A revision of Herennia (Araneae : Nephilidae : Nephilinae), the Australasian ‘coin spiders’
Matjaž KuntnerA Department of Entomology, National Museum of Natural History, Smithsonian Institution, NHB-105, PO Box 37012, Washington, DC 20013-7012, USA and Department of Biological Sciences, George Washington University, 2023 G St. N.W., Washington, DC 20052, USA.
B Present address: Institute of Biology, Scientific Research Centre of the Slovenian Academy of Sciences and Arts, Novi trg 2, PO Box 306, SI-1001 Ljubljana, Slovenia.
C Email: kuntner@gmail.com
Invertebrate Systematics 19(5) 391-436 https://doi.org/10.1071/IS05024
Submitted: 14 June 2005 Accepted: 23 September 2005 Published: 12 December 2005
Abstract
The nephilid ‘coin spiders’ (Herennia Thorell) are known for their arboricolous ladder webs, extreme sexual size dimorphism and peculiar sexual biology. This paper revises Herennia taxonomy, systematics, biology and biogeography. The widespread Asian Herennia multipuncta (Doleschall) ( = H. sampitana Karsch, new synonymy; = H. mollis Thorell, new synonymy) is synanthropic and invasive, whereas the other 10 species are narrowly distributed Australasian island endemics: H. agnarssoni, sp. nov. is known from Solomon Islands; H. deelemanae, sp. nov. from northern Borneo; H. etruscilla, sp. nov. from Java; H. gagamba, sp. nov. from the Philippines; H. jernej, sp. nov. from Sumatra; H. milleri, sp. nov. from New Britain; H. oz, sp. nov. from Australia; H. papuana Thorell from New Guinea; H. sonja, sp. nov. from Kalimantan and Sulawesi; and H. tone, sp. nov. from the Philippines. A phylogenetic analysis of seven species of Herennia, six nephilid species and 15 outgroup taxa scored for 190 morphological and behavioural characters resulted in 10 equally parsimonious trees supporting the monophyly of Nephilidae, Herennia, Nephila, Nephilengys and Clitaetra, but the sister-clade to the nephilids is ambiguous. Coin spiders do not fit well established biogeographic lines (Wallace, Huxley) dividing Asian and Australian biotas, but the newly drawn ‘Herennia line’ suggests an all-Australasian speciation in Herennia. To explain the peculiar male sexual behaviour (palpal mutilation and severance) known in Herennia and Nephilengys, three specific hypotheses based on morphological and behavioural data are proposed: (1) broken embolic conductors function as mating plugs; (2) bulb severance following mutilation is advantageous for the male to avoid hemolymph leakage; and (3) the eunuch protects his parental investment by fighting off rival males.
Acknowledgments
Views, opinions, interpretations and potential errors in this paper are my own, not those of who have commented on and criticised earlier drafts. I thank Jonathan Coddington and Gustavo Hormiga for advice and help, Ingi Agnarsson and Jeremy Miller for their daily help, encouragement and comments, Jutta Schneider and Miquel Arnedo for discussing the ideas and preliminary results from this study, and Marc Allard, Jim Clark, Diana Lipscomb and Chris Thompson for their comments on an early draft. The helpful comments of Camilla Myers, Mark Harvey and Volker Framenau much improved the paper. Fernando Alvarez-Padilla, Lara Lopardo, Dana deRoche and Scott Larcher offered assistance and help; Scott Whittaker and Patrick Herendeen provided SEM help, and Karie Darrow kindly helped with digital image manipulation. Numerous curators, collection managers and other biologists have assisted with loans (see Materials and methods) and by collecting the specimens. Erik J. van Nieukerken kindly helped translate Doleschall’s text and find his original artwork. Gustavo Hormiga kindly provided his unpublished photograph. The fieldwork in Indonesia was done jointly with Irena Kuntner and Matjaž Bedjanič. This project was supported by the USA National Science Foundation (PEET grant DEB-9712353 to Hormiga and Coddington) and partly by the OTS-STRI-Mellon Research Exploration Award (to Kuntner and Šereg). I further acknowledge material and financial support of the George Washington University, Smithsonian Institution, the Ministry of Science of the Republic of Slovenia and the Biological Institute of the Slovenian Academy of Sciences and Arts. At the latter institution, the support (1999–2001) was coordinated by Rajko Slapnik, and the final stages endorsed by Branko Vreš and Oto Luthar. This project would have been impossible without the support of my wife Irena, my parents Sonja and Tone, and my brother Jernej.
Bremer K.
(1988) The limits of amino acid sequence data in angiosperm phylogenetic reconstructions. Evolution 42, 795–803.
