Protecting the innocent: studying short-range endemic taxa enhances conservation outcomes
Mark S. Harvey A B C G , Michael G. Rix A , Volker W. Framenau D A B , Zoë R. Hamilton E B , Michael S. Johnson B , Roy J. Teale E A B , Garth Humphreys E A B and William F. Humphreys A B FA Department of Terrestrial Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australia 6986, Australia.
B School of Animal Biology, University of Western Australia, Crawley, Western Australia 6009, Australia.
C Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 49th Street, New York, NY 10024-5192, USA; and California Academy of Sciences, Golden Gate Park, San Francisco, CA 94103-3009, USA.
D Phoenix Environmental Sciences Pty Ltd, PO Box 857, Balcatta, Western Australia 6914, Australia.
E Biota Environmental Sciences Pty Ltd, PO Box 155, Leederville, Western Australia 6903, Australia.
F School of Earth and Environmental Sciences, University of Adelaide, South Australia 5005, Australia; and Karst Waters Institute, PO Box 4142, Leesburg, VA 20177, USA.
G Corresponding author: Email: mark.harvey@museum.wa.gov.au
Invertebrate Systematics 25(1) 1-10 https://doi.org/10.1071/IS11011
Submitted: 18 March 2011 Accepted: 26 May 2011 Published: 14 July 2011
Abstract
A major challenge confronting many contemporary systematists is how to integrate standard taxonomic research with conservation outcomes. With a biodiversity crisis looming and ongoing impediments to taxonomy, how can systematic research continue to document species and infer the ‘Tree of Life’, and still maintain its significance to conservation science and to protecting the very species it strives to understand? Here we advocate a systematic research program dedicated to documenting short-range endemic taxa, which are species with naturally small distributions and, by their very nature, most likely to be threatened by habitat loss, habitat degradation and climate change. This research can dovetail with the needs of industry and government to obtain high-quality data to inform the assessment of impacts of major development projects that affect landscapes and their biological heritage. We highlight how these projects are assessed using criteria mandated by Western Australian legislation and informed by guidance statements issued by the Environmental Protection Authority (Western Australia). To illustrate slightly different biological scenarios, we also provide three case studies from the Pilbara region of Western Australia, which include examples demonstrating a rapid rise in the collection and documentation of diverse and previously unknown subterranean and surface faunas, as well as how biological surveys can clarify the status of species thought to be rare or potentially threatened. We argue that ‘whole of biota’ surveys (that include all invertebrates) are rarely fundable and are logistically impossible, and that concentrated research on some of the most vulnerable elements in the landscape – short-range endemics, including troglofauna and stygofauna – can help to enhance conservation and research outcomes.
Introduction
Conservation biology strives to understand the processes and mechanisms that can be used to identify and preserve species, ecosystems and the underlying genetic diversity of life (see Meine et al. 2006). Billions of dollars are invested annually around the world to help conserve the most vulnerable of Earth’s inhabitants. Areas are included in reserves intended for the conservation of biodiversity generally, or to protect particular threatened ecosystems, communities or species. Zoos and other organisations engage in breeding programs to maintain captive populations of species at risk or even extinct in the wild. Most of these funds are directed at either habitat protection or restoration or towards so-called ‘charismatic’ species. Who can deny the plight of the tiger, Panthera tigris (Linnaeus, 1758), listed as endangered by the International Union for the Conservation of Nature (IUCN) (Chundawat et al. 2010) and estimated in a media release to become extinct in the wild by 2022 (e.g. Sydney Morning Herald, 22 November 2010)? Or that of Spix’s macaw, Cyanopsitta spixii (Wagler, 1832), listed as critically endangered by the IUCN and likely to be extinct in the wild throughout its native range of eastern Brazil (Anonymous 2010)? These iconic and beautiful species rightly engender sympathy and our greatest efforts to aid their ongoing preservation.
However, most of the world’s organisms live largely unnoticed by humans, with most species – both described and undescribed – known only to taxonomic specialists, and many of those known from only a handful of preserved specimens in museums and herbaria. It is unknown how many of these species are threatened or at risk, and a major challenge confronting many contemporary systematists is how to integrate standard taxonomic research with conservation outcomes (Agnarsson and Kuntner 2007). Although ‘alpha taxonomy’ – i.e. the description of new species, redescription of previously named species and the documentation of their distributions, habitat preferences and seasonality – is sufficient to ensure an ongoing and valuable contribution towards identifying and preserving biodiversity, the vast number of still undescribed species is a daunting hurdle for many taxonomists. Indeed, studying these organisms is challenging and time-consuming and, while the advent of molecular techniques has aided this process considerably, the ongoing ‘taxonomic impediment’ (Taylor 1983) calls into focus the role of modern systematics in the face of a complex biodiversity crisis (Crozier 1992, 1997; Faith 1992, 1994; Agnarsson and Kuntner 2007; de Carvalho et al. 2007). With taxonomic expertise, training and funding dwindling (Agnarsson and Kuntner 2007) and conservation concerns increasing, where should our efforts be concentrated? How can systematic research continue to document biodiversity and infer the ‘Tree of Life’ (Donoghue and Cracraft 2004), and still maintain its significance to conservation science and to protecting the very species it strives to understand?
Here we advocate a systematic research program dedicated to documenting short-range endemic (SRE) taxa, which, by their very nature, are threatened or are most likely to be threatened by habitat loss, habitat degradation and climate change. A focus on short-range endemics dovetails with the needs of industry and government agencies to obtain high-quality data to assess the impacts of major development projects that affect landscapes and their biological heritage. This approach has been widely applied in Western Australia over the past decade, in a landscape where extraordinary biodiversity and natural heritage values (Hopper et al. 1996; Myers et al. 2000; Hopper and Gioia 2004) intersect with an ever-expanding resources sector. These large-scale developments must conform to a legislative framework in Western Australia – the Wildlife Conservation Act 1950 and the Environmental Protection Act 1986 – where the protection of unique habitats and individual taxa (species and subspecies) is promoted and regulated.
