Biodiversity, functional roles and ecosystem services of groundwater invertebrates
Andrew J. Boulton A D , Graham D. Fenwick B , Peter J. Hancock A and Mark S. Harvey CA Ecosystem Management, University of New England, Armidale, New South Wales 2350, Australia.
B National Institute of Water and Atmospheric Research, PO Box 8602, Riccarton, Christchurch, New Zealand.
C Department of Terrestrial Invertebrates, Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australia 6986, Australia.
D Corresponding author. Email: aboulton@une.edu.au
Invertebrate Systematics 22(2) 103-116 https://doi.org/10.1071/IS07024
Submitted: 11 June 2007 Accepted: 28 December 2007 Published: 12 May 2008
Abstract
Recent surveys of groundwater invertebrates (stygofauna) worldwide are yielding rich troves of biodiversity, with significant implications for invertebrate systematists and phylogeneticists as well as ecologists and groundwater managers. What is the ecological significance of this high biodiversity of invertebrates in some aquifers? How might it influence groundwater ecosystem services such as water purification or bioremediation? In terrestrial ecosystems, biodiversity is typically positively correlated with rates of ecosystem functions beneficial to humans (e.g. crop pollination). However, the links between biodiversity, ecosystem function, and ecosystem services in groundwater are unknown. In some aquifers, feeding, movement and excretion by diverse assemblages of stygofauna potentially enhance groundwater ecosystem services such as water purification, bioremediation and water infiltration. Further, as specific taxa apparently play ‘keystone’ roles in facilitating ecosystem services, declines in abundance or even their extinction have serious repercussions. One way to assess the functional significance of biodiversity is to identify ‘ecosystem service providers’, characterise their functional relationships, determine how service provision is affected by community structure and environmental variables, and measure the spatio-temporal scales over which these operate. Examples from Australian and New Zealand alluvial aquifers reveal knowledge gaps in understanding the functional importance of most stygofauna, hampering effective protection of currently undervalued groundwater ecosystem services.
Additional keywords: aquifers, biodiversity, ecosystem goods and services, functional structure, groundwater management, stygofauna.
Introduction
Human appropriation of Earth’s natural resources inevitably impacts on global and local biodiversity. With an annual population increase of 90 million (Cohen 2005) and increasing technological advances in irrigation and agriculture, we consume a disproportionate amount of the world’s primary production while transforming natural environments into human-dominated landscapes (Vitousek et al. 1997; Imhoff et al. 2004). The pressures of our burgeoning population and associated environmental changes are considered responsible for the current ‘sixth great extinction in the history of life on Earth’ (Dirzo and Raven 2003), with numerous examples of precipitous declines in biodiversity across a range of terrestrial, marine, and aquatic ecosystems (Sodhi et al. 2004; Millenium Ecosystem Assessment 2005; Worm et al. 2006). The natural diversity of all organisms is an essential resource for humans, providing food, clean water, oxygen, medical products, and other fundamental requirements (Grifo and Rosenthal 1997; Worm et al. 2006). Humans cannot afford to lose these essential resources yet there is clear evidence that declines in biodiversity threaten the provision of these ecosystem goods and the stability of our life-support system (Lubchenco 1998; Ehrlich and Ehrlich 2004; Millenium Ecosystem Assessment 2005).
Ecosystem services are the conditions and processes by which natural ecosystems and their species sustain and fulfill human life (Daily 1997). They maintain biodiversity and the production of ecosystem goods yet their functional mechanisms are extraordinarily difficult to understand because of the complexity of the interdependent components and their non-linear interactions (Carpenter et al. 2006). There has been extensive work mapping the supply and demand for services, potential threats, and estimates of economic value (Costanza et al. 1997; Heal 2000; Murray et al. 2006) but relatively little research on the specific mechanisms of how the various facets of biodiversity provide these services (Kremen 2005). To date, attention has focused on terrestrial biomes, biodiversity, and their associated ecosystem services such as pollination, crop production, seed dispersal, and soil biogeochemistry (Klein et al. 2003; Larsen et al. 2005; Millenium Ecosystem Assessment 2005; Losey and Vaughan 2006).
In contrast, there are very few specific assessments of groundwater ecosystem services and their biological providers (e.g. biogeochemical water purification, Herman et al. 2001; Danielopol et al. 2003, 2004) although there is a strong growing interest in dependency on groundwater by other ecosystems (e.g. Clifton and Evans 2001; Boulton and Hancock 2006; Bergkamp and Cross 2006). Aquifers and their inhabitants are the perfect example of a completely groundwater dependent ecosystem (Humphreys 2006) yet our understanding of how groundwater invertebrates influence ecosystem services in aquifers is almost nonexistent (Danielopol et al. 2003). Taxonomists and ecologists are now appreciating the unexpectedly-diverse assemblages of groundwater invertebrates (stygofauna) in aquifers across the world (Marmonier et al. 1993; Danielopol et al. 2000; Humphreys 2006), raising the key question: what is the relationship between this biodiversity and groundwater ecosystem services provided or facilitated by stygofauna?
In this paper, we review briefly the principal theories of the relationship between biodiversity and ecosystem function. We extend these to the provision of ecosystem services, assessing their likely applicability to shallow groundwater ecosystems based on case studies from Australian and New Zealand alluvial aquifers. Our focus is on functional aspects of biodiversity rather than solely taxa richness. This functional approach to the provision of groundwater ecosystem services complements current studies of stygofaunal systematics and phylogenetics (e.g. Cooper et al. 2002, 2007; Watts and Humphreys 2006), revealing how groundwater ecosystem services may vary with changes in assemblage composition over time, the likely effects of species loss or invasion, and potential influences of aquifer habitat fragmentation. Such insights can guide protection and restoration of aquifers and their ecosystem services as well as contributing to one of the most exciting fundamental ecological questions of our time.
Biodiversity, ecosystem function and ecosystem services
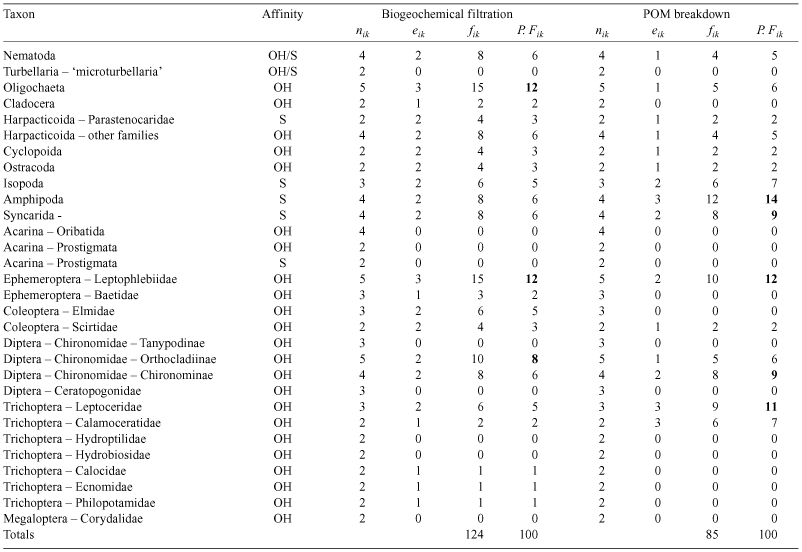
Biodiversity is the total variety of life, including genetic, population, species and ecosystem diversity (Millenium Ecosystem Assessment 2005). This definition is often extended to encompass ecological roles and relationships (e.g. predator–prey) among organisms (Hooper et al. 2005; Kim and Byrne 2006). Most of the research on the relationship between biodiversity and ecosystem function has focused on associations with species richness, resulting in several models and hypotheses. There is, of course, the null hypothesis that no relationship exists between biodiversity and ecosystem function (Fig. 1) but support for this is weak (Giller and O’Donovan 2002). The next simplest model (but also considered unlikely, Tilman 1997) is a linear relationship where each additional species contributes the same amount of ecosystem function (Fig. 1), a situation that might arise in even communities with little functional differentiation (Kremen 2005). A third model recognises that strong ecological interactions among species generate variable ecosystems so that the relationship between biodiversity and ecosystem function depends on which species are present (the ‘idiosyncratic’ model, Lawton 1994). Consequently, the associations yielded from this model are difficult to predict or generalise (Fig. 1).
|
The evocatively-named ‘rivet’ hypothesis (Ehrlich and Ehrlich 1981) likens the ecological function of species to the rivets that attach a wing to a plane; several rivets can be lost before the wing falls off. The implication here is that ecological functions of species overlap so that even if a species goes extinct, ecosystem function continues due to compensation by other species. An ecological function will not disappear until all the species that perform that function are extinct (Peterson et al. 1998). The related ‘redundant species’ hypothesis predicts an association between species richness and ecosystem function that is more strongly saturating (Fig. 1). Above the saturation point, species can be lost without significant effect because some species are functionally redundant. Walker (1992) extended this notion of species complementarity by proposing that ecosystem function is primarily determined by functionally important ‘driver’ species (or groups of those species) whereas ‘passenger’ species have only a minor ecological role. The loss of such ‘drivers’ severely impairs ecosystem function (Walker 1995) so that the relationship is not a simple function of species richness but also depends on the identities, abundance or biomass, activity, efficiency, and interactions of the populations of these species as well as simply richness.
Most studies have demonstrated that a reduction in biodiversity has a negative effect on ecosystem function (reviews in Loreau et al. 2001; Giller and O’Donovan 2002; Hooper et al. 2005). The majority of this work has been done on terrestrial plant communities, and results of 95% of the manipulation studies reviewed by Schwartz et al. (2000) supported the redundant species hypothesis. The comparatively fewer studies on the role of animal biodiversity on ecosystem function also indicate increased rates of ecosystem functioning (e.g. productivity, Naeem et al. 1995; organic matter decomposition, Heneghan et al. 1999; Jonsson et al. 2001) associated with increasing species richness. However, nearly all of these studies of biodiversity–ecosystem function relationships have been synthetic, species-poor experiments, prompting criticism of their direct relevance to natural ecosystems that are more complex, open, and species-rich (Loreau et al. 2001; Ostfeld and LoGiudice 2003). Given the intractable nature of experimentation on entire natural ecosystems, this research gap typically has been addressed by assessing responses to human modification leading to species extinction (e.g. Larsen et al. 2005), revealing that habitat loss often disrupts ecosystem functioning by affecting factors such as extinction order and species abundance as well as species richness.
