 audit. For all swabs processed, the mean age was 9.1 years for Māori and 9.6 years for non-Māori participants.
|
The GAS-positive rates per 100 throat swabs were similar for Māori (19.3) and non-Māori participants (18.3). This is also similar to the GAS- positive rates (18.7 for Māori and 18.6 non-Māori) in the 2012 audit.
Complete prescription data (medication dispensed, collection date and duration of course) was available for only 389/1109 (35.1%) of participants with GAS-positive swabs (Table 2), compared with 55.2% complete prescription data in the 2012 audit. Of participants with complete data, 347/389 (89%) were given recommended antibiotics for 10 days, within 9 days of the swab result, meeting all criteria in the national guidelines. The 2014 updated guidelines recommend treatment without any delay, as the evidence to support delaying treatment is insufficient.5 However, Māori young people were significantly less likely than non-Māori participants to receive antibiotics according to all criteria in the guidelines (P < 0.05; Table 3).

|

|
A small proportion of participants (19/389; 4.9%) received a recommended antibiotic, but not for 10-days’ duration; 21/389 (5.4%) received a non-guideline-recommended antibiotic, and two participants received the recommended antibiotic but did not collect it within 9 days of the GAS-positive swab result.
Dispensing data were missing for 138 (12.4%) participants with GAS-positive swab results; of these, 99/138 (71.7%) were Māori, a significantly greater proportion than for non-Māori (P < 0.001). The duration of antibiotic prescription was not possible to ascertain for the remainder of the dispensing data, most of which were oral suspensions; 576/1109 (52%) had ‘zero’ days recorded as the duration of antibiotic dispensed.
Overall, 944/1109 (85.1%) participants with a GAS-positive result received a recommended antibiotic, but there was a significant difference between Māori and non-Māori participants: 479/528 (90.7%) of non-Māori participants and 465/581 (80.0%) of Māori participants received a recommended antibiotic (P < 0.001). Most (811/1109, 73.1%) received amoxicillin; non-Māori 407/528 (77.1%) and Māori participants 404/581 (69.5%), P = 0.8. The dataset does not specify whether once-daily dosing was prescribed.
If participants prescribed ‘zero’ days of antibiotic treatment were included in the complete prescription dataset, then the proportion who received the recommended antibiotic and duration of course would be slightly lower (923/1109, 83.2%) than estimated from analysis of the ‘complete data’ subset (ie 89.0%).
The mean NZDep201312 score for non-Māori participants was 6.89 (95% CI 6.68 – 7.09), and for Māori participants, it was 8.84 (95% CI 8.70 – 8.98). A logistic regression was completed to analyse the relationship between participants’ demographics (ethnicity, aged over 13 years and NZDep2013 Index) and whether recommended antibiotics were prescribed (assuming prescription annotated for zero days’ supply were in fact for an appropriate duration). There was significant association between Māori (odds ratio 0.43, P < 0.001) or participants aged over 13 years (odds ratio 0.35, P < 0.001) and prescribing of recommended antibiotics. However, there was no significant association for NZDep2013 (odds ratio 0.98, P = 0.726).
Discussion
Although there has been a pleasing reduction in ARF incidence in school-aged children in Northland since 2012, effective management of sore throats in general practice is critical to maintaining this. Sore throat is a prevalent presentation to New Zealand general practices with 2.8–10 per 100 presentations.13
The significant increase in throat swab volumes since 2012 may not represent a true increase in sore throat presentations to general practice, and there are likely to be multifactorial reasons for this. However, ‘zero fees’ access to general practice was extended to children aged <13 years from 1 October 2014 in Northland, pre-empting the national policy implemented from 1 July 2015. An initial analysis of general practice consultations comparing 2014 and 2015 data shows utilisation by children aged <13 years increased by 16%, which could account for some of the volume increase.14 Increased demand from parents and caregivers for throat swabbing as a result of promotional campaigns15 or changes in general practice diagnostic behaviour may also have contributed.
In this audit, 85.1% of participants (80.0% for Māori, 90.7% for non-Māori) with a GAS-positive result received one of the recommended antibiotics, a slightly lower proportion than that estimated in the 2012 audit (89.6% overall; 86.5% of Māori and 92.3% of non-Māori participants). Amoxicillin was more often prescribed in 2016 than in 2012.
Although a higher proportion of the subset of participants with complete prescription data in 2016 than in 2012 received treatment according to guidelines, including correct length of course (89% compared with 80%), this was significantly lower for Māori participants despite their significantly higher risk of ARF. One-in-five Māori participants compared with 1 in 10 non-Māori with laboratory-confirmed GAS pharyngitis did not receive recommended antibiotics in this audit.