Short-range endemism
Species with naturally small distributions are termed short-range endemic species (Harvey 2002). Although often considered to be restricted to invertebrate animals, SREs can in fact be found among all major biological lineages. Any taxon that has a restricted, small distribution qualifies as an SRE, including most threatened species, many of which are under threat simply because they are restricted to small patches of suitable (but often dwindling) habitat. Harvey (2002) noted several life-history features that are characteristic – and may be central to the evolution of – short-range endemism, including poor powers of dispersal, ecological confinement to discontinuous or rare habitats, slow growth and low fecundity. These factors can all lead to evolutionary diversification over time and to highly restricted modern distributions. Harvey (2002) suggested a nominal range of less than 10 000 km2 as a working definition of short-range endemism, but Eberhard et al. (2009), considering stygofauna, recommended a reduced area of less than 1000 km2. Even this may need further refinement as, for example, groundwater calcrete aquifers of the southern Western Shield (Yilgarn) each have unique faunas largely comprising endemic species (Humphreys 2008; Guzik et al. 2011a; and references therein). Of 77 unique calcrete faunas listed as ‘priority communities’ by the Western Australian Department of Environment and Conservation, the mean area of each calcrete is 90.8 km2 (range 0.89–2205 km2) and some of these distinct calcretes are only 1 km apart.
To incorporate short-range endemism into conservation assessments and to produce positive conservation outcomes in accordance with the Wildlife Conservation Act 1950 and the Environmental Protection Act 1986, the Western Australian Environmental Protection Authority (EPA) has produced a guidance statement specific to SREs. This statement requires proponents of large-scale activities, such as mining and associated infrastructure, to survey for SRE species to allow informed assessment of development proposals (Environmental Protection Authority 2009). An associated set of guidance statements dealing with subterranean fauna has also been developed (Environmental Protection Authority 2003, 2007) to aid in the sampling of highly diverse short-range endemic troglofauna and stygofauna that occur throughout large areas of Western Australia (Humphreys 2008). The EPA wishes ‘to ensure adequate protection of important habitats for these species’ (Environmental Protection Authority 2003), and the onus is on proponents to demonstrate that SRE species, including subterranean fauna, are not irreparably affected by the proposed activity. A positive side effect of these requirements is that we are now gaining insights into a previously poorly known and largely undocumented fauna (see review by Guzik et al. 2011a). Collaborative research projects being developed between industry, government and universities foster a unique blend of outcomes that not only document and describe the fauna, but also provide sufficient background data against which regulatory authorities can assess proposals to ensure positive conservation outcomes.
We highlight this scientific and legislative situation in the context of Western Australia’s Pilbara region, an area of some 500 000 km2, which is highly prospective for several minerals, principally iron ore but also gold and other precious metals. The large-scale, even landscape-scale, developments either underway or proposed for the region are now heavily scrutinised by state government agencies before their approval, aiming to ensure ‘the protection of any portion of the environment’ (Section 28, Environmental Protection Act 1986). Several case studies are presented here to demonstrate the conservation benefits that detailed studies on some of the most vulnerable species in the landscape – short-range endemics – can have. These case-studies are not meant to be comprehensive, but provide a vignette of three different investigations that have led to a much greater understanding of individual species, their taxonomy, phylogenetic relationships and their significance to proponents seeking approval for developments that may threaten populations of these and other species. While several short-range endemic invertebrate species from the Pilbara region are listed under Western Australian legislation, there is currently only a single species listed under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999, the remipede crustacean Lasionectes exleyi Yager & Humphreys, 1996 from the Cape Range Peninsula. We therefore restrict our comments to the Western Australian legislation and associated guidance statements that are relevant to the case studies presented below.
Schizomids (family Hubbardiidae)
Schizomida (Fig. 1) is a small arachnid order with ~270 described species in two families: the meso-American Protoschizomidae and the circum-tropical Hubbardiidae (Reddell and Cokendolpher 1995; Harvey 2003, 2007). The Australian fauna comprises 52 named species from eastern and northern Australia, of which nearly one-third (16 species) are restricted to subterranean habitats. The discovery of a troglobitic schizomid from caves in Cape Range represented the first schizomid to be described from Australia (Harvey 1988), and this species was subsequently listed as threatened under the Western Australian Wildlife Conservation Act 1950. The remaining Australian schizomids have been treated in successive revisions (Harvey 1992, 2000a, 2000b, 2001; Harvey and Humphreys 1995; Harvey et al. 2008). The north-western Australian schizomid fauna is of particular interest, as there appear to be no surface representatives in the region, with the entire schizomid fauna consisting of a variety of troglobites. This fauna is thought to represent a relictual vestige of a widespread tropical fauna that was extirpated in surface environments due to the progressive drying of the region since the Miocene (Humphreys 1993). This Tertiary drying (Byrne et al. 2008) ameliorated habitats that were suitable for moisture-dependent animals, such as schizomids.

|
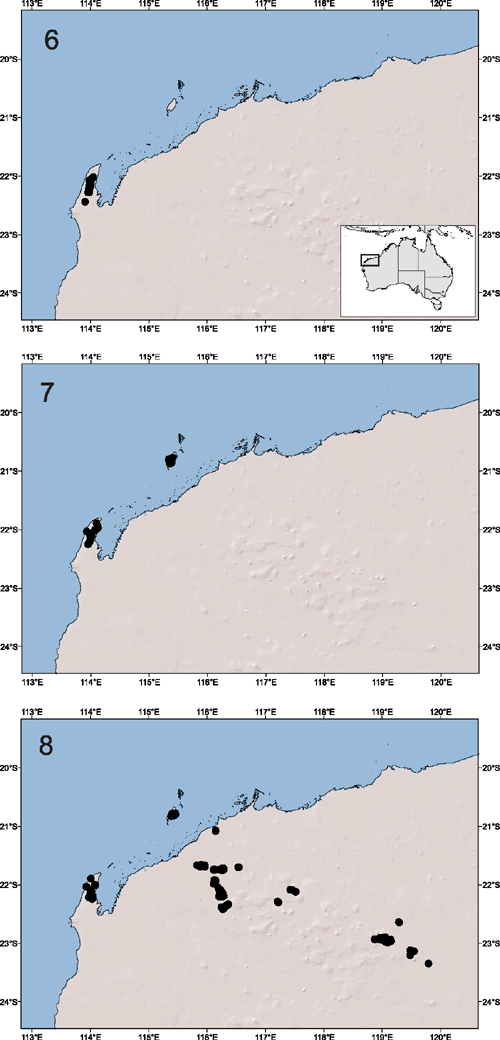
The requirement for all resource development proponents in Western Australia to sample subterranean fauna, wherever they are likely to occur (Environmental Protection Authority 2003, 2007), has resulted in the collection of numerous schizomids from across the Pilbara during the past decade. Indeed, since the description of the first schizomid from Cape Range, now known as Draculoides vinei (Harvey, 1988), a surprising diversity of schizomids has been detected in the Pilbara region. The description of five newly discovered species of Draculoides Harvey, 1992 (Fig. 1), two new species of Bamazomus Harvey, 1992 and four new species of Paradraculoides Harvey, Berry, Edward & Humphreys, 2008 (Harvey and Humphreys 1995; Harvey 2001; Harvey et al. 2008) has brought the total number of named species in the Pilbara to 12. This incremental increase in our knowledge is portrayed in Figs 6–8, with relatively few schizomids collected during the 1980s and 1990s, followed by a rapid increase in sampling success during the 2000s. At least 15 new species are thought to be represented among these new collections (MSH, VWF, unpubl. data), bringing considerable insight to a poorly known group of arachnids. Many species are known to be endemic to individual geological formations, such as pisolitic mesas only a few hundred hectares in area (Harvey et al. 2008), and no species are known from an area greater than 500 km2, clearly conforming to current definitions of short-range endemism (Harvey 2002; Eberhard et al. 2009; Environmental Protection Authority 2009). Knowledge of the taxonomy and distribution of different species and genera has been refined with each new discovery, and recent molecular phylogeographic research has highlighted the evolutionary inter-relationships among species (Harvey et al. 2008) and the remarkable subterranean biogeography of the region (summarised in Guzik et al. 2011a).