Another major research gap relates to the link between biodiversity–ecosystem function and the provision of ecosystem services by natural ecosystems (Kremen 2005). As distinct from ecosystem goods (e.g. fibre, wood, meat), most ecosystem services are functions that are essential to human life (e.g. climate regulation, air and water purification, crop pollination). Given the importance of many of these ecosystem services, understanding the relationship between biodiversity and ecosystem function is crucial, especially for their effective conservation and management. Groundwater ecosystem services include prevention of land subsidence, erosion and flood control through absorption of runoff, reception and bioremediation of wastes and other by-products of human economic activity, and improvement in water quality through biogeochemical water purification (Boulton 2000a; Herman et al. 2001; Danielopol et al. 2003). Almost nothing is known of how biodiversity in groundwaters affects ecosystem functioning and therefore the ecosystem services provided by this immense biome. However, reliance on this resource and its undervalued ecosystem services is steadily increasing (Danielopol et al. 2003; Hancock et al. 2005; Bergkamp and Cross 2006).
Once considered virtually lifeless semi-deserts, groundwater ecosystems are now recognised as often supporting diverse metazoan faunal assemblages and associated consortia of active microbes (Rouch 1977; Marmonier et al. 1993; Danielopol et al. 2003; Hancock et al. 2005). These groundwater metazoans (stygofauna) include many unique representatives of lineages from various geological periods and can exhibit extraordinary endemicity (Poore and Humphreys 1992; Harvey 2002; Humphreys 2006, 2008). Globally, stygofauna comprises a significant component of total biodiversity (Rouch and Danielopol 1997; Sket 1999) and there are several ‘hot-spots’ of high subterranean biodiversity (Culver and Sket 2000; Danielopol and Pospisil 2001; Castellarini et al. 2007), including parts of Australia (Bradbury and Williams 1997; Humphreys 2001; Karanovic and Marmonier 2003; Karanovic 2007). The arid zone in Western Australia is yielding especially interesting and diverse groundwater faunas (Humphreys 2001; Cooper et al. 2002; Leys et al. 2003) yet these regions are also where human pressures on groundwater are heaviest (Humphreys 2000; Boulton et al. 2003; Hancock et al. 2005).
Our knowledge of this biodiversity is fragmentary (Humphreys this issue); our understanding of its functional significance is virtually nonexistent. Yet, if stygofauna maintain or influence groundwater ecosystem functions in such a way as to provide crucial ecosystem goods and services, a strategic research agenda is essential to combine our growing phylogenetic and systematic knowledge of groundwater invertebrate biodiversity with ecological understanding of its functional significance.
Assessing stygofaunal biodiversity and its role in providing groundwater ecosystem services
Kremen (2005) proposed several approaches to develop this research agenda and bridge the gap between biodiversity– ecosystem function and provision of ecosystem services. Although her focus was on terrestrial ecosystems and their ecosystem services, such as pollination and pest control, we suggest these approaches can be extended to understanding the relationship between biodiversity, ecosystem function, and provision of ecosystem services in groundwaters. Although each of these approaches comprises a research topic in its own right in the discipline of ecology, their integration is where the real challenge lies, especially when the information must be interpreted in a management context to provide laws and policies that protect crucial groundwater ecosystem services (e.g. Herman et al. 2001; Danielopol et al. 2004). For maximum effect, this agenda must include socio-economic aspects (Millenium Ecosystem Assessment 2005) but in this paper, we only consider the ecological components and focus on the potential role of stygofauna in provision of groundwater ecosystem services. These ecological components, modified from Kremen (2005), are: (1) identifying the species or other entities that are the key ‘ecosystem service providers’ (ESPs); (2) characterising their functional relationships and functional structure; (3) assessing how aspects of community structure of ESPs and its changes affect provision of services; (4) identifying how key environmental factors affect ESPs and their provision of services; and (5) measuring the spatio-temporal scales over which ESPs and their services operate.
Identifying ‘ecosystem service providers’
The level at which to identify a particular ESP depends on the specific ecosystem service, and this may not necessarily equate to the taxonomic characterisation of the species. For example, the genetic level may be the appropriate level for maintaining disease resistance (e.g. Luck et al. 2003) whereas the functional group level may be best for characterising suites of microbes that cycle nutrients or break down organic matter. In this latter case, logistic impediments to species-level taxonomy of most microorganisms may mean that the functional level is adequate for categorising the ESP but this does not exclude the use of population- or species-level categorisation.
Depending on the scale of the assessment, it may be most pragmatic to consider some ESPs at the community level where an entire assemblage carries out a specific ecosystem service (Kremen 2005). Functional classifications are popular in aquatic ecology, although seldom couched in terms of ecosystem service provision. For example, detritivorous stream invertebrates commonly are classified into functional feeding groups of ‘shredders’ capable of feeding on coarse particulate organic matter >1 mm such as leaves or bark, ‘collector-gatherers’ that feed on fine particulate organic matter (FPOM, <1 mm) that settles on the stream-bed and ‘collector-filterers’ that filter FPOM from the water column (Cummins and Klug 1979). Despite some limitations with this classification (Boulton and Brock 1999), use of these functional groups has facilitated comparisons of invertebrate community structure and organic matter breakdown across river systems as well as empirical tests of conceptual models of how stream ecosystems function (e.g. the River Continuum Concept, Vannote et al. 1980). Functional feeding groups could also be considered as ESPs, essential in the ecosystem service of organic matter decomposition. Loss of ‘shredders’, for example, would inhibit removal of accumulations of leaf litter in streams with concomitant impacts on water quality and reduction of a crucial source of carbon for the stream foodweb, as observed in some streams where urbanisation has markedly reduced shredder biodiversity and densities (Miller and Boulton 2005). Functional classifications of groundwater invertebrates (e.g. Claret et al. 1999) are still in their infancy and unavailable for most poorly known groundwater faunas. None has explored the potential for these to be considered as groupings of ESPs in terms of their roles in facilitating groundwater ecosystem services such as water purification, toxin and waste material breakdown, maintenance of hydraulic conductivity and connectivity, and organic matter decomposition.
Characterising functional relationships – a ‘functional inventory’
An ecosystem service can be characterised by conducting a ‘functional inventory’ to estimate the importance of each ESP’s contribution to the aggregate function (Kremen 2005). The functional importance fik of ESPi in an environment k will reflect its abundance in that environment (nik) and its efficiency (eik) at performing that service (Balvanera et al. 2005). Changes in resources, predators, competitors and environmental variables potentially affect the abundance and efficiency of each ESP, and hence, their contribution to a given ecosystem service. Individual functional importance fik reveals which ESPs are disproportionately important relative to their abundance, revealed by deviation from the null hypothesis that relative importance equates to relative abundance (Balvanera et al. 2005). Thus, species can be ranked by their functional importance (i.e. ‘functional structure’) to illustrate the vulnerability of the ecosystem service to loss or declines of particular species. For example, an analysis of the functional structure of native bees pollinating watermelon crops in California within a conserved forest matrix revealed that the first two species contributed 80% of the function. However, under conventional agriculture without forest, there was a loss of 60% of the total species pool, declines in the abundance of functionally important species, and decreased evenness in functional structure, resulting in loss of 60–80% of the entire pollination function (Balvanera et al. 2005).
The aggregate function Fk of environment k is simply the sum of the contributions of each ESPik. This enables prediction of how the aggregate function might vary as assemblage composition of ESPs change over space or time. By measuring individual functional contributions and estimating aggregate function, the relationships between biodiversity and ecosystem function (Fig. 1) now translate into those of ESPs and their aggregate functions (Kremen 2005). The effects of taxonomic richness sum across the species while specific characteristics of efficiency and abundance are incorporated into the functional importance (fik). This process is demonstrated in the alluvial aquifer case study below.
Aspects of community structure of ESPs affect provision of services
As mentioned above, ecosystem function depends on identities, densities and biomasses, activity, efficiencies, and interactions of the populations of ESPs within an environment as well as their species richness. To manage ecosystem services properly requires understanding how changes in all these aspects of community structure, acting alone or together, affects provision of each service. Broadly, the responses to change in community structure are variously compensatory, stabilising, or result in a rapid loss of function.
High diversity can have a compensatory effect whereby statistical averaging, more diverse communities provide more stable services just as diverse stock holdings minimise volatility and thus investment risk (the ‘portfolio effect’, Tilman et al. 1998). Density compensation also stabilises ecosystem service provision where there are negative interactions among ESPs. Species manipulation experiments demonstrated stability in above-ground biomass in temperate grasslands where reduced abundance of one species enabled competitive release of others (Tilman 1996). Functional compensation occurs when individuals’ efficiencies increase as aggregate abundance declines. For example, honey bees in an apple orchard were found to carry more pollen when bee densities were low, interpreted as functionally compensating for lower numbers of overall pollinators (Harder and Thomson 1989).