In the best-case scenario, that is, including all participants who were prescribed the correct antibiotic but with no ‘duration of course’ data (83.2% in 2016 compared with 82.4% in 2012), the estimate of one-in-five children and young people aged 3–20 years seen in general practice in Northland with laboratory-confirmed GAS pharyngitis receiving inadequate treatment is unchanged between the two audits.
Māori participants were significantly more likely to have no prescription data available than non-Māori, and the proportion overall in this category was higher than in the 2012 audit (12.4% in 2016 compared with 6.8% in 2012). In our previous study, we suggested that one reason for this may be greater use of MPSO medications than in 2012, and dispensing antibiotics directly to Māori patients. Given the promotion of empiric treatment and MPSO dispensing for high-risk children by the RFPP, this is a possibility. However, earlier research has shown that even when MPSO dispensing is taken into account, Māori have lower rates of being prescribed antibiotics.16 The other possibilities are that some Māori participants were given a prescription but did not collect medications from a community pharmacy or that some did not receive either a prescription or a MPSO-dispensed antibiotic.
People living in the most socioeconomically deprived areas of New Zealand are less likely to collect prescriptions due to cost (more than four-fold for adults and 5.6-fold for children) compared with the people living in the least deprived areas, and both Māori adults and children are over-represented in this category.17 Another concern is that Māori patients are more likely to delay obtaining prescriptions due to associated costs, compared to non-Māori.18 Given the limitations of the prescribing data in this study, it is not possible to ascertain the true reason for differences in antibiotic dispensing, but logistic regression analysis of our data indicated that age (≥13 years, where there is a prescription charge) and ethnicity contributed significantly to whether antibiotics were dispensed, whereas the NZDep2013 score did not.
Our study has similar limitations to the earlier study; we were able to analyse only the management of children and young people with laboratory-proven GAS pharyngitis, rather than all people presenting with sore throat to general practices in Northland. A 2005 study about sore throat management by New Zealand general practices showed that the rates of throat swabbing were up to 10-fold lower than antibiotic prescribing rates.13 It may be that higher-risk children (according to Heart Foundation Guidelines) are being prescribed antibiotics appropriately without having throat swabs taken, although the volume of swabs taken has more than doubled since 2012, suggesting otherwise. This is also a greater proportionate increase than the increase in general practice consultations for this age group in the same time period.
The proportion of participants with a GAS positive throat swab remains at similar levels to the 2012 audit, indicating that children and young people aged 3–20 years attending general practice are more likely to have GAS pharyngitis than children having a throat swab in schools, but it also suggests that GAS pharyngitis in general practice in Northland may have been underdiagnosed previously.
Conclusion
High rates of ARF continue to affect Māori children and young people in Northland. This repeat audit suggests that general practice management of sore throat remains significantly poorer in quality than management in school-based throat swabbing programmes in Northland, where more than 98% of school-aged children receive treatment according to guidelines, including once-daily amoxicillin dosage to support adherence.
Prescribers in general practice should ensure that all children and young people presenting with sore throat are managed appropriately. CME workshops, dissemination of national guidelines and promotion of nurse-led care since 2012 appear to have been ineffective in changing general practice prescribing behaviour. Electronic clinical pathways, recently implemented in Northland, may support improved adherence to guidelines.19 While promoting empirical treatment and MPSO dispensing for high-risk children as recommended by the Ministry of Health is important,10,19,20 this does not address the issue of non-guideline prescribing.
There may be implicit health provider bias operating in general practice, negatively impacting on engagement, communication and treatment of their patients, as suggested by recent qualitative research on ARF in Northland.21,22 Further exploration of this is needed. In addition, focused engagement with identified subgroups of general practices or GPs who disproportionately contribute to non-guideline prescribing should be attempted.
COMPETING INTERESTS
None.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the New Zealand Health Information Service, and Northland District Health Board laboratory and Northland Pathology laboratory staff for their support in providing data for this audit.
References
[1] Ministry of Health, New Zealand. Progress on the better public services rheumatic fever target. Wellington: Ministry of Health. [cited 2017 April 14]. Available from: http://www.health.govt.nz/about-ministry/what-we-do/strategic-direction/better-public-services/progress-better-public-services-rheumatic-fever-target[2] Statistics New Zealand. Population projections prepared for Ministry of Health - Northland DHB projected population aged 0–20 years by sex at 30 June 2014–43 (2013-Base). Wellington: Statistics New Zealand; 2015.
[3] Robin A, Mills C, Lennon D, Tuck R. The epidemiology of rheumatic fever in Northland, 2002 to 2011. N Z Med J. 2013; 126 46–52.