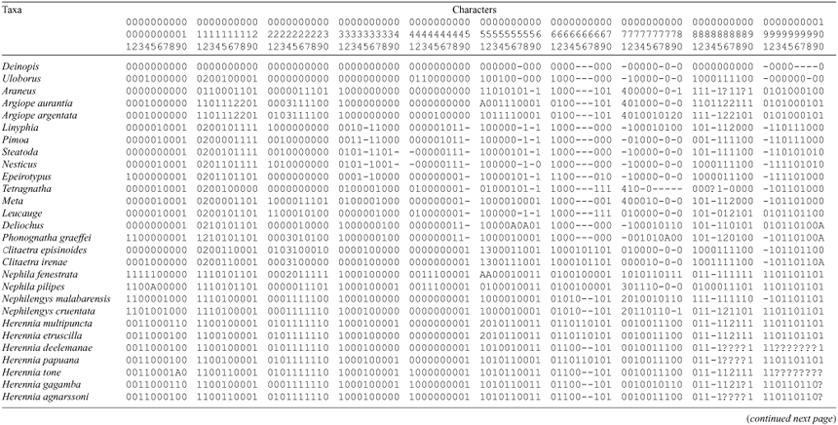
and characters 28 and 29 in Griswold et al. (1998). Herennia lacks eye tapeta.
(18) PLE canoe tapetum: 0, absent; 1, full; 2, narrow.
Note: as above.
(19) Female clypeus height: 0, low (less than three AME diameters); 1, equal or more than three AME diameters.
(20) Endites: 0, very long (> 2 × width); 1, short (length < 2 × width).
(21) Labium and sternum: 0, separate; 1, fused.
(22) Female sternum: 0, longer than wide; 1, as wide as or wider than long.
(23) Sternal slit sensilla: 0, present (Figs 6E–F, 20F); 1, absent.
(24) Female sternum colour pattern: 0, inconspicuously coloured; 1, uniformly orange/red (Fig. 26B); 2, medially dark, laterally pale; 3, medially light, laterally dark; 4, with yellow spots corresponding to tubercles.
(25) Sternal white pigment: 0, absent; 1, present.
(26) Female sternal tubercle I: 0, absent; 1, present.
Note: paired elevations of the female sternum adjacent to coxae I-IV are termed sternal tubercles (Fig. 6E – arrows).
(27) Female sternal tubercle II: 0, absent; 1, present (Fig. 6E – arrows).
(28) Female sternal tubercle III: 0, absent; 1, present (Fig. 6E – arrows).
(29) Female sternal tubercle IV: 0, absent; 1, present (Fig. 6E – arrows).
(30) Female frontal sternal tubercle: 0, absent; 1, present.
(31) Female chilum: 0, absent; 1, present.
Note: The chilum is present as a paired sclerite (Fig. 1B) at the base of chelicerae, just under the clypeus in most nephilids (not in certain Clitaetra species).
(32) Female chelicerae: 0, massive (width > 1/2 length); 1, slender (width < 1/2 length).
Note: nephilids have massive chelicerae with the width from profile more than 1/2 length (Figs 1A, 20A).
(33) Cheliceral ectal margins: 0, smooth; 1, with stridulatory striae.
(34) Cheliceral boss: 0, present (Figs 6B, 20A–C); 1, absent.
(35) Cheliceral boss surface: 0, smooth; 1, striated (Figs 6B, 20A–C).
(36) Prosomal supracheliceral lobe (PSL): 0, present (Fig. 20B); 1, absent.
(37) Cheliceral furrow: 0, denticulated (Fig. 20E); 1, smooth.
(38) Female first femur: 0, more/less straight; 1, sigmoidal.
(39) Femoral macrosetae: 0, present (Figs 5B, D, 19B); 1, absent.
(40) Femoral (I, II) macrosetae length: 0, long (Fig. 19B); 1, short, stout (Fig. 5D).
(41) Female femur 1 group of prolateral long spines: 0, absent; 1, present (Figs 16A–B, 31B).
(42) Dorsal femoral trichobothria: 0, absent; 1, present.
(43) Female tibia I tufts: 0, absent; 1, present.
Note: all Nephila species at some stage possess dense tibial setae on legs I, II, IV.
(44) Female tibia II tufts: 0, absent; 1, present.
(45) Female tibia IV tufts: 0, absent; 1, present.
(46) Patella-tibia autospasy: 0, absent; 1, present.
(47) Ventral tarsus IV setae: 0, irregular; 1, comb-like.
(48) Tarsus IV median claw: 0, long (as long or longer than the main claw; Fig. 7A–D); 1, short (shorter than the paired main claw).
(49) Sustentaculum: 0, present (Fig. 7B–D); 1, absent.