Conversely, no density compensation was seen in other temperate bee communities where reduced species richness due to habitat loss diminished crop pollination (Larsen et al. 2005). When there are no compensatory mechanisms, changes in community composition or loss of species lead to rapid loss of function. This loss of function may be due to extinction of particular species with especially great functional importance fik or of other species that facilitate or complement these important ones. Alternatively, factors that affect efficiency or abundance of a functionally important species cause rapid loss of function without necessarily involving species loss. Order of extinction is a non-random event, especially in response to human impact; the most important crop pollinators or most active dung beetles are first to be lost due to agriculture or habitat fragmentation (Larsen et al. 2005).
In groundwater ecosystems, larger invertebrates such as amphipods are more susceptible than agile, small copepods to swift changes in groundwater level due to intensive water extraction (M. Tomlinson unpublished data). However, these larger invertebrates also play a greater role in bioturbation and compaction of fine sediments into faecal pellets than smaller ones (copepods), making the loss of amphipods and other relatively big stygofauna potentially more significant. Amphipods are also likely to be more active in processing coarser organic matter in groundwater ecosystems than copepods so that the ecosystem service of organic decomposition in groundwaters could be impacted by selective extinction through excessive falls in water tables caused by over-extraction. There was a greater loss in function than expected in assemblages of shredding stream invertebrates experimentally manipulated to mimic the order of extinction due to acidification and pollution observed in northern Europe (Jonsson et al. 2002). This rapid loss of ecosystem function was attributed to the loss of inter-specific interactions that facilitated leaf-shredding, and similar interactions may occur in species-rich downwelling zones of alluvial aquifers.
Dramatic changes in abundance rather than outright extinction can also lead to rapid loss of function. In the North Pacific Ocean, industrial whaling reduced numbers of great whales, a preferred food of killer whales, causing the killer whales to switch to eating sea otters. This, in turn, took predation pressure off a keystone herbivore, sea urchins, that then overgrazed kelp beds, transforming them into ‘urchin barrens’ dominated by crustose algae (Springer et al. 2003). Predicting the outcomes of changes in abundance of ESPs in natural ecosystems is difficult, often yielding rather nasty ‘ecological surprises’ (Carpenter et al. 2006).
Key environmental factors affecting ESPs and their provision of services
Depending on the results of the functional inventory above, the effects of environmental factors can either be assessed for the functionally important ESPs (ESP-centred approach) or on the ecosystem function as a whole (function-centred approach, Kremen 2005). The ESP-centred approach is most effective when a single or a few ESPs contribute disproportionately to the ecosystem function. The conventional approach in ecology to assessing the effects of environmental factors affecting individual species uses field surveys to identify strong correlations, followed by manipulative experiments to determine causality and interactions among two or more variables. Observations of the apparent significance of several groups of common stream invertebrates such as amphipods and some case-building caddisfly larvae in shredding leaves (Cummins 1974; Graça et al. 2001) led to their inclusion as a keystone functional group in conceptual models of organic matter processing along rivers (e.g. Vannote et al. 1980). Although their functional significance varies between streams (Cheshire et al. 2005), numerous field and laboratory experiments have demonstrated how environmental factors such as leaf species, diversity and chemical composition, flow, water quality and human impacts affect the diversity, abundance and activity of shredders (Graça et al. 2001; Gessner and Chauvet 2002; Boyero et al. 2006).
Conversely, the ecosystem function-centred approach would be used when there are large numbers of functionally important ESPs and mechanisms are poorly known yet there are good data on rates of ecosystem processes and the environmental factors associated with them. Multivariate methods are ideal to identify and prioritise suites of environmental variables that are most highly correlated with aggregate function and its variability. Kremen et al. (2004) used this approach to show that the stability and extent of crop pollination by native bees correlated more with the proportion of upland natural habitat within several kilometres than with other local or landscape scale variables. Experimental manipulations of entire ecosystem functions are more challenging at this broad scale. Nonetheless, when the roles of environmental variables in controlling rates of ecosystem services can be identified, conservation and management targets are more readily established. Plausibly, these can be done before precise mechanisms are worked out – especially in such poorly known environments as groundwaters – and the responses to management will provide further insights into environmental controls of ecosystem function via adaptive management (Boulton 2005; Seward et al. 2006).
Spatio-temporal scales over which ESPs and their services operate
Ecosystem services and their ESPs operate across local, regional and global scales, and usually a combination of these. Spatially, ESPs are affected by environmental filters (sensu Poff 1997). For example, in rivers at the landscape scale, historical, climatic, geological and human land-use patterns dictate species pools (biogeography, evolutionary genetics) as well as environmental features such as thermal regimes, water chemistry, and flow variability. Nested within the landscape scale lies the valley/reach scale which imposes geomorphic and catchment vegetation controls (e.g. sediment size, channel morphology, riparian vegetation) that directly control ecosystem functions such as secondary productivity and the supply and breakdown of organic matter. The next two scales – channel unit and microhabitat – govern finer-scale constraints such as the availability of instream physical habitat, substrate stability, and food for stream life. Extending the example of organic matter processing introduced above, the landscape scale governs the species pools of leaf litter and shredding invertebrates, the valley scale controls leaf input and retention, and channel units and microhabitats dictate patterns of leaf retention and species interactions, abundance and efficiency of leaf shredding by individual ESPs (Graça et al. 2001; Boyero et al. 2006).
The time-scales over which ESPs and their services operate can vary from less than an hour (e.g. microbial processing of organic matter) through to centuries (e.g. breakdown of wood, colonisation and local extinction of ESPs). In groundwater ecosystems, these time-scales are especially relevant because water flows and ecosystem functions are slower than at the surface (Hancock et al. 2005). Thus, temporal ‘lag effects’ of response by ESPs to environmental changes at broader spatial scales are probably common, confounding conclusions from correlative studies of ecosystem functions in aquifers. Mining ‘fossil’ groundwater recharged centuries previously exemplifies unsustainable use of an ecosystem good provided over a long time-scale; most examples of aquifer over-allocation and over-exploitation (Boulton et al. 2003) reflect a lack of appreciation of the time-scales of provision of this resource.
Stygofauna biodiversity and ecosystem service provision in shallow alluvial aquifers of a subtropical Australian river
Groundwater ecosystem services of lateral gravel bars in rivers
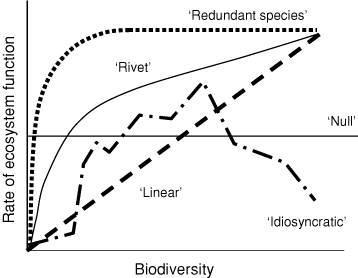
The groundwater of shallow alluvial aquifers of many gravel-bed rivers exchanges with surface water in the saturated sediments below the river and its banks in the hyporheic zone (Orghidan 1959). Along the leading edges of lateral gravel bars, surface water inwells into the sediments where it spends varying periods of time as groundwater before emerging in outwelling zones at the downstream end of the bar (Fig. 2). Inwelling surface water carries dissolved oxygen, coarse and fine particulate organic matter and even stream invertebrates into the hyporheic zone whereas outwelling groundwater is usually hypoxic and often enriched in nutrients, promoting local patches of enhanced primary productivity in the surface stream (Coleman and Dahm 1990; Boulton et al. 1998). Along these interstitial flow paths lie environmental gradients in redox potential, organic matter quantity and quality, nutrient forms and concentrations, and stygofaunal assemblage composition (Fig. 2, reviews in Brunke and Gonser 1997; Boulton 2000b; Hancock et al. 2005). The invertebrates of the hyporheic zone, collectively called the hyporheos, comprise a mixture of surface invertebrates temporarily occupying the sediments (‘occasional hyporheos’, Williams and Hynes 1974), permanent hyporheos and obligate stygofauna.
|
These lateral bars provide several crucial groundwater ecosystem services, potentially facilitated or driven by invertebrates within the sediment voids. Biogeochemical filtration of water during its travel through the hyporheic zone is largely a microbial process (Jones and Holmes 1996) but there is experimental evidence that hyporheic invertebrates can affect nitrogen cycling and respiration in the sediments, potentially by stimulating microbial activity (Marshall and Hall 2004). Microbial biofilms coating the large interstitial surface areas of sediment particles provide food for grazing invertebrates (Bärlocher and Murdoch 1989). Microbial activity may be enhanced by this feeding activity (Danielopol 1989) as well as fueled by nutrients excreted by hyporheic invertebrates (Boulton 2000b, Marshall and Hall 2004). Interstitial bacterial activity can also be increased by invertebrate bioturbation in finer sediments (e.g. oligochaete worms, Mermillod-Blondin et al. 2000) while invertebrate faeces potentially ‘seed’ the substrate with bacteria and themselves provide further substrate for microbial exploitation.
Another ecosystem service performed by lateral gravel bars is the retention and decomposition of particulate organic matter such as leaf litter and wood that falls into the stream and becomes buried (e.g. Metzler and Smock 1990) or downwells as fragments into the hyporheic zone. Carbon and nutrients mobilised by groundwater invertebrate and microbial activity (Lenting et al. 1997; Boulton and Foster 1998; Crenshaw et al. 2002) are eventually released back to the surface stream ecosystem, and the lateral gravel bars act as subsurface reservoirs of retention and processing of organic matter that gradually spiral carbon downstream (Tillman et al. 2003). Like their surface water counterparts, groundwater amphipods may play an active role in shredding buried leaf litter while the grazing activity of smaller hyporheic ‘collectors’ such as detritivorous harpacticoid copepods focuses on smaller leaf fragments or invertebrate fecal pellets.