The conservation implications of these research outcomes are manifold. The discovery of subterranean short-range endemic invertebrates in areas that are ear-marked for mining activity brings considerable uncertainty to the proponents. In contrast, fine-scale studies on the taxonomy and molecular relationships of SREs, combined with extensive sampling programs, can provide sufficient information for the regulatory agencies to approve mining proposals with conservation outcomes embedded within each proposal. The application to extract iron ore from ‘Mesa A’ and ‘Warramboo’, west of Pannawonica, was initially opposed by the Western Australian EPA, but was ultimately approved by the Minister for the Environment in 2007 (Ministerial Approval Statement 756, available from http://www.epa.wa.gov.au/peia/approvalstatements, accessed 19 February 2011) after the extent and scope of the mine was modified by the proponent to include conservation strategies for the schizomid (and the other subterranean species) known to be endemic to Mesa A. Project approval also required additional conservation offsets and research funding to be committed to by the proponent. Detailed research into these obligate troglobites has provided a snapshot of a varied and hitherto unknown fauna, as well as placing them at the centre of a conservation strategy aimed at ensuring viable populations of these taxa persist during and after the development of the iron ore mine. The course of the proposal assessment and associated taxonomic work also served to substantially improve the profile of these little known animals: industry proponents and government regulators alike are now far more aware of their potential significance and relevance to proposal assessment than they were before the EPA’s assessment of the Mesa A project.
As a footnote, Draculoides vinei was eventually removed from the list of Western Australian threatened species after the discovery that it was more widespread than previously suspected and under no imminent danger of extinction. However, 10 other shorter-range endemic schizomid species are currently listed as either vulnerable or endangered by the Minister for the Environment due to their occurrence at few sites and, in many cases, the perceived threat from mining or other destructive activities.
Fire millipedes (family Pachybolidae)
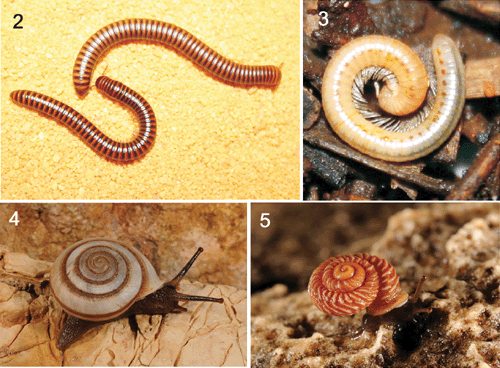
Fire millipedes of the family Pachybolidae (Figs 2, 3) include some large and spectacular millipedes of the order Spirobolida. They appear to represent an ancient radiation, with individual genera restricted to continental masses (Hoffman 1980). In Australia, they occur in the northern tropical zones, as well as in the forests of eastern Australia. Three strange, brightly-coloured pachybolids were collected from the Burrup Peninsula, in the Pilbara region of north-western Australia in 2002, and represented the first epigean spirobolids collected from this region. Prior to this discovery, specimens of a small troglobitic spirobolid were collected from Barrow Island (Hoffman 1994). The large epigean species was subsequently named by Hoffman (2003) as Austrostrophus stictopygus Hoffman, 2003 (Fig. 2). Due to their large size (~5 cm) and distinctive tan and black stripes, it was thought that if the species was widely distributed it would have been collected elsewhere. Indeed, MSH considered nominating A. stictopygus as a threatened species under the Wildlife Conservation Act 1950. However, the guidelines used by the Threatened Species Scientific Committee (which advises the Minister for the Environment on which taxa are to be included as ‘specially protected fauna’ or ‘rare flora’) are rigorous in ascertaining whether a species should be listed as threatened, and it was considered that the nomination would fail the ‘adequacy of survey’ guideline required before listing (available from http://www.dec.wa.gov.au/content/view/866/2006, accessed 19 February 2011).
|
|
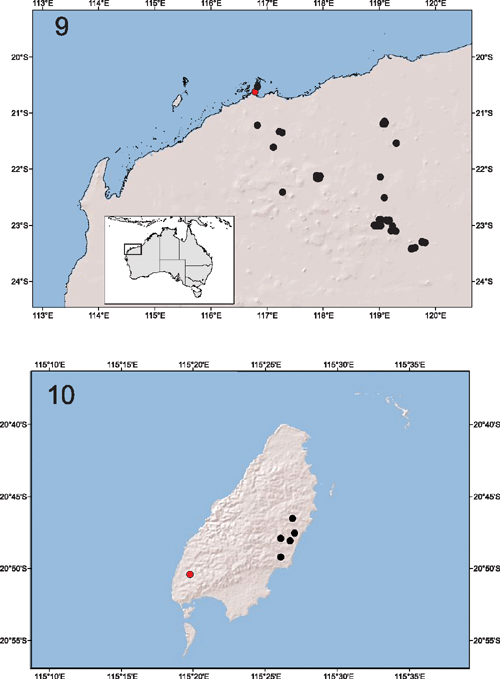
The decision not to list A. stictopygus was, in hindsight, fortuitous. During subsequent environmental surveys before mining activities in the Pilbara region, numerous pachybolids have been collected and submitted to the Western Australian Museum for identification. All males have extremely similar gonopod morphologies to that described for the holotype (Hoffman 2003) and are all deemed to represent the same species. These records indicate that A. stictopygus – originally thought to represent a short-range endemic species restricted to the Burrup Peninsula – is actually widespread across the Pilbara (Fig. 9) and found at a variety of locations where suitable microclimatic conditions occur. The species is clearly not a short-range endemic, and does not need special protection. Similar rigorous sampling and detailed taxonomic research are critical to accurately identify SREs, and to avoid ‘Type I’ errors in the recognition of taxa of conservation concern. In all cases, good taxonomy must operate in concert with adequate environmental surveys of impact regions. Listing SREs of conservation concern is as important as de-listing those that are subsequently recognised as secure (e.g. Draculoides vinei, see above) and never listing those that do not satisfy the Threatened Species Scientific Committee’s guidelines.
