Implementation of an antimicrobial stewardship program in an Australian metropolitan private hospital: lessons learned
Jeannine A. M. Loh A E , Jonathan D. Darby A B , John R. Daffy A B , Carolyn L. Moore A C , Michelle J. Battye A , Yves S. Poy Lorenzo D and Peter A. Stanley A BA St Vincent’s Private Hospital Melbourne, Vic. 3065, Australia.
B Infectious Diseases Unit, St Vincent’s Hospital Melbourne, Vic. 3065, Australia.
C Australian Catholic University (Honorary Clinical Fellow), Vic. 3065, Australia.
D Pharmacy Department, St Vincent’s Hospital Melbourne, Vic. 3065, Australia.
E Corresponding author. Email: jeannine.loh@svha.org.au
Healthcare Infection 20(4) 134-140 https://doi.org/10.1071/HI15015
Submitted: 17 July 2015 Accepted: 31 August 2015 Published: 14 September 2015
Journal Compilation © Australasian College for Infection Prevention and Control 2015
Abstract
Introduction: While there is literature on the implementation and efficacy of antimicrobial stewardship (AMS) programs in the public hospital setting, there is little concerning their implementation in the private hospital setting. Resources to guide the implementation of such programs often fail to take into consideration the resource limitations and cultural barriers faced by private hospitals. In this paper we discuss the main obstacles encountered when implementing an AMS program at a private hospital and methods that were used to overcome them.
Methods: In 2012, St Vincent’s Private Hospital Melbourne implemented an AMS program that was tailored to suit the requirements and limitations faced by private hospitals. Baseline data was collected to determine areas of priority. Cultural barriers were overcome by forming relationships between AMS and non-AMS personnel, involving key clinical stakeholders when developing hospital policies, and having ample support from hospital executives. We also modified our approach to conventional AMS interventions so that typically resource-intensive projects could be carried out with minimal resources, such as the restriction of antimicrobials via a two-stage post-prescription review model.
Results: Through our AMS program, we have been able to implement multiple initiatives including a formulary restriction, significantly reduce aminoglycoside use, develop hospital guidelines and regularly contribute data to national surveillance programs.
Conclusion: While there are guidelines available to help develop an AMS program, these guidelines need to be adapted to suit different hospital settings. Private hospitals present a unique challenge in the implementation of AMS programs. Identifying and addressing barriers specific to an individual institution is vital.
Implications
-
Antimicrobial stewardship programs need to be tailored to suit the needs and available resources of individual institutions.
-
Endorsement and support from hospital executives, involvement of key clinical stakeholders and awareness of prescribing etiquette is crucial for successful implementation of antimicrobial stewardship programs.
Introduction
Antimicrobial resistance has been heralded as one of the greatest challenges to human health today.1 The indiscriminate use of antibiotics has led to the development of antibiotic-resistant organisms which has been associated with increased morbidity, mortality and healthcare costs.2–4 It is estimated that up to 50% of antimicrobial courses prescribed in hospitals overseas and in Australia are inappropriate,1,5 and there is evidence to support the ability of Antimicrobial Stewardship (AMS) programs to improve the quality of antimicrobial use, improve patient outcomes, minimise resistance,6–9 and reduce excessive antimicrobial prescribing without worsening patient outcomes.10 In 2012 the Australian Commission on Safety and Quality in Health Care (ACSQHC) introduced the AMS criterion in the new National Safety and Quality in Health Service (NSQHS) Standards.11 This required all Australian hospitals, both public and private, to implement an AMS program in order to meet hospital accreditation standards.