Finally, pervious lateral gravel bars play a role in moderating the variability of stream discharge because of their ability to absorb and release water during changes in stream height (e.g. Fernald et al. 2006). They also mediate recharge of alluvial aquifers from the stream. This ecosystem service may be influenced by groundwater invertebrates because of their ability to maintain or create voids by pelletising fine interstitial materials and biofilms (Danielopol 1989) that might otherwise clog up the hyporheic flow paths (i.e. colmation, Brunke and Gonser 1997). The movement by groundwater invertebrates, especially larger ones such as amphipods, isopods and syncarids, also would help prevent clogging (Boulton 2000b; Song et al. 2007), sustaining the interstitial environmental gradients responsible for biogeochemical filtration (Fig. 2). Loss of this porosity and flow-buffering effect intensifies the scouring effect of spates and removes supply of a significant baseflow contribution (Boulton and Hancock 2006) during drought and low flows, impacting on surface stream biota and ecosystem processes (Lake 2003).
ESPs and their controls in lateral gravel bars of a subtropical river
The subtropical Never Never River in New South Wales, Australia, is lined with lateral gravel bars along much of its mid-lower reaches and drains a largely native forest of mixed eucalypt and rainforest species (Boulton and Foster 1998). Studies of hyporheos from gravel bars near Tallowood Point (30°21′S, 152°54′E) over five years, including assessments of their roles in organic matter processing (Boulton and Foster 1998) and responses to experimental spates (Boulton and Harvey 2003; Boulton et al. 2004; Claret, Dole-Olivier and Marmonier unpublished data) and interstitial de-siltation (Morris unpublished data) have yielded a reasonably complete species list. From these data, we derived a functional inventory of hyporheic invertebrates that are potentially important ESPs in the provision of the groundwater ecosystem services of biogeochemical filtration and particulate organic matter decomposition (Table 1). Their functional importance fik was estimated as the product of a ranked measure of their relative abundance and biomass (nik) multiplied by ranked probable efficiency (eik) for each of the two ecosystem services where the gravel bar at Tallowood Point is environment k. Rank abundance/biomass (nik) was derived from the combination of three size classes (<250 µm, 250 µm–1000 µm, >1000 µm) and three levels of abundance of individuals in a composite sample of c. 300 L (<10, 11–500, >500 individuals). Probable efficiency (eik) was categorised into four groups (no or unknown direct role, minor role, moderate role, major role). Although inwelling and outwelling zones of the gravel bar constitute discrete hyporheic functional zones (sensu Boulton 2007), data were pooled to simplify this example.
The likelihood of compensatory, stabilising community responses v. rapid loss of an ecosystem function through non-random extinction of a disproportionately important ESP was inferred for the two ecosystem services listed in Table 1 based on the functional inventory. Finally, key environmental factors affecting the main ESPs and hence, their provision of ecosystem services were predicted based on results of experimental and field studies in the Never Never River and from the published literature. Both of these services operate at multiple spatial (e.g. <10 mm along a single flow path to sequences of gravel bars along the river) and temporal (e.g. <1 day for microbial and invertebrate processing of organic matter to 102 years for complete breakdown of buried wood) scales.
Thirty taxa were sufficiently common in the composite sample to be considered in this analysis (Table 1). Species diversity within some of the groups (e.g. Nematoda, Oligochaeta, Prostigmata, Chironomidae) is known to be much higher than reported here, but, for the purposes of this exercise, these groups were considered at the functional level because of their shared role in the provision of the two ecosystem services. The dominant leptophlebiid mayflies were Austrophlebioides and Atalophlebia (collector and shredder detritivores, Boyero et al. 2006) and all the leptocerid caddisflies collected were shredders (i.e. not the predatory Oecetis). Of the thirty taxa, only three contributed substantially (proportion of Fik ≥8%) to the ecosystem service of biogeochemical filtration, based on their abundance-biomass ranking and predicted efficiency (Table 1). One of these taxa, the leptophlebiid mayfly nymph, was also disproportionately important in particulate organic matter breakdown. The other four functionally-important ESPs in particulate organic matter breakdown (proportion of Fik ≥ 8%, Table 1) were amphipods, syncarids, chironomid midge larvae and leptocerid caddisflies.
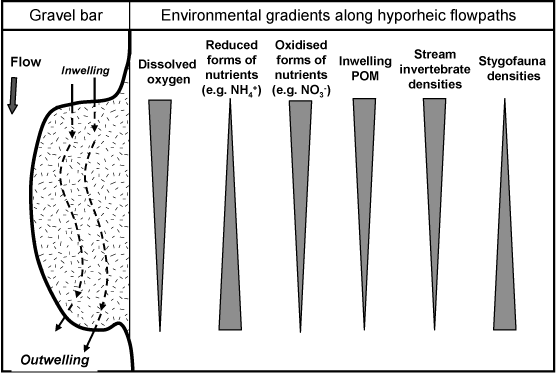
Based on this functional inventory, a conceptual model is derived that illustrates likely roles and interactions of these key ESPs, and the environmental variables that potentially affect their provision of the two ecosystem services (Fig. 3). There is also scope to include other hyporheic taxa that may not influence the ecosystem services directly but whose presence could affect the key ESPs (e.g. predation and parasitism via water mites, predation by tanypodid midges and hydrobiosid caddisflies, Fig. 3). Experimental studies are needed to test the associations proposed in the conceptual model but this provides a starting place for research priorities and setting conservation targets for protecting key ESPs.
|
Some of the key ESPs in the lateral bar at Tallowood Point are common and/or species-diverse (e.g. oligochaetes, leptophlebiid mayflies, chironomid midges), implying a high likelihood of compensatory, stabilising community responses if their hyporheic abundances changed or local extinction occurred. Conversely, amphipods and syncarids are relatively rare at this site. This suggests that non-random extinction of either of these two taxa, especially the amphipod, would impair the breakdown of particulate organic matter. However, because there are other ESPs capable of this ecosystem service (Table 1), the breakdown of buried leaf litter in lateral gravel bars at Tallowood Point is unlikely to cease completely.
Stygofauna and ecosystem service provision in alluvial aquifers of the South Island, New Zealand
Hydrological and environmental features of South Island alluvial aquifer ecosystems
The large aquifers of deep alluvial outwash plains dominating the eastern side of New Zealand’s South Island receive water from the relatively impervious greywacke hills and mountains to the west. Rivers flowing from these mountains also contribute to the aquifer, especially where coarser gravels dominate the riverbeds near the foothills (Taylor et al. 1989; Larned et al. in press). Conversely, groundwater recharge of these rivers increases near the coast, where the water table intersects the more gently sloping land surface. In many places, subsurface impervious silty-clay lenses intervene to force groundwater to the surface (Bowden et al. 1983).
As groundwater traverses the plains within the upper aquifer over tens to hundreds of years (Bowden et al. 1983), the aquifer ecosystem transforms the water. The resulting environmental gradients probably resemble those described above for lateral gravel bars in rivers (Fig. 2). As water remains within the aquifer, concentrations of dissolved oxygen, oxidised forms of nutrients and organic carbon (both particulate and dissolved) are expected to decline. Concurrently, reduced forms of nutrients, notably ammonium, are hypothesised to increase in concentration, whilst total stygofaunal densities decline (Hancock et al. 2005).
The reality is less straightforward. Horizontal distance along a gradient is generally equated with groundwater residence time within the groundwater so that greater transformations are expected to correlate with residence time and, hence, horizontal distance along the gradient. However, these alluvial aquifers are far from homogenous, horizontally or vertically. Detailed investigations within a 38 km × 26 km area of the plains (Thorley et al. unpublished data) revealed flow paths that differ substantially in velocity and discharge at any one time, as well as varying temporally as aquifer levels change. Indeed, this aquifer resembles a three-dimensional braided river, with some flow paths flowing and water velocities constant through time whereas others, notably vertical flow paths, decrease in velocity, disconnect or dry during periods of low aquifer levels. For example, water takes ~14 years to travel from its departure point from the adjacent Waimakariri River to the coast along some flow paths, whereas the same passage requires >60 years along other flow paths (Thorley et al. unpublished data). Thus, there are significant practical difficulties in attempting to determine true gradients along flow paths in aquifer ecosystems because the complexity of flow paths frequently confounds sampling along any putative gradient over scales of more than a few tens of metres.
Superimposed on the gradients of mountain-coast and time are additional ones that contribute to the heterogeneity. Rivers crossing the Canterbury Plains lose varying volumes of water to the underlying aquifer along their length, at times with both gains and losses occurring simultaneously (e.g. the Selwyn River (Taylor et al. 1989; Larned et al. in press). An initial survey of a similar North Island alluvial aquifer where suitable sampling wells were arrayed at increasing distances from a river showed a gradient associated with recharge of the aquifer from the river along at least part of its route across an alluvial plain (Scarsbrook and Fenwick 2003). This gradient spanned horizontal scales of up to 2 km and may extend further, particularly alongside rivers with larger volumes of water or where differences between riverine and aquifer conditions (e.g. dissolved organic carbon [DOC] concentrations) are greater.