[4] Ministry of Health. Previous BPS target: reduce rheumatic fever. Wellington: Ministry of Health; 2017. (Updated 09 June). [cited 2017 November 22]. Available from: https://www.health.govt.nz/about-ministry/what-we-do/strategic-direction/better-public-services/previous-bps-target-reduce-rheumatic-fever
[5] Heart Foundation of New Zealand. Group A Streptococcal Sore Throat Management Guideline. 2014 Update. Auckland: Heart Foundation of New Zealand; 2014.
[6] New Zealand Guidelines Group. New Zealand Primary Care Handbook 2012. 3rd ed. Wellington: Ministry of Health; 2012. [cited 2017 January 30]. Available from: www.health.govt.nz/publication/new-zealand-primary-care-handbook-2012
[7] Shetty A, Mills C, Eggleton K. Primary care management of group A streptococcal pharyngitis in Northland. J Prim Health Care. 2014; 6 189–94.
[8] Lennon D, Kerdemelidis M, Arroll B, Sharpe N. Once-daily amoxicillin for group A streptococcal (GAS) sore throat as the other first-line option: a clarification of the NZ sore throat guidelines. N Z Med J. 2011; 124 116–9.
[9] Ministry of Health. Rheumatic fever. Wellington: Ministry of Health; 2017. [cited 2017 November 22]. Available from: https://www.health.govt.nz/our-work/diseases-and-conditions/rheumatic-fever
[10] Ministry of Health. New Zealand. Rheumatic fever resources for health professionals. Wellington: Ministry of Health. [cited 2017 April 14]. Available from: http://www.health.govt.nz/our-work/diseases-and-conditions/rheumatic-fever/rheumatic-fever-resources/rheumatic-fever-resources-health-professionals.
[11] Ministry of Health. New Zealand. Pharmaceutical collection. Updated 24/06/15. Wellington: Ministry of Health. [cited 2017 April 14]. Available from: http://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/pharmaceutical-collection
[12] Atkinson J, Salmond C, Crampton P. NZDep2013 Index of Deprivation. Wellington: Department of Public Health, University of Otago; 2014.
[13] Kljakovic M, Crampton P. Sore throat management in New Zealand general practice. N Z Med J. 2005; 118 U1609.
[14] Shetty A, Mills C. Primary care utilisation by 6 to 12 year old children in Northland: pre- and post-implementation of zero-fee visits. Whangarei, Northland: Public and Population Health Unit, Northland DHB; 2016.
[15] Mardani J, Calder L, Haydon-Carr J, et al. Throat swabbing for the primary prevention of rheumatic fever following health information. N Z Med J. 2011; 124 46–51.
[16] Norris P, Horsburgh S, Keown S, et al. Too much and too little? Prevalence and extent of antibiotic use in a New Zealand region. J Antimicrob Chemother. 2011; 66 1921–6.
| Too much and too little? Prevalence and extent of antibiotic use in a New Zealand region.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFClsr0%3D&md5=59d5c82f02b53c89fe2d07eacc76975bCAS |
[17] Ministry of Health. New Zealand. Annual update of Key Results 2015/16. New Zealand Health Survey. Wellington: Ministry of Health. [cited 2017 April 14]. Available from: http://www.health.govt.nz/ publications/annual-update-key-results-2015–16-new-zealand-health-survey
[18] Jatrana S, Crampton P, Norris P. Ethnic differences in access to prescription medication because of cost in New Zealand. J Epidemiol Community Health. 2011; 65 454–60.
| Ethnic differences in access to prescription medication because of cost in New Zealand.Crossref | GoogleScholarGoogle Scholar |
[19] Bhattacharyya O, Reeves S, Zwarenstein M. What is implementation research? Rationale, concepts, and practices. Res Soc Work Pract. 2009; 19 491–502.
| What is implementation research? Rationale, concepts, and practices.Crossref | GoogleScholarGoogle Scholar |
[20] Ministry of Health. Using Practitioner Supply Orders and Standing Orders in the Rheumatic Fever Prevention Programme. Published online 17 February 2015. Wellington: Ministry of Health, 2015. [cited 2017 April 14]. Available from: http://www.health.govt.nz/publication/using- practitioner-supply-orders-and-standing-orders- rheumatic-fever-prevention-programme
[21] Anderson A, Mills C, Eggleton K, et al. Northland District Health Board Research Report: Whanau experiences of the diagnosis and management of Acute Rheumatic Fever for tamariki in Te Tai Tokerau, Aotearoa. Te Kupenga Hauora Māori. Auckland: University of Auckland; 2015.
[22] Hokomau CA. What you can’t see can hurt you: how do stereotyping, implicit bias and stereotype threat affect Māori health? MAI J. 2016; 5 124–136.
| What you can’t see can hurt you: how do stereotyping, implicit bias and stereotype threat affect Māori health?Crossref | GoogleScholarGoogle Scholar |