|
Austrostrophus stictopygus is not the only fire millipede in the Pilbara bioregion. Speleostrophus nesiotes Hoffman, 1994 (Fig. 3) was described from specimens collected in Ledge Cave on Barrow Island, a continental island situated 130 km off the Pilbara coast. The island is known to harbour an assortment of relictual species, and a significant and highly unusual subterranean community was discovered in the 1990s (Humphreys 2000, 2001), which included various arachnids (e.g. Harvey and Humphreys 1995; Edward and Harvey 2008; Volschenk and Prendini 2008), myriapods (Hoffman 1994), insects (Humphreys 2000), crustaceans (Bradbury and Williams 1996; Bradbury 2000) and fish (Humphreys 2000), most of which are endemic to the island. Although originally known from only a single location, S. nesiotes has since been found at several different sites on Barrow Island (Fig. 10), during environmental surveys commissioned before the approval for the construction of a liquefied natural gas plant on the east coast of Barrow Island (see http://www.chevronaustralia.com/ourbusinesses/gorgon.aspx). While some of the locations at which S. nesiotes has been found occur underneath the footprint of the gas plant, others are found outside the area, suggesting that if the plant has a detrimental effect on the subterranean fauna directly below it, at least some populations of this species will be unaffected (Ministerial Approval Statements 748 and 800, available from http://www.epa.wa.gov.au/peia/approvalstatements/).
The final safeguard in the case of Austrostrophus and Speleostrophus would be molecular analyses to test for the presence of cryptic species, as reported within the Caribbean spirobolid millipede genus Anadenobolus Silvestri, 1897 by Bond (2002) and Bond and Sierwald (2003). Molecular systematic and population genetic methods are crucial to unearthing cryptic taxa (Cook et al. 2010), owing at least, in some taxonomic groups, to their morphological constancy. This has been recently documented among the Western Australian subterranean fauna by Bradford et al. (2010) and Guzik et al. (2011b).
Pilbara land snails (family Camaenidae)
In northern Western Australia, the Camaenidae (Figs 4, 5) are the dominant group of land snails, with greatest diversity in the Kimberley region (Solem 1981a, 1991, 1997). Solem (1997) highlighted that 19 of the 25 camaenid genera found in the Kimberley are restricted SREs. In the Ningbing Ranges east of Kununurra, for example, the median geographical range of the 26 species occupying the area is 0.8 km2 (Cameron 1992). Many species have ranges of only a few square kilometres and, although the Kimberley fauna as a whole contains over 40 species, no more than a few are found at any one site (see Solem 1985). Solem (1997) noted that many of these ranges were already shrinking, due to threatening processes, such as grazing and fire. This resulted in the listing of 31 camaenid species under the ‘specially protected fauna notice’ of the Wildlife Conservation Act 1950.
In response to very clear conservation concerns for species with narrow ranges (e.g. Harvey 2002), combined with an accelerated mining boom in the Pilbara region since the early 2000s, camaenid land snails became a key target of inventory surveys carried out for the purpose of environmental impact assessment (EIA). Solem (1997) provided the only taxonomic framework for the Pilbara camaenids, which comprised 27 species from six genera, distributed between Port Hedland in the north and Cape Range in the south. A striking feature of this diversity is that congeneric species occur as geographic replacements, as Solem (1997) had no examples of sympatry between congeneric species. The documented ranges of several species qualified them as SREs (sensu Harvey 2002); for example, Rhagada pilbarana Solem, 1997 and Strepsitaurus milyeringus Solem, 1997 (Fig. 5) had documented ranges of less than 5 km2. In addition, there were several shell forms from different localities that did not correspond to any of Solem’s (1997) descriptions, and some of these taxa were known from only a few collections over distances of less than 1 km.
The expectation of the EPA is that all potential SRE specimens be identified to the lowest possible taxonomic level when assessing the impact of development proposals (Environmental Protection Authority 2009). This requirement, combined with an incomplete taxonomic framework and lack of relevant taxonomic expertise, led to the use of molecular genetic approaches to address questions concerning camaenid diversity and endemism, initially allozymes (Johnson et al. 2004, 2006), then mitochondrial DNA (Biota Environmental Sciences 2007, 2008; O’Neill 2008; Taylor 2010) and more recently microsatellite DNA (Eades et al. 2010). These analyses are usually informative at relatively small spatial scales, and often at scales not much larger than the proponent’s project footprint. Certainly, to meet the conservation objectives of the EPA, mining companies and other stakeholders, a wider phylogenetic framework is required, especially since EIA surveys continued to discover undescribed forms with abutting but non-overlapping distributions. The lack of co-occurrence of congeneric species complicates assessments of taxonomy, as only where species occur together is there direct evidence of whether they can interbreed, and therefore warrant recognition as separate species. Thus, nearly all taxonomic decisions must be based on indirect evidence of ‘distinctiveness’. With camaenid land snails, this has historically been determined from shell morphology and reproductive anatomy (e.g. Solem 1997), although more recent work using DNA sequences has proved especially useful in searching for such distinct groups.
Targeted sampling of gaps in the known distributions of camaenid land snails has revealed that many forms are parapatric, rather than allopatric, allowing direct genetic tests of reproductive isolation and indicating the inadequacy of the sampling available to Solem (1997). The combination of increased geographic sampling with molecular phylogenetics has revealed that some species have broader distributions than formerly thought (e.g. Rhagada convicta (Cox, 1870)), while other described species are actually complexes of multiple species, some with very narrow distributions (e.g. Strepsitaurus species; Fig. 5). The molecular analyses have also shown the unreliability of shell characteristics on their own for assessing species taxonomy in these snails. For example, Rosemary Island, in the Dampier Archipelago, has five morphologically described species of Rhagada, which span the range of shell variation in the entire genus (Solem 1997). However, detailed molecular and morphological analyses have shown continuity among these forms, indicating that they are a single species (S. Stankowski, unpubl. data). In contrast, other populations with no apparent morphological differences are highly divergent lineages (e.g. Rhagada spp. from the central Pilbara, Fig. 4). In addition to revealing the taxonomic complexities of the camaenids in the Pilbara region, the phylogeographic perspective from wider geographic sampling and molecular phylogenetics is helping to understand the geological and landscape features shaping the evolution of these snails. This understanding can allow predictions of where novel forms might occur, and to accordingly target new areas for biodiscovery (e.g. Biota Environmental Sciences 2008).