Additionally, land use effects on groundwater quality and ecosystems may be substantial and occur over widely differing spatial scales (e.g. Sinton 1984; Hayward and Hansen 2004). Marked land-use effects were apparent at two sites in Canterbury where oxidation pond effluent was discharged to land, and these effects extended for more than 1.1 km horizontally (Sinton 1984). Less obvious land-use activities may have significant cumulative effects, superimposing gradients of their impact onto natural groundwater ecosystem gradients. For example, leaching losses from animal waste and fertiliser into the aquifer accumulate across the Canterbury Plains, with resultant high nitrate and nitrite concentrations near the coast (Hayward and Hansen 2004). This effect alone appears to impose gradients over scales of >50 km and the gradients may be even more pronounced where there are discharges of industrial wastewater onto the land (Hayward and Hansen 2004).
Stygofaunal responses to changes in groundwater gradients
As a result of this natural hydrological and environmental complexity in alluvial aquifers, the extent to which stygofaunal invertebrates influence groundwater ecosystem services is very difficult to deduce from correlative field investigations. This is especially true where these ecosystems lie 10–50 m below ground (as across much of the Canterbury Plains) because of the high costs of installing sampling wells. Despite these difficulties, a few studies have gathered some relevant observations on groundwater ecosystem services and stygofaunal responses to changes in groundwater gradients, and the likely impacts of stygofauna on these ecosystem services within Canterbury’s large aquifer system.
One example is at Templeton where there are wells at increasing distances downstream of an oxidation pond effluent disposal area some 10 km from the nearest surface water body. Using bacteria and bacteriophages as tracers, effluent and its associated POM and DOC was found to percolate from the ground surface through highly porous, poorly sorted alluvial gravels into the aquifer some 12 m below the ground surface (Sinton et al. 1997, 2005). Some microbes reached the aquifer 30 m down-gradient of the disposal site within 10 h of irrigation. Highest concentrations of the tracers reached this well after 15 h, and persisted for 80 h (Sinton et al. 1997, 2005). Horizontal transport of these tracers indicated groundwater velocities of up to 8 m/h (Martin and Noonan 1977). There was an inconsistent gradient in water quality but a more consistent gradient in bacterial (coliforms, faecal coliforms, faecal streptococci, Clostridium perfringens) concentrations with increasing distance over ~1100 m downstream of the Templeton disposal area (Sinton 1984; Fenwick unpublished data). Water quality in a single upstream well was consistently better and bacterial concentrations always lower than those in the farthest downstream well (Sinton 1984; Fenwick unpublished data).
Although physical processes, specifically pore size exclusion, may explain the attenuation in microbe numbers (e.g. Sinton et al. 2005), groundwater ecosystem services also are implicated in this natural, downstream remediation of groundwater due to the very high numbers of typically rare stygofaunal species along this gradient. Large numbers, notably crustaceans up to 20 mm long, occurred in these wells at Templeton, with numbers more than ten times higher immediately downstream of the effluent disposal area, compared with upstream or further downstream (Sinton 1984; Scarsbrook and Fenwick 2003). The data are partly confounded by differences in well casing slot areas and sizes (Sinton et al. 1997) and limited replication of wells yet abundance tended to decrease with increasing distance away from the disposal area (Sinton 1984; Scarsbrook and Fenwick 2003). Additionally, the guts of more than half (n = 100) of all individuals of the two dominant crustaceans tested positive for presumptive coliforms and 12% of these had coliform bacteria in their guts (Sinton 1984). The dominant crustacean, Phreatoicus typicus Chilton 1882 (Isopoda: Phreatoicidae), was found to both ingest live bacteria and to digest these (Fenwick et al. 2004). Here then, the stygofauna contributes to groundwater ecosystem services by consuming natural and contaminant microbes.
This large (up to 20 mm long) isopod feeds by ingesting clay-sized particles and digesting organic carbon from the associated biofilms (Fenwick et al. 2004). Extrapolations from experimentally-measured consumption rates and crude density estimates indicate that populations of these animals process 7–28 tonnes of sediment ha–1 y–1 and assimilate 120–650 g of organic carbon ha–1 y–1 (Fenwick et al. 2004; Fenwick unpublished data). Another estimate based on applying amphipod assimilation rates from the literature suggested that up to 20% of the calorific value of the effluent applied to the disposal area was assimilated by Phreatoicus and the two most abundant amphipods present beneath the 14 ha site (Sinton 1984). Despite the approximations involved in these estimates of stygofauna population feeding and assimilation, it is clear that significant grazing effects and bioturbation occurred in the vicinity of the Templeton site. This level of stygofaunal browsing would remove biofilm on one hand and enhance biofilm activity on the other (Hancock et al. 2005). As a result, stygofaunal grazing of these finer sediments provides further ecosystem services by maintaining water flow through fine pore spaces, promoting the aerobic nature of these finer sediments, and removing potentially harmful microbes, in turn, maintaining water quality.
These studies show that some stygofaunal species fulfill keystone roles within this alluvial groundwater ecosystem. The large isopod Phreatoicus acted as a keystone species in the organically-enriched aquifer at Templeton because its increased population densities played a significant role in processing contaminants. It is probably just one ESP within this ecosystem, vitally important to the sustainability of this economically invaluable aquifer, yet we know little of even its geographic range, let alone its general biology (Wilson and Fenwick 1999) and tolerances to the increasing concentrations of land-derived contaminants such as nitrate in Canterbury’s aquifer system.
Conclusions
The generally positive association of invertebrate biodiversity with rates of ecosystem functioning and provision of ecosystem services observed in surface terrestrial ecosystems appears to hold true in these two groundwater ecotonal ecosystems. Thus, the high biodiversity reported from various subterranean ‘hotspots’ probably sustains high levels of valuable ecosystem services, such as water purification, bioremediation, and water infiltration and transport. More importantly, this high biodiversity potentially confers resilience of the groundwater ecosystem functions to natural and anthropogenic disturbance because it facilitates compensatory community responses to loss of some of the ESPs. However, where stygofauna biodiversity is reduced by human pressures or is naturally low, these ecosystem services may be extremely vulnerable to disappearance of even a single ESP. This has implications for the resilience of groundwater ecosystems to climate change and human resource use, as well as their ability to continue to provide crucial ecosystem services, many of which cannot be readily replaced by technology.
We modified and extended the strategic research agenda proposed by Kremen (2005), applying it to an example from an Australian alluvial aquifer. This example implied that several ESPs are disproportionately important in facilitating provision of different ecosystem services, suggesting they may be good starting points for addressing our abysmal ignorance of the ecology of stygofauna. The functional inventories and conceptual models from our preliminary data are over-simplistic; indirect controls by predation or occasional environmental events (e.g. 1-in-100 year bed-moving spates for shallow alluvial aquifers) must also be factored into assessing controls and scales of the provision of ecosystem services. Further, we only considered two groundwater ecosystem services in the example. Groundwater invertebrates that are functionally unimportant for these two services may be key ESPs for others, and functional inventories must span the complete range of services if feasible. However, we see merit in adopting this approach because it highlights the functional aspects of invertebrate biodiversity in groundwaters (especially those linked to surface ecosystems such as alluvial aquifers and rivers), and it provides a coordinated agenda for strategic taxonomic and ecological research on stygofauna and individual species’ potential value as ESPs. The resulting insights into fundamental questions in theoretical ecology (cf. river restoration, Lake et al. 2007) will be directly relevant to managing sustainable groundwater ecosystems and their ecosystem services.
We do not wish to leave the impression that groundwater invertebrates should be considered only from the perspective of their potential to facilitate or provide ecosystem services for humans. Ecosystem values extend beyond the ‘use values’ of direct use, ecological function value, and option value (i.e. future drugs, large genetic pools) to include the ‘non-use values’ of existence value and bequest value (Edwards and Abivardi 1998). Bequest value is an altruistic one, acknowledging rights of intergenerational equity and their entitlement to a healthy environment (Pearce and Moran 1994). Existence value refers to the satisfaction that humans derive from simply knowing that an entity exists (Goulder and Kennedy 1997) and can be communicated via images of photogenic stygofauna to enhance policy documents (e.g. Department of Land and Water Conservation 2002) and popular accounts of discovering species new to science (Pain 2005). Groundwaters are, to some degree, the final unexplored aquatic frontier on Earth. Their biodiversity and ecosystem services seem presently undervalued, jeopardising their effective protection and wise management. The use of functional inventories as exemplified here is one way to help suitably revise groundwater management policies while documenting the potential role of stygofaunal diversity in promoting groundwater goods and services in Australia and overseas.
Acknowledgements
We appreciate the invitation to present these ideas on groundwater biodiversity and ecosystem services at the 5th ‘Southern Connections Conference’ (Adelaide, January 2007) and in this Special Issue. Funding from the Australian Research Council is gratefully acknowledged, and we thank Darren Ryder, Moya Tomlinson, Rhiannon Smith and four anonymous referees for comments on early drafts. Graham Fenwick thanks Mike Scarsbrook for valuable discussions of groundwater ecosystems, and gratefully acknowledges funding from New Zealand’s Foundation for Research, Science and Technology (notably Contract C01X0503) that supported much of the research into New Zealand’s groundwater ecosystems.
Balvanera P.,
Kremen C., Martinez-Ramos M.
(2005) Applying community structure analysis to ecosystem function: Examples from pollination and carbon storage. Ecological Applications 15, 360–375.
| Crossref | GoogleScholarGoogle Scholar |