Despite the abundance of literature on AMS,12 there is little concerning the implementation of an AMS program in the private hospital setting. A survey of Australian hospitals13 found that only 4.8% of private hospitals restricted the use of broad-spectrum antimicrobials versus 93.8% in the public metropolitan sector. Resources available to guide the implementation and development of AMS programs5 often centre around the public healthcare sector and fail to take into consideration the difference in patient case-mix. For example, national guidelines often place emphasis on policies for medical conditions, such as community-acquired pneumonia, as most public hospital patients (74%) are admitted for medical treatment.14 In contrast, 41% of private hospital patients are admitted for surgery and only 38% admitted for medical treatment.14
Unlike the public hospital, where medical staff work in speciality teams, in the private healthcare sector medical specialists admit their own patients and are individually responsible for their care. Problems may arise in private hospitals as a result of this difference in workplace dynamics as long-standing cultures of ‘prescribing etiquette’ are amplified.15 In an institution that deals predominantly with doctors at the top of the medical hierarchy, an environment of autonomous decision-making with regard to prescriptions is often widely accepted and unchallenged by other healthcare staff.15 These unwritten rules often lead to an ethos of ‘non interference’ with prescriptions written by other medical officers which may result in suboptimal prescribing of antimicrobials.15
A recent survey of healthcare workers at Australian private hospitals16 identified the following as attitudes to AMS which could prove to be barriers when implementing an AMS program in a private hospital: (i) a low proportion of healthcare staff (nursing staff in particular) being aware of AMS, (ii) the challenge of making antimicrobial resistance a relevant local issue among health professionals at the hospital in which they practice, and (iii) significant disengagement in issues revolving around antimicrobial use amongst clinical stakeholders at the hospital, despite formal endorsement and sponsorship of AMS by the hospital executive.
Reviews of AMS programs from around the world have shown that the most successful interventions are those that have been tailored to local conditions.12 The likelihood of producing behavioural change in professional practice improves if interventions are adapted to address institution-specific barriers and limitations.17,18 Qualitative research in AMS has largely been performed in public hospital settings; as such, finding an optimal and sustainable AMS program model for a private hospital setting and its prescribing culture presents unique challenges.17 Literature exists which demonstrates the barriers that may be encountered in the private hospital system16 and possible methods to overcome them.19 The aim of our paper is to demonstrate one model for implementing an AMS program at a private hospital based upon our experiences.
Methods
Setting
St Vincent’s Private Hospital Melbourne (SVPHM), is a metropolitan private hospital comprised of three campuses (Fitzroy, East Melbourne and Kew) with ~400 overnight-stay beds, 70 day-case beds and eight ICU beds. Medical and surgical specialties are represented, including cardiothoracics, neurosurgery and obstetrics. In 2012, SVPHM started the development of its AMS program.
Development of the AMS committee (AMSC) and AMS team (AMST)
In preparation for the new AMS criterion in the 2012 NSQHS standards,11 hospital executives approved funding for the implementation of an AMS program at SVPHM. Three infectious diseases (ID) physicians who were already well known to the institution were selected to participate in the AMS program on a consultative basis. Together with a medical microbiologist, pharmacist, nursing representatives from infection prevention, and executive representatives (the director of medicine and general nursing director), they formed the AMS committee (AMSC). The AMSC was responsible for ensuring compliance with the NSQHS standards11 with a smaller subgroup, the AMS team (AMST), being responsible for implementing and directing the activities of the AMS program. The AMST comprised of the ID physicians, pharmacist and infection control nurses. While the AMSC would meet on a quarterly basis, the AMST would meet regularly to discuss projects.
Following recommendations set out by the NSQHS standards,11 the AMSC was integrated into the hospital’s organisational structure and reported to the Infection Prevention Committee, the Drugs and Therapeutics Committee and other various groups or committees when required.
Implementation of an AMS program in the private hospital system
From the outset, we identified the importance of the program being inclusive of all healthcare workers, in particular nurses, pharmacists, physicians, surgeons and anaesthetists. To make staff more aware of the presence of the AMST in the hospital, an AMS bulletin introducing the AMS team and topics relating to AMS was produced and circulated to all accredited medical practitioners (AMPs) at SVPHM. Cotta et al.16 identified the importance of nursing staff in particular as playing an important role in AMS interventions in private hospitals. To ensure that nursing staff were educated on AMS, lectures on AMS were incorporated into the nursing graduate program, in-house education was held for existing nursing staff and nursing unit managers engaged to promote awareness of issues surrounding antimicrobial use. Prescribers were educated on issues pertaining to AMS to overcome the barrier of prescribing autonomy. ID physicians repeatedly attended all medical craft groups (which is a group of prescribers from that speciality who meet regularly to discuss issues relevant to their field). Where no craft groups existed, prescribers were engaged on a one-to-one basis. When implementing AMS projects, we focused on patient safety and emphasised the possible ramifications of inappropriate prescribing to individual prescribers. In this way, we were able to better engage AMPs in activities and issues concerning AMS as projects were made more meaningful to them as individual prescribers. We also modified our approach to typical AMS interventions so that projects could be carried out with limited resources. Cultural barriers, such as a lack of ‘clinician buy-in’ to alter prescribing practice, were addressed by involving key clinical stakeholders in the development of hospital AMS guidelines. This can be seen in the following AMS projects that we have launched at SVPHM.