Conservation outcomes
Targeted surveys of short-range endemic species potentially have many long-lasting conservation benefits. First, we are gaining an unparalleled insight into the diversity, phylogenetic relationships and, in some cases, genetic characteristics of a previously undocumented fauna. The sheer number of new species and the work needed to prepare full taxonomic descriptions is still daunting and challenging, but interactions between government and industry are providing new avenues for SRE-based research collaboration and species discovery.
Second, the discovery of extensive radiations of subterranean (e.g. Leys et al. 2003; Edward and Harvey 2008; Guzik et al. 2008; Harvey et al. 2008; Guzik et al. 2011a) and epigean short-range endemic taxa (e.g. Solem 1979, 1981a, 1981b, 1984, 1985, 1988, 1991, 1993, 1997; Johnson et al. 2006; Edward and Harvey 2010; Rix and Harvey 2010) provides insights into the diversification of the Western Australian biota under increasingly arid influences since the late Oligocene and Miocene. These influences are now thought to have driven the speciation process throughout much of Australia (e.g. Byrne et al. 2008) and to have had a considerable impact on communities of organisms that are susceptible to reduced moisture and increased temperature.
Third, the accurate identification and description of individual species and detailed documentation of their geographic ranges and habitat requirements provide regulatory authorities the necessary background data to assess major development proposals against as much relevant knowledge as possible. If the goal of government conservation agencies is to reduce, in a sustainable fashion, the impact that humans are having on a stressed landscape, then providing information on those species that are likely to be most affected is a laudable achievement. The guidelines used by the Western Australian government (i.e. those provided by the Environmental Protection Authority 2003, 2007, 2009) to benchmark proposals before development approval ensure transparency in the process. Notably, these guidelines are globally unique in their insistence that biological surveys include short-range endemic invertebrates, specifically including troglofauna and stygofauna, as well as more standard surveys for plants and vertebrate animals.
Concluding remarks
While our knowledge of the identity and distribution of many short-range endemic species is being considerably improved by targeted industry-funded SRE surveys, they are providing data on only a small suite of co-occurring species and do little to understand functional ecosystems or the services they provide (e.g. Boulton et al. 2008; Mooney 2010). This is quite different from the requirements in some other regions of the world, where the emphasis is rightly placed on ecosystem conservation (e.g. Job and Simons 1994) under a legislative framework (e.g. GSchV 1998). Majer (2009) also lamented that the funds available for short-range endemic research are not made available to other components of the terrestrial invertebrate fauna. We agree, but argue that research to improve the rate of discovery and the conservation status of any invertebrate species is a good aim, and what better targets than those species that we already know from their life history traits (e.g. Harvey 2002) are highly restricted in distribution and susceptible to many forms of land alteration? Research targeting SRE taxa also has greater potential to yield insights into phylogeographic patterns, and relationships with historical and contemporary landscape-scale processes, than do most studies of more vagile, less habitat-specific and more widespread invertebrate species. Furthermore, given the current biodiversity crisis (Laurance and Wright 2009; Mooney 2010; Stork 2010) and the ongoing impediments to taxonomy, ‘whole of biota’ surveys (that include all invertebrates) are rarely fundable and are logistically impossible. Clearly, we must find a way to focus our funding and research efforts on those taxa that are potentially most at risk, most likely to be undescribed, and most likely to have distributions small enough that development proposals could significantly impact their total known range. This is especially true in landscapes that may be severely modified by large-scale resource and development projects, where a ‘business as usual’ approach may result in extinctions of short-range endemic species. Like Western Australia, all Australian states and territories and many other countries have legislation aimed at protecting biodiversity and natural heritage values, and all have the potential to recognise and mandate for the survey and protection of short-range endemic taxa.
Acknowledgements
Funds to study the short-range endemic fauna of the Pilbara region were supplied by Rio Tinto, BHP Billiton Iron Ore, Chevron Australia, Woodside Energy and Australian Premium Iron Joint Venture. John Dell (Office of the Environmental Protection Agency) kindly commented on a draft of the manuscript. MGR is funded by the Australian Biological Resources Study (ABRS Grant No. 209–08).
References
Agnarsson, I., and Kuntner, M. (2007). Taxonomy in a changing world: seeking solutions for a science in crisis. Systematic Biology 56, 531–539.| Taxonomy in a changing world: seeking solutions for a science in crisis.Crossref | GoogleScholarGoogle Scholar | 17562477PubMed |
Anonymous (2010). Cyanopsitta spixii. In ‘IUCN 2010. IUCN Red List of Threatened Species’. Version 2010.4. Available at http://www.iucnredlist.org [Verified 20 February 2011].
Biota Environmental Sciences (2007). ‘Molecular systematics of Rhagada from the Pilbara and the Brockman Syncline 4 Project Area.’ Unpublished report for Pilbara Iron.
Biota Environmental Sciences (2008). ‘Brockman Rhagada – a molecular investigation of the ‘BROMD’ population.’ Unpublished report for Pilbara Iron.
Bond, J. E. (2002). Cryptic speciation in the Anadenobolus excisus millipede species complex on the island of Jamaica. Evolution 56, 1123–1135.
| 1:CAS:528:DC%2BD38XlvFKmur0%3D&md5=feb786b70ad434b1748b93e520a4d961CAS | 12144014PubMed |
Bond, J. E., and Sierwald, P. (2003). Molecular taxonomy of the Anadenobolus excisus (Diplopoda: Spirobolida: Rhinocricidae) species-group on the Caribbean island of Jamaica. Invertebrate Systematics 17, 515–528.
| Molecular taxonomy of the Anadenobolus excisus (Diplopoda: Spirobolida: Rhinocricidae) species-group on the Caribbean island of Jamaica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnsVOqu74%3D&md5=2b78a66bee4d114cd16df7dc35f7b551CAS |
Boulton, A. J., Fenwick, G., Hancock, P. J., and Harvey, M. S. (2008). Biodiversity, functional roles and ecosystem services of groundwater invertebrates. Invertebrate Systematics 22, 103–116.
| Biodiversity, functional roles and ecosystem services of groundwater invertebrates.Crossref | GoogleScholarGoogle Scholar |
Bradbury, J. H. (2000). Western Australian stygobiont amphipods (Crustacea: Paramelitidae) from the Mt Newman and Millstream regions. Records of the Western Australian Museum 60, 1–102.