Bärlocher F., Murdoch L. H.
(1989) Hyporheic biofilms – a potential food source for interstitial animals. Hydrobiologia 184, 61–67.

Boulton A. J.
(2000a) River ecosystem health down under: assessing ecological condition in riverine groundwater zones in Australia. Ecosystem Health 6, 108–118.
| Crossref | GoogleScholarGoogle Scholar |

Boulton A. J.
(2005) Chances and challenges in the conservation of groundwater-dependent ecosystems. Aquatic Conservation: Marine & Freshwater Ecosystems 15, 319–323.
| Crossref | GoogleScholarGoogle Scholar |

Boulton A. J.
(2007) Hyporheic rehabilitation in rivers: restoring vertical connectivity. Freshwater Biology 52, 632–650.
| Crossref | GoogleScholarGoogle Scholar |

Boulton A. J., Foster J. G.
(1998) Effects of buried leaf litter and vertical hydrologic exchange on hyporheic water chemistry and fauna in a gravel-bed river in northern New South Wales, Australia. Freshwater Biology 40, 229–243.
| Crossref | GoogleScholarGoogle Scholar |

Boulton A. J., Hancock P. J.
(2006) Rivers as groundwater dependent ecosystems: a review of degrees of dependency, riverine processes, and management implications. Australian Journal of Botany 54, 133–144.
| Crossref | GoogleScholarGoogle Scholar |

Boulton A. J.,
Harvey M. S., Proctor H.
(2004) Of spates and species: responses by interstitial water mites to simulated spates in a subtropical Australian river. Experimental & Applied Acarology 34, 149–169.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Boulton A. J.,
Humphreys W. F., Eberhard S. M.
(2003) Imperilled subsurface waters in Australia: biodiversity, threatening processes and conservation. Aquatic Ecosystem Health & Management 6, 41–54.
| Crossref | GoogleScholarGoogle Scholar |

Boulton A. J.,
Findlay S.,
Marmonier P.,
Stanley E. H., Valett H. M.
(1998) The functional significance of the hyporheic zone in streams and rivers. Annual Review of Ecology and Systematics 29, 59–81.
| Crossref | GoogleScholarGoogle Scholar |

Boyero L.,
Pearson R. G., Camacho R.
(2006) Leaf breakdown in tropical streams: the role of different species in ecosystem functioning. Archiv für Hydrobiologie 166, 453–466.
| Crossref | GoogleScholarGoogle Scholar |

Bradbury J. H., Williams W. D.
(1997) Amphipod (Crustacea) diversity in underground waters in Australia: an Aladdin’s cave. Memoirs of Museum Victoria 56, 513–519.

Brunke M., Gonser T.
(1997) The ecological significance of exchange processes between rivers and groundwater. Freshwater Biology 37, 1–33.
| Crossref | GoogleScholarGoogle Scholar |

Castellarini F.,
Malard F.,
Dole-Olivier M.-J., Gibert J.
(2007) Modelling the distribution of stygobionts in the Jura Mountains (eastern France). Implications for the protection of ground waters. Diversity & Distributions 13, 213–224.

Cheshire K.,
Boyero L., Pearson R. G.
(2005) Food webs in tropical Australian streams: shredders are not scarce. Freshwater Biology 50, 748–769.
| Crossref | GoogleScholarGoogle Scholar |

Claret C.,
Marmonier P.,
Dole-Olivier M. -J.,
Creuzé des Châtelliers M.,
Boulton A. J., Castella E.
(1999) A functional classification of interstitial invertebrates: supplementing measures of biodiversity using species traits and habitat affinities. Archiv für Hydrobiologie 145, 385–403.

Cohen J. E.
(2005) Human population grows up. Scientific American 293, 48–55.

Coleman R. L., Dahm C. N.
(1990) Stream geomorphology: effects on periphyton standing crop and primary production. Journal of the North American Benthological Society 9, 293–302.
| Crossref | GoogleScholarGoogle Scholar |

Cooper S. J. B.,
Hinze S.,
Leys R.,
Watts C. H. S., Humphreys W. F.
(2002) Islands under the desert: molecular systematics and evolutionary origins of stygobitic water beetles (Coleoptera: Dytiscidae) from central Western Australia. Invertebrate Systematics 16, 589–598.
| Crossref | GoogleScholarGoogle Scholar |

Cooper S. J. B.,
Bradbury J. H.,
Saint K. M.,
Leys R.,
Austin A. D., Humphreys W. F.
(2007) Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from central Western Australia. Molecular Ecology 16, 1533–1544.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Costanza R.,
d’Arge R.,
de Groot R.,
Farber S., Grasso M. , et al..
(1997) The value of the world’s ecosystem services and natural capital. Nature 387, 253–260.
| Crossref | GoogleScholarGoogle Scholar |

Crenshaw C. L.,
Valett H. M., Webster J. R.
(2002) Effects of augmentation of coarse particulate organic matter on metabolism and nutrient retention in hyporheic sediments. Freshwater Biology 47, 1820–1831.
| Crossref | GoogleScholarGoogle Scholar |

Culver D. C., Sket B.
(2000) Hotspots of subterranean biodiversity in caves and wells. Journal of Cave and Karst Studies 62, 11–17.