I. Restriction of antimicrobials
Guidelines for AMS set out by the ACSQHC5 suggest five essential strategies for all hospitals (such as monitoring of resistance and establishing an approval-based formulary restriction system) and several extra activities that can be implemented according to local priorities and resources. While some of these strategies are common to most hospitals, for example, selective reporting of susceptibility testing results,13 others are more suited to public metropolitan hospitals and are less applicable to the limited resources and structure of the private hospital setting. An example of this is the establishment of a formulary restriction and approval system. Whilst widely adopted at public tertiary hospitals and considered an essential AMS activity, private hospitals often lack the resources and ability to perform this intervention, making this type of intervention unsustainable. Furthermore, preauthorisation and restriction methods come with their own disadvantages such as a loss of autonomy for prescribers, being resource-intensive (e.g. the potential need for all-hours decision support)20 and possibly compromising patient care if there is a significant delay in the administration of an antimicrobial.5,21 The impact of restrictive interventions is also short-lived if used alone.10 Qualitative studies have found restrictive interventions which limit prescribing via an approval process are often least favoured by clinical staff.16 Senior physicians, in particular, are more likely to override recommendations from an approval-based process,22 making this type of intervention even harder for private hospitals which mainly deal with medical consultants. To overcome these barriers at SVPHM, a formulary restriction was implemented via a two-step post-prescription review model which has been shown to be sustainable, cost-effective and easily applied even in the setting of limited resources.23 In this method, a group of 23 antimicrobials was classified as ‘restricted,’ with a subset of those antimicrobials, for example linezolid and amphotericin B, being further restricted for use only after approval by an ID physician. In the first stage, if one of these restricted antimicrobials was dispensed by a pharmacist, they would alert the AMS pharmacist who would review the patient individually. In the second stage, information about the case would be conveyed to the ID physicians who would then provide immediate feedback and intervene if required. In this way, patients prescribed restricted antimicrobials were able to be reviewed by the AMST in a timely manner without diminishing prescriber autonomy or creating a barrier to initiating therapy. This made the intervention more acceptable to prescribers and provided opportunities for face-to-face feedback and education of prescribers, post-prescription review.22,23 By using a non-confrontational approach, we were able to encourage prescribers to engage with AMS practitioners if they had questions, thereby projecting the image of a facilitator, as opposed to a policing body.24
II. Development of adult surgical antibiotic prophylaxis guidelines and reduction of prophylactic gentamicin
In 2013 a point prevalence survey (n = 324) on antimicrobial use at our hospital was performed as part of the National Antimicrobial Prescribing Survey (NAPS). It showed that 54% of all antimicrobials were prescribed for surgical prophylaxis. Other audits showed a disparity in regimens used for standard surgical prophylaxis, with a significant proportion of patients (44%) receiving prophylactic antibiotics beyond 24 h. Baseline usage data also showed that aminoglycoside use at SVPHM was intrinsically linked to surgical prophylaxis, with 70% of gentamicin being prescribed for the insertion and removal of indwelling urinary catheters between 2012 and 2013 (an indication that essentially does not require an antimicrobial). The need to change this prescribing practice was spurred on by a research article in the Medical Journal of Australia which highlighted the toxicities of gentamicin following 103 patients who developed severe gentamicin-associated vestibulotoxicity even if they only received a single low dose.25
Consequently, the development of a local, aminoglycoside-free surgical antibiotic prophylaxis guideline became a priority. While the core of our advice regarding surgical antibiotic prophylaxis was based on the Therapeutic Guidelines: Antibiotic,26 the AMST made some alterations based on local resistance data. Recognising the central importance of ‘clinician buy-in’ to alter prescribing practice, when developing the adult surgical antibiotic prophylaxis guidelines, the AMST liaised with all speciality craft groups and involved key prescribing stakeholders to encourage greater acceptance of the final publication.24,27 The most established craft groups included specialties such as orthopaedics, internal medicine and peri-operative physicians, anaesthetics and obstetrics. Upon its release, formal support of the guidelines from the medical director was instrumental in giving it credibility, as well as providing the authority to question prescribers who chose not to follow either the Therapeutic Guidelines: Antibiotic26 or the SVPHM surgical antibiotic prophylaxis guidelines.