Bradbury, J. H., and Williams, W. D. (1996). Freshwater amphipods from Barrow Island, Western Australia. Records of the Australian Museum 48, 33–74.
| Freshwater amphipods from Barrow Island, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bradford, T., Adams, M., Humphreys, W. F., Austin, A. D., and Cooper, S. J. B. (2010). DNA barcoding of stygofauna uncovers cryptic amphipod diversity in a calcrete aquifer in Western Australia’s arid zone. Molecular Ecology Resources 10, 41–50.
| DNA barcoding of stygofauna uncovers cryptic amphipod diversity in a calcrete aquifer in Western Australia’s arid zone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1ans7k%3D&md5=ad9b543ceacbacf9d3013968b102e199CAS | 21564989PubMed |
Byrne, M., Yeates, D. K., Joseph, L., Kearney, M., Bowler, J., Williams, M. A. J., Cooper, S., Donnellan, S. C., Keogh, J. S., Leys, R., Melville, J., Murphy, D. J., Porch, N., and Wyrwoll, K.-H. (2008). Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cjhvFGruw%3D%3D&md5=2bb4d789a88b0bd02263023fa38a4f00CAS | 18761619PubMed |
Cameron, R. A. D. (1992). Land snail faunas of the Napier and Oscar ranges, Western Australia – diversity, distribution and speciation. Biological Journal of the Linnean Society. Linnean Society of London 45, 271–286.
| Land snail faunas of the Napier and Oscar ranges, Western Australia – diversity, distribution and speciation.Crossref | GoogleScholarGoogle Scholar |
de Carvalho, M. R., Bockmann, F. A., Amorim, D. S., Brandão, C. R. F., de Vivo, M., de Figueiredo, J. L., Britski, H. A., de Pinna, M. C. C., Menezes, N. A., Marques, F. P. L., Papavero, N., Cancello, E. M., Crisci, J. V., McEachran, J. D., Schelly, R. C., Lundberg, J. G., Gill, A. C., Britz, R., Wheeler, Q. D., Stiassny, M. L. J., Parenti, L. R., Page, L. M., Wheeler, W. C., Faivovich, J., Vari, R. P., Grande, L., Humphries, C. J., DeSalle, R., Ebach, M. C., and Nelson, G. J. (2007). Taxonomic impediment or impediment to taxonomy? A commentary on systematics and the cybertaxonomic-automation paradigm. Evolutionary Biology 34, 140–143.
| Taxonomic impediment or impediment to taxonomy? A commentary on systematics and the cybertaxonomic-automation paradigm.Crossref | GoogleScholarGoogle Scholar |
Chundawat, R. S., Habib, B., Karanth, U., Kawanishi, K., Ahmad Khan, J., Lynam, T., Miquelle, D., Nyhus, P., Sunarto, S., Tilson, R., and Wang, S. (2010). Panthera tigris. In ‘IUCN 2010. IUCN Red List of Threatened Species’. Version 2010.4. Available at http://www.iucnredlist.org [Verified 19 February 2011].
Cook, L. G., Edwards, R. D., Crisp, M. D., and Hardy, N. B. (2010). Need morphology always be required for new species descriptions? Invertebrate Systematics 24, 322–326.
| Need morphology always be required for new species descriptions?Crossref | GoogleScholarGoogle Scholar |
Crozier, R. H. (1992). Genetic diversity and the agony of choice. Biological Conservation 61, 11–15.
| Genetic diversity and the agony of choice.Crossref | GoogleScholarGoogle Scholar |
Crozier, R. H. (1997). Preserving the information content of species: genetic diversity, phylogeny, and conservation worth. Annual Review of Ecology and Systematics 28, 243–268.
| Preserving the information content of species: genetic diversity, phylogeny, and conservation worth.Crossref | GoogleScholarGoogle Scholar |
Donoghue, M. J., and Cracraft, J. (2004). Charting the tree of life. In ‘Assembling the Tree of Life’. (Eds J. Cracraft, and M. Donoghue.) pp. 1–4. (Oxford University Press: New York, NY.)
Eades, A., Kuhar, A., and Penwarden, K. (2010). Evidence for genetic exchange between parapatric populations of Rhagada land snails with divergent mitochondrial DNA. Group Honours Thesis, School of Animal Biology, University of Western Australia, Crawley, Australia.
Eberhard, S. M., Halse, S. A., Williams, M. A., Scanlon, M. D., Cocking, J., and Barron, H. J. (2009). Exploring the relationship between sampling efficiency and short-range endemism for groundwater fauna in the Pilbara region, Western Australia. Freshwater Biology 54, 885–901.
| Exploring the relationship between sampling efficiency and short-range endemism for groundwater fauna in the Pilbara region, Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltVOqurg%3D&md5=eae0a443b652843e1a7fff5dd170905fCAS |
Edward, K. L., and Harvey, M. S. (2008). Short-range endemism in hypogean environments: the pseudoscorpion genera Tyrannochthonius and Lagynochthonius (Pseudoscorpiones: Chthoniidae) in the semiarid zone of Western Australia. Invertebrate Systematics 22, 259–293.
| Short-range endemism in hypogean environments: the pseudoscorpion genera Tyrannochthonius and Lagynochthonius (Pseudoscorpiones: Chthoniidae) in the semiarid zone of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Edward, K. L., and Harvey, M. S. (2010). A review of the Australian millipede genus Atelomastix (Diplopoda: Spirostreptida: Iulomorphidae). Zootaxa 2371, 1–63.
Environmental Protection Authority (2003). Consideration of subterranean fauna in groundwater and caves during environmental impact assessment in Western Australia. Guidance for the assessment of environmental factors (in accordance with the Environmental Protection Act 1986). No. 54, pp. 1–12. Perth.
Environmental Protection Authority (2007). Consideration of subterranean fauna in groundwater and caves during environmental impact assessment in Western Australia. Guidance for the assessment of environmental factors (in accordance with the Environmental Protection Act 1986). No. 54a, pp. 1–32. Perth.
Environmental Protection Authority (2009). Sampling of short range endemic invertebrate fauna for environmental impact assessment in Western Australia. Guidance for the assessment of environmental factors (in Accordance with the Environmental Protection Act 1986). No. 20, pp. 1–31. Perth.