Cummins K. W.
(1974) Structure and function of stream ecosystems. Bioscience 24, 631–641.
| Crossref | GoogleScholarGoogle Scholar |

Cummins K. W., Klug M. J.
(1979) Feeding ecology of stream invertebrates. Annual Review of Ecology and Systematics 10, 147–172.
| Crossref | GoogleScholarGoogle Scholar |

Danielopol D. L.
(1989) Groundwater fauna associated with riverine aquifers. Journal of the North American Benthological Society 8, 18–35.
| Crossref | GoogleScholarGoogle Scholar |

Danielopol D. L., Pospisil P.
(2001) Hidden biodiversity in the groundwater of the Danube Flood Plain National Park (Austria). Biodiversity and Conservation 10, 1711–1721.
| Crossref | GoogleScholarGoogle Scholar |

Danielopol D. L.,
Pospisil P., Rouch R.
(2000) Biodiversity in groundwater: a large-scale view. Trends in Ecology & Evolution 15, 223–224.
| Crossref | GoogleScholarGoogle Scholar |

Danielopol D. L.,
Griebler C.,
Gunatilaka A., Notenboom J.
(2003) Present state and future prospects for groundwater ecosystems. Environmental Conservation 30, 104–130.
| Crossref | GoogleScholarGoogle Scholar |

Danielopol D. L.,
Gibert J.,
Griebler C.,
Gunatilaka A.,
Hahn H. J.,
Messana G.,
Notenboom J., Sket B.
(2004) Incorporating ecological perspectives in European groundwater management policy. Environmental Conservation 31, 185–189.
| Crossref | GoogleScholarGoogle Scholar |

Dirzo R., Raven P. H.
(2003) Global state of biodiversity and loss. Annual Review of Environment and Resources 28, 137–167.
| Crossref | GoogleScholarGoogle Scholar |

Edwards P. J., Abivardi C.
(1998) The value of biodiversity: Where ecology and economy blend. Biological Conservation 83, 239–246.
| Crossref | GoogleScholarGoogle Scholar |

Fernald A. G.,
Landers D. H., Wigington P. J.
(2006) Water quality changes in hyporheic flow paths between a large gravel bed river and off-channel alcoves in Oregon, USA. River Research and Applications 22, 1111–1124.
| Crossref | GoogleScholarGoogle Scholar |

Gessner M. O., Chauvet E.
(2002) A case for using litter breakdown to assess functional stream integrity. Ecological Applications 12, 498–510.
| Crossref | GoogleScholarGoogle Scholar |

Giller P. S., O’Donovan G.
(2002) Biodiversity and ecosystem function: Do species matter? Biology and Environment: Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science 103B, 129–139.

Graça M. A. S.,
Cressa C.,
Gessner M. O.,
Feio M. J.,
Callies K. A., Barrios C.
(2001) Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshwater Biology 46, 947–957.
| Crossref | GoogleScholarGoogle Scholar |

Hancock P. J.,
Boulton A. J., Humphreys W. F.
(2005) Aquifers and hyporheic zones: Towards an ecological understanding of groundwater. Hydrogeology Journal 13, 98–111.
| Crossref | GoogleScholarGoogle Scholar |

Harder L. D., Thomson J. D.
(1989) Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. American Naturalist 133, 323–344.
| Crossref | GoogleScholarGoogle Scholar |

Harvey M. S.
(2002) Short-range endemism among the Australian fauna: some examples from non-marine environments. Invertebrate Systematics 16, 555–570.
| Crossref | GoogleScholarGoogle Scholar |

Hayward S. A., Hansen C. R.
(2004) Nitrate contamination of groundwater in the Ashburton-Rakaia plains. Environment Canterbury R04(9), 1–45.

Heal G.
(2000) Valuing ecosystem services. Ecosystems 3, 24–30.
| Crossref | GoogleScholarGoogle Scholar |

Heneghan L.,
Coleman D. C.,
Zou X.,
Crossley D. A., Haines B. L.
(1999) Soil microarthropod contributions to decomposition dynamics: tropical-temperate comparisons of a single substrate. Ecology 80, 1873–1882.

Herman J. S.,
Culver D. C., Salzman J.
(2001) Groundwater ecosystems and the service of water purification. Stanford Environmental Law Journal 20, 479–495.

Hooper D. U.,
Chapin F. S.,
Ewel J. J.,
Hector A., Inchausti P. , et al..
(2005) Effects of biodiversity on ecosystem functioning: A consensus of current knowledge Ecological Monographs 75, 3–35.
| Crossref | GoogleScholarGoogle Scholar |

Humphreys W. F.
(2001) Groundwater calcrete aquifers in the Australian arid zone: the context to an unfolding plethora of stygal biodiversity. Records of the Western Australian Museum Supplement 64 , 63–83.

Humphreys W. F.
(2006) Aquifers: the ultimate groundwater-dependent ecosystems. Australian Journal of Botany 54, 115–132.
| Crossref | GoogleScholarGoogle Scholar |

Humphreys W. F.
(2008) Rising from Down Under: developments in subterranean biodiversity in Australia from a groundwater fauna perspective. Invertebrate Systematics 22, 85–101.
| Crossref | GoogleScholarGoogle Scholar |

Imhoff M. L.,
Bounoua L.,
de Fries R.,
Lawrence W. T.,
Stutzer D.,
Tucker C. J., Ricketts T.
(2004) The consequences of urban land transformation on net primary productivity in the United States. Remote Sensing of Environment 89, 434–443.
| Crossref | GoogleScholarGoogle Scholar |

Jones J. B., Holmes R. M.
(1996) Surface-subsurface interactions in stream ecosystems. Trends in Ecology & Evolution 11, 239–242.
| Crossref | GoogleScholarGoogle Scholar |

Jonsson M.,
Malmqvist B., Hoffsten P. O.
(2001) Leaf litter breakdown rates in boreal streams: does shredder species richness matter? Freshwater Biology 46, 161–171.
| Crossref | GoogleScholarGoogle Scholar |

Jonsson M.,
Dangles O.,
Malmqvist B., Guerold F.
(2002) Simulating species loss following perturbation: assessing the effects on process rates. Proceedings of the Royal Society of London. Series B. Biological Sciences 269, 1047–1052.
| Crossref | GoogleScholarGoogle Scholar |

Karanovic I.
(2007) Candoninae ostracodes from the Pilbara Region in Western Australia. Crustaceana Monographs 7, 1–432.

Karanovic I., Marmonier P.
(2003) Three new genera and nine new species of the subfamily Candoninae (Crustacea, Ostracoda, Podocopida) from the Pilbara region (Western Australia). Beaufortia 53, 1–51.

Kim K. C., Byrne L. B.
(2006) Biodiversity loss and the taxonomic bottleneck: emerging biodiversity science. Ecological Research 21, 794–810.
| Crossref | GoogleScholarGoogle Scholar |

Klein A. M.,
Steffan-Dewenter I., Tscharntke T.
(2003) Fruit set of highland coffee increases with the diversity of pollinating bees. Proceedings of the Royal Society of London. Series B. Biological Sciences 270, 955–961.
| Crossref | GoogleScholarGoogle Scholar |

Kremen C.
(2005) Managing ecosystem services: what do we need to know about their ecology? Ecology Letters 8, 468–479.
| Crossref | GoogleScholarGoogle Scholar |

Kremen C.,
Williams N. M.,
Bugg R. L.,
Fay J. P., Thorp R. W.
(2004) The area requirements of an ecosystem service: crop pollination by native bee communities in California. Ecology Letters 7, 1109–1119.
| Crossref | GoogleScholarGoogle Scholar |

Lake P. S.
(2003) Ecological effects of perturbation by drought in flowing waters. Freshwater Biology 48, 1161–1172.
| Crossref | GoogleScholarGoogle Scholar |

Lake P. S.,
Bond N., Reich P.
(2007) Linking ecological theory with stream restoration. Freshwater Biology 52, 597–615.
| Crossref | GoogleScholarGoogle Scholar |

Larned S. T.,
Hicks D. M.,
Schmidt J.,
Davey A. J. H.,
Dey K.,
Scarsbrook M.,
Arscott D. B., Woods R. A.
(2008) The Selwyn River of New Zealand: a benchmark system for alluvial plain rivers. River Research and Applications 24, 1–21.
| Crossref | GoogleScholarGoogle Scholar |

Larsen T. H.,
Williams N. M., Kremen C.
(2005) Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecology Letters 8, 538–547.
| Crossref | GoogleScholarGoogle Scholar |

Lawton J.
(1994) What do species do in ecosystems? Oikos 71, 367–374.
| Crossref | GoogleScholarGoogle Scholar |

Lenting N.,
Williams D. D., Fraser B. G.
(1997) Qualitative differences in interstitial organic matter and their effect on hyporheic colonization. Hydrobiologia 344, 19–26.
| Crossref | GoogleScholarGoogle Scholar |

Leys R.,
Watts C. H. S.,
Cooper S. J. B., Humphreys W. F.
(2003) Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution 57, 2819–2834.
| PubMed |

Loreau M.,
Naeem S.,
Inchausti P.,
Bengtsson J., Grime J. P. , et al..
(2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Losey J. E., Vaughan M.
(2006) The economic value of ecological services provided by insects. Bioscience 56, 311–323.
| Crossref | GoogleScholarGoogle Scholar |

Lubchenco J.
(1998) Entering the century of the environment: a new social contract for science. Science 279, 491–497.
| Crossref | GoogleScholarGoogle Scholar |

Luck G. W.,
Daily G. C., Ehrlich P. R.
(2003) Population diversity and ecosystem services. Trends in Ecology & Evolution 18, 331–336.
| Crossref | GoogleScholarGoogle Scholar |

Marmonier P.,
Vervier P.,
Gibert J., Dole-Olivier M.-J.
(1993) Biodiversity in groundwater. Trends in Ecology & Evolution 8, 392–395.
| Crossref | GoogleScholarGoogle Scholar |

Marshall M. C., Hall R. O.
(2004) Hyporheic invertebrates affect N cycling and respiration in stream sediment microcosms. Journal of the North American Benthological Society 23, 416–428.
| Crossref | GoogleScholarGoogle Scholar |

Mermillod-Blondin F.,
Creuzé des Châtelliers M.,
Gerino M., Gaudet J. P.
(2000) Testing the effect of Limnodrilus sp. (Oligochaeta, Tubificidae) on organic matter and nutrient processing in the hyporheic zone: a microcosm method. Archiv für Hydrobiologie 149, 467–487.