Following concerns of toxicity, gentamicin was removed from all ward medication rooms (with the exception of theatres) to reduce ‘easy access’ and improve prescribing accountability as the AMST was alerted each time gentamicin was dispensed. While this reduced gentamicin use rapidly, persuasive interventions were also employed to produce longer lasting effects. A recent Cochrane review demonstrated that while restrictive interventions produced immediate significant effects, if used alone, the effects of these interventions diminish over time whilst persuasive interventions may produce greater effects after 12 months.10 As part of the educational process, a memo concerning gentamicin use at SVPHM and its associated toxicities was written by one of the AMS ID physicians and circulated to all doctors practicing at SVPHM. Intensive education was carried out by the AMST with nursing and pharmacy staff educated to raise awareness of issues surrounding gentamicin use, whilst the AMS ID physicians would approach individual prescribers to discuss their use of gentamicin and suggest alternative antibiotics if needed.
III. Switching from intravenous to oral antibiotics
An early switch from intravenous (IV) to oral antibiotics has demonstrated several benefits including reduced costs,28 shorter length of stay, improved patient comfort,29 and fewer complications as a result of IV therapy without negatively affecting clinical outcome.30 When we implemented a program to promote the timely conversion from IV to oral antimicrobials, we employed several strategies including development of posters with an IV to oral guide, use of screensavers on hospital computers to remind clinical staff to check if their patients are eligible to change to oral antibiotics, and production of lanyard cards for pharmacy staff to guide the IV to oral decision process. All pharmacists were educated on how to identify patients who may be eligible to change to oral antibiotics and were given stickers to place on medical charts to highlight eligible patients to prescribers. Education of medical staff was achieved through a memo sent out by the medical director with the accompanying guideline. Widespread education of nurses was achieved through the infection control team who educated ward nurses of the risk of intravascular line infections and need to change to oral preparations earlier.
Results
Seven months following the launch of the SVPHM surgical antibiotic prophylaxis guidelines, adherence to either the Therapeutic Guidelines: Antibiotic26 or the SVPHM guidelines for surgical prophylaxis increased from 73% pre-intervention to 87% post-intervention. The prolonged use of surgical antimicrobial prophylaxis past 24 h also decreased from 44% to 38%. However, our sample size was small, with only 60 patients being audited. We expect a larger sample size when we perform our next whole hospital point prevalence survey for NAPS 2015. Through our audits and continued monitoring of ‘restricted’ antimicrobials, we also identified that during surgery it was often the anaesthetists who decided which surgical antibiotic prophylaxis regimen the patient would receive as well as duration in many cases. These results highlighted to us the important role that anaesthetists played in surgical prophylaxis. As a result, more effort was dedicated to engaging anaesthetists to champion adherence to surgical antibiotic prophylaxis guidelines.