Faith, D. P. (1992). Conservation evaluation and phylogenetic diversity. Biological Conservation 61, 1–10.
| Conservation evaluation and phylogenetic diversity.Crossref | GoogleScholarGoogle Scholar |
Faith, D. P. (1994). Genetic diversity and taxonomic priorities for conservation. Biological Conservation 68, 69–74.
| Genetic diversity and taxonomic priorities for conservation.Crossref | GoogleScholarGoogle Scholar |
GSchV (1998). ‘Gewässerschutzverordnung (Swiss Water Ordinance) 814.210.’ (Der Schweizer Bundesrat: Bern, Switzerland.)
Guzik, M. T., Austin, A. D., Cooper, S. J. B., Harvey, M. S., Humphreys, W. F., Bradford, T., Eberhard, S. M., King, R., Leys, R., Muirhead, K., and Tomlinson, M. (2011a). Is the Australian subterranean fauna uniquely diverse? Invertebrate Systematics 24, 407–418.
| Is the Australian subterranean fauna uniquely diverse?Crossref | GoogleScholarGoogle Scholar |
Guzik, M. T., Cooper, S. J. B., Humphreys, W. F., and Austin, A. D. (2008). Phylogeography of the ancient Parabathynellidae (Crustacea: Syncarida) from the Yilgarn region of Western Australia. Invertebrate Systematics 22, 205–216.
| Phylogeography of the ancient Parabathynellidae (Crustacea: Syncarida) from the Yilgarn region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Guzik, M. T., Cooper, S. J. B., Humphreys, W. F., Ong, S., Kawakami, T., and Austin, A. D. (2011b). Evidence for population fragmentation within a subterranean aquatic habitat in the Western Australian desert. Heredity , .
| Evidence for population fragmentation within a subterranean aquatic habitat in the Western Australian desert.Crossref | GoogleScholarGoogle Scholar |
Harvey, M. S. (1988). A new troglobitic schizomid from Cape Range, Western Australia (Chelicerata: Schizomida). Records of the Western Australian Museum 14, 15–20.
Harvey, M. S. (1992). The Schizomida (Chelicerata) of Australia. Invertebrate Taxonomy 6, 77–129.
| The Schizomida (Chelicerata) of Australia.Crossref | GoogleScholarGoogle Scholar |
Harvey, M. S. (2000a). Brignolizomus and Attenuizomus, new schizomid genera from Australia (Arachnida: Schizomida: Hubbardiidae). Memorie della Società Entomologica Italiana. Supplemento 78, 329–338.
Harvey, M. S. (2000b). A review of the Australian schizomid genus Notozomus (Hubbardiidae). Memoirs of the Queensland Museum 46, 161–174.
Harvey, M. S. (2001). New cave-dwelling schizomids (Schizomida: Hubbardiidae) from Australia. Records of the Western Australian Museum 64, 171–185.
Harvey, M. S. (2002). Short-range endemism in the Australian fauna: some examples from non-marine environments. Invertebrate Systematics 16, 555–570.
| Short-range endemism in the Australian fauna: some examples from non-marine environments.Crossref | GoogleScholarGoogle Scholar |
Harvey, M. S. (2003). ‘Catalogue of the Smaller Arachnid Orders of the World: Amblypygi, Uropygi, Schizomida, Palpigradi, Ricinulei and Solifugae.’ (CSIRO Publishing: Melbourne.)
Harvey, M. S. (2007). The smaller arachnid orders: diversity, descriptions and distributions from Linnaeus to the present (1758 to 2007). Zootaxa 1668, 363–380.
Harvey, M. S., Berry, O., Edward, K. L., and Humphreys, G. (2008). Molecular and morphological systematics of hypogean schizomids (Schizomida: Hubbardiidae) in semiarid Australia. Invertebrate Systematics 22, 167–194.
| Molecular and morphological systematics of hypogean schizomids (Schizomida: Hubbardiidae) in semiarid Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlslajsr8%3D&md5=21cec7432d015799786af7b7a053f51aCAS |
Harvey, M. S., and Humphreys, W. F. (1995). Notes on the genus Draculoides Harvey (Schizomida: Hubbardiidae), with the description of a new troglobitic species. Records of the Western Australian Museum 52, 183–189.
Hoffman, R. L. (1980). ‘Classification of the Diplopoda.’ (Muséum d’Histoire Naturelle: Geneva, Switzerland.)
Hoffman, R. L. (1994). Studies on spirobolid millipeds. XVIII. Speleostrophus nesiotes, the first known troglobitic milliped, from Barrow Island, Western Australia (Spirobolida: Pachybolidae: Trigoniulinae). Myriapodologica 3, 19–24.
| Studies on spirobolid millipeds. XVIII. Speleostrophus nesiotes, the first known troglobitic milliped, from Barrow Island, Western Australia (Spirobolida: Pachybolidae: Trigoniulinae).Crossref | GoogleScholarGoogle Scholar |
Hoffman, R. L. (2003). A new genus and species of trigonuiline milliped from Western Australia (Spirobolida: Pachybolidae: Trigoniulinae). Records of the Western Australian Museum 22, 17–22.
Hopper, S. D., and Gioia, P. (2004). The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology and Systematics 35, 623–650.
| The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity.Crossref | GoogleScholarGoogle Scholar |
Hopper, S. D., Harvey, M. S., Chappill, J. A., Main, A. R., and Main, B. Y. (1996). The Western Australian biota as Gondwanan heritage – a review. In ‘Gondwanan Heritage: Past, Present and Future of the Western Australian Biota’. (Eds S. D. Hopper, J. A. Chappill, M. S. Harvey, and A. S. George.) pp. 1–46. (Surrey Beatty & Sons: Chipping Norton, NSW.)
Humphreys, W. F. (1993). Cave fauna in semi-arid tropical Western Australia: a diverse relict wet-forest litter fauna. Memoires de Biospeologie 20, 105–110.
Humphreys, W. F. (2000). The hypogean fauna of the Cape Range Peninsula and Barrow Island, northwestern Australia. In ‘Subterranean Ecosystems’. (Eds H. Wilkens, D. C. Culver, and W. F. Humphreys.) pp. 581–601. (Elsevier: Amsterdam.)
Humphreys, W. F. (2001). The subterranean fauna of Barrow Island, northwestern Australia, and its environment. Memoires de Biospeologie 28, 107–127.