Metzler G. M., Smock L. A.
(1990) Storage and dynamics of subsurface detritus in a sand-bottomed stream. Canadian Journal of Fisheries and Aquatic Sciences 47, 588–594.

Miller W. M., Boulton A. J.
(2005) Managing and rehabilitating ecosystem processes in regional urban streams in Australia. Hydrobiologia 552, 121–133.
| Crossref | GoogleScholarGoogle Scholar |

Murray B. R.,
Hose G. C.,
Eamus D., Licari D.
(2006) Valuation of groundwater-dependent ecosystems: a functional methodology incorporating ecosystem services. Australian Journal of Botany 54, 221–229.
| Crossref | GoogleScholarGoogle Scholar |

Naeem S.,
Thompson L. J.,
Lawler S. P.,
Lawton J. H., Woodfin R. M.
(1995) Empirical evidence that declining species diversity may alter the performance of terrestrial ecosystems. Philosophical Transactions of the Royal Society of London 347, 249–262.
| Crossref | GoogleScholarGoogle Scholar |

Orghidan T.
(1959) Ein neuer Lebensraum des unterirdischen Wassers: Der hyporheische Biotop. Archiv für Hydrobiologie 55, 392–414.

Ostfeld R. S., LoGiudice K.
(2003) Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology 84, 1421–1427.
| Crossref | GoogleScholarGoogle Scholar |

Pain S.
(2005) Underground Australia. New Scientist 6(August), 28–33.

Peterson G.,
Allen C. R., Holling C. S.
(1998) Ecological resilience, biodiversity, and scale. Ecosystems 1, 6–18.
| Crossref | GoogleScholarGoogle Scholar |

Poff N. L.
(1997) Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. Journal of the North American Benthological Society 16, 391–409.
| Crossref | GoogleScholarGoogle Scholar |

Poore G. C. B., Humphreys W. F.
(1992) First record of Thermosbaenacea (Crustacea) from the southern hemisphere: a new species from a cave in tropical Western Australia. Invertebrate Taxonomy 6, 719–725.
| Crossref | GoogleScholarGoogle Scholar |

Rouch R.
(1977) Considérations sur l’écosystème karstique. Comptes Rendus Hebdomadaires des Seances. Academie des Sciences. Serie D. Sciences Naturelles 284, 1101–1103.

Rouch R., Danielopol D. L.
(1997) Species richness of microcrustacea in subterranean freshwater habitats. Comparative analysis and approximate evaluation. Internationale Revue der Gesamten Hydrobiologie 82, 121–145.
| Crossref | GoogleScholarGoogle Scholar |

Scarsbrook M. R., Fenwick G. D.
(2003) A preliminary assessment of crustacean distribution patterns in New Zealand groundwater aquifers. New Zealand Journal of Marine and Freshwater Research 37, 405–413.

Schwartz M. W.,
Brigham C. A.,
Hoeksema J. D.,
Lyons K. G.,
Mills M. H., van Mantgem P. J.
(2000) Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia 122, 297–305.
| Crossref | GoogleScholarGoogle Scholar |

Seward P.,
Xu Y. X., Brendonck L.
(2006) Sustainable groundwater use, the capture principle, and adaptive management. Water S.A. 32, 473–482.

Sinton L. W.
(1984) The macroinvertebrates of a sewage-polluted aquifer. Hydrobiologia 119, 161–169.
| Crossref | GoogleScholarGoogle Scholar |

Sinton L. W.,
Finlay R. K.,
Pang L., Scott D. M.
(1997) Transport of bacteria and bacteriophages in irrigated effluent into and through an alluvial gravel aquifer. Water, Air, and Soil Pollution 98, 17–42.

Sinton L. W.,
Braithwaite R. R.,
Hall C. H.,
Pang L.,
Close M. E., Noonan M. J.
(2005) Tracing the movement of irrigated effluent into an alluvial aquifer. Water, Air, and Soil Pollution 166, 287–301.
| Crossref | GoogleScholarGoogle Scholar |

Sket B.
(1999) The nature of biodiversity in hypogean waters and how it is endangered. Biodiversity and Conservation 8, 1319–1338.
| Crossref | GoogleScholarGoogle Scholar |

Sodhi N. S.,
Liow L. H., Bazzaz F. A.
(2004) Avian extinctions from tropical and subtropical forests. Annual Review of Ecology, Evolution and Systematics 35, 323–345.
| Crossref | GoogleScholarGoogle Scholar |

Song J. X.,
Chen X. H.,
Cheng C.,
Summerside S., Wen F. J.
(2007) Effects of hyporheic processes on streambed vertical hydraulic conductivity in three rivers of Nebraska. Geophysical Research Letters 34, L07409.
| Crossref | GoogleScholarGoogle Scholar |

Springer A. M.,
Estes J. A.,
van Vliet G. B.,
Williams T. M.,
Doak D. F.,
Danner E. M.,
Forney K. A., Pfister B.
(2003) Sequential megafaunal collapse in the North Pacific Ocean: an ongoing legacy of industrial whaling? Proceedings of the National Academy of Sciences of the United States of America 100, 12 223–12 228.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Taylor C. B.,
Wilson D. D.,
Brown L. J.,
Stewart M. K.,
Burden R. J., Brailsford G. W.
(1989) Sources and flow of north Canterbury Plains groundwater, New Zealand. Journal of Hydrology 106, 311–340.
| Crossref | GoogleScholarGoogle Scholar |

Tillman D. C.,
Moerke A. H.,
Ziehl C. L., Lamberti G. A.
(2003) Subsurface hydrology and degree of burial affect mass loss and invertebrate colonisation of leaves in a woodland stream. Freshwater Biology 48, 98–107.
| Crossref | GoogleScholarGoogle Scholar |

Tilman D.
(1996) Biodiversity: populations versus ecosystem stability. Ecology 77, 350–363.
| Crossref | GoogleScholarGoogle Scholar |

Tilman D.
(1997) Distinguishing between the effects of species diversity and species composition. Oikos 80, 185–193.
| Crossref | GoogleScholarGoogle Scholar |

Tilman D.,
Lehmann C., Bristow C.
(1998) Diversity-stability relationships: statistical inevitability or ecological consequence. American Naturalist 151, 277–282.
| Crossref | GoogleScholarGoogle Scholar |

Vannote R. L.,
Minshall G. W.,
Cummins K. W.,
Sedell J. R., Cushing C. E.
(1980) The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37, 130–137.

Vitousek P. M.,
Mooney H. A.,
Lubchenco J., Melillo J. M.
(1997) Human domination of Earth’s ecosystems. Science 277, 494–499.
| Crossref | GoogleScholarGoogle Scholar |

Walker B. H.
(1992) Biodiversity and ecological redundancy. Conservation Biology 6, 18–23.
| Crossref | GoogleScholarGoogle Scholar |

Walker B. H.
(1995) Conserving biological diversity through ecosystem resilience. Conservation Biology 9, 747–752.
| Crossref | GoogleScholarGoogle Scholar |

Watts C. H. S., Humphreys W. F.
(2006) Twenty-six new Dytiscidae (Coleoptera) of the genera Limbodessus Guignot and Nirripirti Watts & Humphreys from underground waters in Australia. Transactions of the Royal Society of South Australia 130, 123–185.

Williams D. D., Hynes H. B. N.
(1974) The occurrence of benthos deep in the substratum of a stream. Freshwater Biology 4, 233–256.
| Crossref | GoogleScholarGoogle Scholar |

Wilson G. D. F., Fenwick G. D.
(1999) Taxonomy and ecology of Phreatoicus typicus Chilton, 1883 (Crustacea, Isopoda, Phreatoicidae). Journal of the Royal Society of New Zealand 29, 41–64.

Worm B.,
Barbier E. B.,
Beaumont N.,
Duffy J. E., Folke C. , et al..
(2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790.
| Crossref | GoogleScholarGoogle Scholar | PubMed |