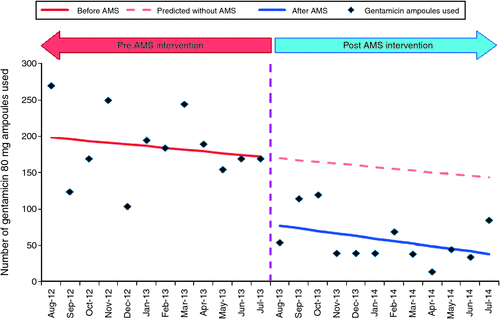
The use of prophylactic gentamicin at SVPHM also significantly decreased following the combination of persuasive and restrictive approaches to minimise its use. A comparison of the 12-month period before the intervention to the 12-month period following the intervention demonstrated a decrease in gentamicin use to approximately one-third of the original quantity at the Fitzroy campus (Fig. 1) with a mean monthly usage of 80 mg gentamicin ampoules reducing from 186 ± 49 to 58 ± 33 [mean difference 128 (P < 0.001; 95% CI, 92 – 163)]. In theatres at East Melbourne and Fitzroy, where the use of gentamicin would be primarily for surgical prophylaxis (in particular, for the insertion of indwelling urinary catheters), the combined number of gentamicin 80 mg ampoules requested for supply fell from 420 ampoules for the month of August 2012 to 15 ampoules for the month of August 2014. The use of gentamicin has since continued to trend downwards, with low rates of aminoglycoside use at SVPHM being sustained.
|
Results of other projects, such as the IV to oral conversion project, are not yet available. However, our continued participation in NAPS has shown an improvement in the appropriateness of antimicrobial prescribing, from 60% in 2013 to 73% in 2014.
As a result of the implementation of our AMS program at SVPHM, we have been able to engage in the following activities: the development of adult surgical antibiotic prophylaxis guidelines, minimisation of inappropriate aminoglycoside use, formulary restriction via a two-stage prospective audit and feedback model, the development of guidelines such as the guideline for the screening and management of perinatal Group B Streptococcus (GBS), the development of patient information (on GBS, antibiotic use in the hospital and on antibiotic resistance), and a project to promote the timely conversion from IV to oral antibiotics. The AMST has also become a part of the hospital’s support framework. This has allowed the AMST to be available for consult when a healthcare provider has a query relating to antibiotic use or when new issues surrounding antibiotic use arise. For example, when there was a query regarding whether to introduce a new antibiotic-impregnated product to the hospital, the AMST was available for consult and provided evidence-based feedback for its recommendations. Our hospital also contributes antimicrobial usage data to the National Antimicrobial Utilisation Surveillance Program (NAUSP) on an ongoing basis, and participates annually in NAPS. We are also committed to providing on-going education to hospital staff members, as well as to members of the community through our annual participation in events such as Antibiotic Awareness Week.
Discussion
We have described the establishment of an AMS program in a private hospital. This is one of several different approaches and still in its early development phase. While we have adopted many approaches commonly employed by AMS programs, we believe that what makes our approach to AMS unique is the way in which our AMS program was integrated into the infection prevention team. This incorporation of the AMS program into an already well established hospital division allowed our AMS program to have a greater impetus in the hospital as we had access to a wider range of contact networks (whether nursing, clinician or executive) throughout the organisation.
Most guidelines on implementing AMS programs5,13 focus on resource limitations that a hospital may face (such as a lack of access to ID physicians or information technology resources), while limitations faced by cultural barriers are often overlooked. These cultural barriers may include a lack of executive leadership to promote AMS, resistance from doctors towards AMS guidelines31 and a lack of willingness from doctors to change their prescribing practices, especially if it goes against engrained prescribing etiquette. From our experience to date, these hospital cultural barriers often play a more significant role than resource limitations in the success of an AMS program as they determine how well the program is received by clinical staff and their subsequent support of the program and its projects.
Early in the program, we identified potential institution-specific barriers and tailored our AMS program to address them. The initial formation of strong relationships between AMS and other personnel was identified as a key factor for the implementation of an AMS program to be successful. By choosing ID physicians who were already well known by medical staff at the hospital, the SVPHM AMS program was able to benefit from the existing associations the ID physicians had with clinicians from other specialties. Similarly, the integration of the AMS program into already existing committee structures, particularly the infection prevention team, allowed the AMS program to obtain a wider exposure to hospital staff, particularly nursing staff. Hospital executive involvement was a crucial driving force in establishing this AMS program.
We feel that the success of an AMS program is ultimately dependent on the education of prescribers to influence change on the prescribing culture of an institution. While many AMS programs may focus on restricting antibiotics or the development of policies, if prescribers themselves are not actively engaged, effects of AMS interventions will most likely be minimal and short-lived, and efforts may outweigh the benefits of such interventions.