Humphreys, W. F. (2008). Rising from Down Under: developments in subterranean biodiversity in Australia from a groundwater fauna perspective. Invertebrate Systematics 22, 85–101.
| Rising from Down Under: developments in subterranean biodiversity in Australia from a groundwater fauna perspective.Crossref | GoogleScholarGoogle Scholar |
Job, C. A., and Simons, J. J. (1994). Ecological basis for management of groundwater in the United States: statutes, regulations, and a strategic plan. In ‘Groundwater Ecology’. (Eds J. Gibert, D. L. Danielopol, and J. A. Stanford.) pp. 523–540. (Academic Press: San Diego, CA.)
Johnson, M. S., Hamilton, Z. R., and Fitzpatrick, J. (2006). Genetic diversity of Rhagada land snails on Barrow Island. Journal of the Royal Society of Western Australia 89, 45–50.
Johnson, M. S., Hamilton, Z. R., Murphy, C. E., MacLeay, C. A., Roberts, B., and Kendrick, P. G. (2004). Evolutionary genetics of island and mainland species of Rhagada (Gastropoda: Pulmonata) in the Pilbara Region, Western Australia. Australian Journal of Zoology 52, 341–355.
| Evolutionary genetics of island and mainland species of Rhagada (Gastropoda: Pulmonata) in the Pilbara Region, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Laurance, W. F., and Wright, S. J. (2009). New insights into the tropical biodiversity crisis. Conservation Biology 23, 1382–1385.
| 20078638PubMed |
Leys, R., Watts, C. H. S., Cooper, S. J. B., and Humphreys, W. F. (2003). Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution 57, 2819–2834.
| 14761060PubMed |
Majer, J. (2009). Saga of the short-range endemic. Australian Journal of Entomology 48, 265–268.
| Saga of the short-range endemic.Crossref | GoogleScholarGoogle Scholar |
Meine, C., Soulé, M., and Noss, R. F. (2006). “A mission-driven discipline”: the growth of conservation biology. Conservation Biology 20, 631–651.
| “A mission-driven discipline”: the growth of conservation biology.Crossref | GoogleScholarGoogle Scholar | 16909546PubMed |
Mooney, H. A. (2010). The ecosystem-service chain and the biological diversity crisis. Philosphical Transactions of the Royal Society. Biological Sciences 365, 31–39.
| The ecosystem-service chain and the biological diversity crisis.Crossref | GoogleScholarGoogle Scholar |
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhs1Olsr4%3D&md5=54b901243208fc675c7e7c122d0bda02CAS | 10706275PubMed |
O’Neill, C. (2008). The relationship between morphology and genetics in Quistrachia (Gastropoda: Pulmonata) from the Pilbara, Western Australia. Honours Thesis, School of Animal Biology, University of Western Australia, Crawley, Australia.
Reddell, J. R., and Cokendolpher, J. C. (1995). Catalogue, bibliography, and generic revision of the order Schizomida (Arachnida). Texas Memorial Museum. Speleological Monographs 4, 1–170.
Rix, M. G., and Harvey, M. S. (2010). The spider family Micropholcommatidae (Arachnida, Araneae, Araneoidea): a relimitation and revision at the generic level. Zookeys 36, 1–321.
| The spider family Micropholcommatidae (Arachnida, Araneae, Araneoidea): a relimitation and revision at the generic level.Crossref | GoogleScholarGoogle Scholar |
Solem, A. (1979). Camaenid land snails from western and central Australia (Mollusca: Pulmonata: Camaenidae). I. Taxa with trans-Australian distributions. Records of the Western Australian Museum 10, 1–142.
Solem, A. (1981a). Camaenid land snails from western and central Australia (Mollusca: Pulmonata: Camaenidae). II. Taxa from the Kimberley, Amplirhagada Iredale, 1933. Records of the Western Australian Museum 11, 147–320.
Solem, A. (1981b). Camaenid land snails from western and central Australia (Mollusca: Pulmonata: Camaenidae). III. Taxa from the Ningbing Ranges and nearby areas. Records of the Western Australian Museum 11, 321–425.
Solem, A. (1984). Camaenid land snails from western and central Australia (Mollusca: Pulmonata: Camaenidae). IV. Taxa from the Kimberley, Westraltrachia Iredale, 1933 and related genera. Records of the Western Australian Museum 17, 427–705.
Solem, A. (1985). Camaenid land snails from western and central Australia (Mollusca: Pulmonata: Camaenidae). V. Remaining Kimberley genera and addenda to the Kimberley. Records of the Western Australian Museum 20, 707–981.
Solem, A. (1988). Maximum in the minimum: biogeography of land snails from the Ningbing Ranges and Jeremiah Hills, northeast Kimberley, Western Australia. Journal of the Malacological Society of Australia 9, 59–113.
Solem, A. (1991). Land snails of Kimberley rainforest patches and biogeography of all Kimberley land snails. In ‘Kimberley Rainforests’. (Eds N. L. McKenzie, R.B. Johnston, and P. G. Kendrick.) pp. 145–246. (Surrey Beatty & Sons: Chipping Norton, NSW.)
Solem, A. (1993). Camaenid land snails from western and central Australia (Mollusca: Pulmonata: Camaenidae). VI. Taxa from the red centre. Records of the Western Australian Museum 43, 983–1459.
Solem, A. (1997). Camaenid land snails from western and central Australia (Mollusca: Pulmonata: Camaenidae). VII. Taxa from Dampierland through the Nullarbor. Records of the Western Australian Museum 50, 1461–1906.
Stork, N. E. (2010). Re-assessing current extinction rates. Biodiversity and Conservation 19, 357–371.
| Re-assessing current extinction rates.Crossref | GoogleScholarGoogle Scholar |
Taylor, J. P. A. (2010). Systematics of the land snail genera Plectorhagada and Strepsitaurus (Gastropoda: Camaenidae). Honours Thesis, School of Animal Biology, University of Western Australia, Australia.
Taylor, R. W. (1983). Descriptive taxonomy: past, present and future. In ‘Australian Systematic Entomology: a Bicentenary Perspective’. (Eds E. Highley, and R. W. Taylor.) pp. 93–134. (CSIRO: Canberra.)
Volschenk, E. S., and Prendini, L. (2008). Aops oncodactylus, gen. et sp. nov., the first troglobitic urodacid (Urodacidae: Scorpiones), with a re-assessment of cavernicolous, troglobitic and troglomorphic scorpions. Invertebrate Systematics 22, 235–257.
| Aops oncodactylus, gen. et sp. nov., the first troglobitic urodacid (Urodacidae: Scorpiones), with a re-assessment of cavernicolous, troglobitic and troglomorphic scorpions.Crossref | GoogleScholarGoogle Scholar |