While there are early signs of significant medical staff involvement in AMS projects at SVPHM, particularly from the anaesthetists, there is also some individual resistance. To what extent this program will need to move to be more restrictive, for example restriction of selected antibiotics past 48 h, is yet to be determined. However, further education of different clinical groups will be our main priority. The AMS program is still evolving as we gain more experience. Results of audits have highlighted to us areas that we need to focus on in the future and the need for further audits to review the success of our projects and interventions. By reflecting on the results of our initial efforts, barriers encountered, and the experience of others, we envisage that we will continue to adapt our methods to develop a program to better suit the needs (and barriers) encountered in a private hospital.
Conclusion
The primary goal of AMS programs is to improve patient outcomes by promoting the appropriate use of antimicrobials. While there are guidelines available to help develop an AMS program, these guidelines need to be adapted to suit different hospital settings. Private hospitals present a unique challenge in the implementation of AMS programs. Identifying and addressing barriers specific to an individual institution is vital. By tailoring our AMS program to accommodate the needs and barriers at our institution, we have managed to implement several AMS initiatives including restriction of selected antibiotics via a post-prescription review, significantly decrease inappropriate aminoglycoside use and develop several hospital guidelines such as surgical antibiotic prophylaxis, management of perinatal Group B Streptococcus and IV to oral swap over guidelines, which have overall been well received by medical staff.
Conflicts of interest
None declared.
Funding
All members of the St Vincent’s Private Hospital Melbourne Antimicrobial stewardship committee are employed by St Vincent’s Private Hospital Melbourne. No further funding was received.
References
[1] Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States, 2013. Atlanta: CDC; 2013. Available from: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf [verified 7 April 2015][2] Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006; 42 S82–9.
| The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs.Crossref | GoogleScholarGoogle Scholar | 16355321PubMed |
[3] Tsang JK, Tsang OT, Yao R, Lai S. The Antimicrobial Stewardship Programme: where have we been...where are we going? World Hosp Health Serv 2013; 49 18–21.
| 24228343PubMed |
[4] Ohl CA, Luther VP. Antimicrobial stewardship for inpatient facilities. J Hosp Med 2011; 6 S4–15.
| Antimicrobial stewardship for inpatient facilities.Crossref | GoogleScholarGoogle Scholar | 21225949PubMed |
[5] Duguid M, Cruickshank M. Antimicrobial stewardship in Australian hospitals. Canberra: Commonwealth of Australia; 2011. Available from: http://safetyandquality.gov.au/wp-content/uploads/2011/01/Antimicrobial-stewardship-in-Australian-Hospitals-2011.pdf [verified 25 February 2015]
[6] Nowak MA, Nelson RE, Breidenbach JL, Thompson PA, Carson PJ. Clinical and economic outcomes of a prospective antimicrobial stewardship program. Am J Health Syst Pharm 2012; 69 1500–8.
| Clinical and economic outcomes of a prospective antimicrobial stewardship program.Crossref | GoogleScholarGoogle Scholar | 22899745PubMed |
[7] Malcolm W, Nathwani D, Davey P, Cromwell T, Patten A, Reilly J, Cairns S, Bennie M. From intermittent antibiotic point prevalence surveys to quality improvement: experience in Scottish hospitals. Antimicrob Resist Infect Control 2013; 2 3
| From intermittent antibiotic point prevalence surveys to quality improvement: experience in Scottish hospitals.Crossref | GoogleScholarGoogle Scholar | 23320479PubMed |
[8] Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother 2014; 69 1748–54.
| Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVWms73N&md5=bef483d73daeedebfb05552d0af28297CAS | 24633207PubMed |
[9] Rosa RG, Goldani LZ, dos Santos RP. Association between adherence to an antimicrobial stewardship program and mortality among hospitalised cancer patients with febrile neutropenia: a prospective cohort study. BMC Infect Dis 2014; 14 286
| Association between adherence to an antimicrobial stewardship program and mortality among hospitalised cancer patients with febrile neutropenia: a prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 24884397PubMed |
[10] Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, Ramsay CR, Wiffen PJ, Wilcox M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013; CD003543
| Interventions to improve antibiotic prescribing practices for hospital inpatients.Crossref | GoogleScholarGoogle Scholar | 23633313PubMed |
[11] Australian Commission on Safety and Quality in Health Care. Safety and Quality Improvement Guide Standard 3: Preventing and Controlling Healthcare Associated Infections, October 2012. Sydney: ACSQHC; 2012. Available from: http://www.safetyandquality.gov.au/wp-content/uploads/2012/10/Standard3_Oct_2012_WEB.pdf [verified 25 February 2015]
[12] Huttner B, Harbarth S, Nathwani D. (European Society of Clinical Microbiology and Infectious Diseases). Success stories of implementation of antimicrobial stewardship: a narrative review. Clin Microbiol Infect 2014; 20 954–62.
| (European Society of Clinical Microbiology and Infectious Diseases). Success stories of implementation of antimicrobial stewardship: a narrative review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2M7ot1Whsg%3D%3D&md5=a15847358907a09d52a2a976268d61b5CAS | 25294340PubMed |
[13] James RS, McIntosh KA, Luu SB, Cotta MO, Marshall C, Thursky KA, Buising KL. Antimicrobial stewardship in Victorian hospitals: a statewide survey to identify current gaps. Med J Aust 2013; 199 692–5.
| Antimicrobial stewardship in Victorian hospitals: a statewide survey to identify current gaps.Crossref | GoogleScholarGoogle Scholar | 24237101PubMed |
[14] Australian Government Productivity Commission. Public and Private Hospitals, Research Report. Canberra; Australian Government; 2009. Available from: http://www.pc.gov.au/inquiries/completed/hospitals/report/hospitals-report.pdf [verified 25 February 2015]
[15] Charani E, Castro-Sanchez E, Sevdalis N, Kyratsis Y, Drumright L, Shah N, Holmes A. Understanding the determinants of antimicrobial prescribing within hospitals: the role of ‘prescribing etiquette’. Clin Infect Dis 2013; 57 188–96.
| Understanding the determinants of antimicrobial prescribing within hospitals: the role of ‘prescribing etiquette’.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3srjvVakug%3D%3D&md5=ab316e8e1a486b4efe5d3bdedccdec3fCAS | 23572483PubMed |
[16] Cotta MO, Robertson MS, Tacey M, Marshall C, Thursky KA, Liew D, Buising KL. Attitudes towards antimicrobial stewardship: results from a large private hospital in Australia. Healthc Infect 2014; 19 89–94.
| Attitudes towards antimicrobial stewardship: results from a large private hospital in Australia.Crossref | GoogleScholarGoogle Scholar |
[17] Chaves NJ, Cheng AC, Runnegar N, Kirschner J, Lee T, Buising K. Analysis of knowledge and attitude surveys to identify barriers and enablers of appropriate antimicrobial prescribing in three Australian tertiary hospitals. Intern Med J 2014; 44 568–74.
| Analysis of knowledge and attitude surveys to identify barriers and enablers of appropriate antimicrobial prescribing in three Australian tertiary hospitals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cbotVynsQ%3D%3D&md5=a21fd45b7ae35b593c24d8d419e18d89CAS | 25083531PubMed |
[18] Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, Robertson N. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews; 2005. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005470.pub2/pdf [verified 31 March 2015]
[19] Cotta MO, Robertson MS, Marshall C, Thursky KA, Liew D, Buising KL. Implementing antimicrobial stewardship in the Australian private hospital system: a qualitative study. Aust Health Rev 2015; 39 315–22.
| Implementing antimicrobial stewardship in the Australian private hospital system: a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[20] Cairns KA, Jenney AWJ, Abbot IJ, Skinner MJ, Doyle JS, Dooley M, Cheng AC. Prescribing trends before and after implementing an antimicrobial stewardship program. Med J Aust 2013; 198 262–6.
| Prescribing trends before and after implementing an antimicrobial stewardship program.Crossref | GoogleScholarGoogle Scholar | 23496402PubMed |
[21] Johannsson B, Beekmann SE, Srinivasan A, Hersh A, Laxminarayan R, Polgreen PM. Improving antimicrobial stewardship: the evolution of programmatic strategies and barriers. Infect Control Hosp Epidemiol 2011; 32 367–74.
| Improving antimicrobial stewardship: the evolution of programmatic strategies and barriers.Crossref | GoogleScholarGoogle Scholar | 21460488PubMed |
[22] Chow A, Lye DC, Arah OA. Psychosocial determinants of physicians’ acceptance of recommendations by antibiotic computerised decision support systems: a mixed methods study. Int J Antimicrob Agents 2015; 45 295–304.
| Psychosocial determinants of physicians’ acceptance of recommendations by antibiotic computerised decision support systems: a mixed methods study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFCnsrnE&md5=4d64a3e39752307a11856630d3b65165CAS | 25434998PubMed |
[23] Chung GW, Wu JE, Yeo CL, Chan D, Hsu LY. Antimicrobial stewardship: a review of prospective audit and feedback systems and an objective evaluation of outcomes. Virulence 2013; 4 151–7.
| Antimicrobial stewardship: a review of prospective audit and feedback systems and an objective evaluation of outcomes.Crossref | GoogleScholarGoogle Scholar | 23302793PubMed |
[24] Pakyz AL, Moczygemba LR, VanderWielen LM, Edmond MB, Stevens MP, Kuzel AJ. Facilitators and barriers to implementing antimicrobial stewardship strategies: Results from a qualitative study. Am J Infect Control 2014; 42 S257–63.
| Facilitators and barriers to implementing antimicrobial stewardship strategies: Results from a qualitative study.Crossref | GoogleScholarGoogle Scholar | 25239719PubMed |
[25] Ahmed RM, Hannigan IP, MacDougall HG, Chan RC, Halmagyi GM. Gentamicin ototoxicity: a 23-year selected case series of 103 patients. Med J Aust 2012; 196 701–4.
| Gentamicin ototoxicity: a 23-year selected case series of 103 patients.Crossref | GoogleScholarGoogle Scholar | 22554194PubMed |
[26] Antibiotic Expert Groups. Therapeutic guidelines: antibiotic. Version 15. Melbourne: Therapeutic Guidelines Limited; 2014.
[27] Broom A, Broom J, Kirby E. Cultures of resistance? A Bourdieusian analysis of doctors’ antibiotic prescribing. Soc Sci Med 2014; 110 81–8.
| Cultures of resistance? A Bourdieusian analysis of doctors’ antibiotic prescribing.Crossref | GoogleScholarGoogle Scholar | 24727665PubMed |
[28] Sevinç F, Prins JM, Koopmans RP, Langendijk PNJ, Bossuyt PM, Dankert J, Speelman P. Early switch from intravenous to oral antibiotics: guidelines and implementation in a large teaching hospital. J Antimicrob Chemother 1999; 43 601–6.
| Early switch from intravenous to oral antibiotics: guidelines and implementation in a large teaching hospital.Crossref | GoogleScholarGoogle Scholar | 10350396PubMed |
[29] Ramirez JA, Vargas S, Ritter GW, Brier ME, Wright A, Smith S, Newman D, Burke J, Mushtaq M, Huang A. Early switch from intravenous to oral antibiotics and early hospital discharge. A prospective observational study of 200 consecutive patients with community-acquired pneumonia. Arch Intern Med 1999; 159 2449–54.
| Early switch from intravenous to oral antibiotics and early hospital discharge. A prospective observational study of 200 consecutive patients with community-acquired pneumonia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7islKmuw%3D%3D&md5=c65e81fe694e9d27cd36c8ff5c2e4dd6CAS | 10665893PubMed |
[30] Mertz D, Koller M, Haller P, Lampert ML, Plagge H, Hug B, Koch G, Battegy M, Fluckiger U, Bassetti S. Outcomes of early switching from intravenous to oral antibiotics on medical wards. J Antimicrob Chemother 2009; 64 188–99.
| Outcomes of early switching from intravenous to oral antibiotics on medical wards.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntlGgsLc%3D&md5=b24aa13b55bacf09929556fa41b2510dCAS | 19401304PubMed |
[31] Duane TM, Zuo JX, Wolfe LG, Bearman G, Edmond MB, Lee K, Cooksey L, Stevens MP. Surgeons do not listen: evaluation of compliance with antimicrobial stewardship program recommendations. Am Surg 2013; 79 1269–72.
| 24351354PubMed |