(50) Sustentaculum angle: 0, wide, diverging from other setae; 1, narrow, parallel to other setae (Fig. 7B–D).
(51) Female abdomen length: 0, very long (> 2 × width); 1, long (longer than wide, but < 2 × width); 2, short (as wide as long or wider).
(52) Female abdomen width: 0, elliptical; 1, widest anteriorly; 2, widest posteriorly; 3, pentagonal.
(53) Female lateral abdominal margin: 0, smooth; 1, with 3–4 pairs of lobes (Figs 1A–D, 8A–C, 9A–B, E, 10A–D).
(54) Female anterior abdominal humps: 0, absent; 1, present.
(55) Female abdomen tip: 0, rounded; 1, truncated (Fig. 9B – arrow).
(56) Female ventro-median sclerotisations: 0, absent; 1, paired sclerotisations (Figs 1D, 8A–C, 9B–D, 10A).
Note: all nephilids possess a row of conspicuous sclerotised apodemes medially on venter and one to several rows of sclerotisations laterally on venter.
(57) Female ventro-median sclerotisations: 0, 1–5 pairs; 1, 6–11 pairs.
(58) Female ventro-lateral abdominal sclerotisations: 0, present (Figs 1D, 8, 10A–D); 1, absent.
(59) Ventro-lateral abdominal sclerotisations: 0, one paired line of small dots; 1, sclerotisations in several lines (Figs 1D, 8, 10A–D).
(60) Female dorso-median abdominal apodemes: 0, absent; 1, 3–5 prominent pairs (Figs 1A, C, 16A, D, 22B–D, 27A, 28A, 29A, 31A–B).
(61) Female dorso-lateral abdominal sclerotisations: 0, present (Figs 1A, C, 16A, D, 22B–D, 27A, 28A, 29A, 31A–B); 1, absent.
(62) Female dorso-central abdominal sclerotisations: 0, absent; 1, present (Figs 1C, 22C, 31A).
(63) Female abdominal sigillae: 0, absent; 1, present (Figs 14, 23, 24A).
(64) Female anterior abdomen: 0, without; 1, with a broad light-pigmented band.
(65) Female abdominal dorsal pattern: 0, inconspicuous; 1, conspicuous.
(66) Female dorsum dark spots: 0, absent; 1, present (Figs 14, 25, 26A).
(67) Female dorsum ‘butterfly’ pattern: 0, absent; 1, present.
(68) Female abdomen tip color: 0, no different to the subapical abdomen; 1, paired white dots around spinnerets.
(69) Female abdomen silver pigment spots: 0, absent; 1, present.
(70) Female venter light pigmented pattern: 0, absent; 1, present (Fig. 1D, 29B).
(71) Female venter light pigmented pattern form: 0, one central light area; 1, transverse line(s); 2, four large spots; 3, numerous spots; 4, longitudinal lines.
(72) Book lung cover: 0, grooved (Figs 1D, 10A–C); 1, smooth.
(73) Area around female book lung spiracle: 0, little sclerotised; 1, strongly sclerotised (Fig. 1D).
(74) Posterior epigynal plate: 0, round; 1, grooved.
Note: in some species of Nephila and Nephilengys the epigynal plate posterior edge is grooved and leads to lateral copulatory openings.
(75) Epigynal ventral area: 0, low; 1, swollen.
(76) Epigynal openings: 0, simple; 1, in chambers (Fig. 11).
Note: copulatory openings are within larger chambers in Nephilengys, Herennia, some Nephila species and in some outgroups.
(77) Chamber opening position: 0, medial (Fig. 27D); 1, lateral (Figs 2A–B, 10E–F, 11).
(78) Epigynal septum: 0, absent; 1, present (Figs 11, 18C, 28C).
(79) Epigynal septum shape: 0, simple border between chambers (Figs 11, 18C, 28C); 1, extensive, broader posteriorly (Fig. 27D); 2, extensive, broader anteriorly.
(80) Epigynal paired sclerotised pocket: 0, absent; 1, present.
(81) Anterior epigynal area: 0, with a pair of apodemes; 1, round.
(82) Cuticle anterior to the epigynal area: 0, rounded; 1, depressed.
(83) Copulatory opening position: 0, caudal; 1, ventral.
(84) Caudal copulatory openings: 0, on the posterior sclerotised epigynal margin; 1, anterior to the posterior margin.
(85) Copulatory opening form: 0, elongated slit openings; 1, rounded openings (Fig. 11).
(86) Copulatory duct morphology: 0, flattened duct (longer than wide, flat); 1, tube (longer than wide, cylindrical); 2, broad attachment to body wall (wider than long).
(87) Spermathecae: 0, lobed; 1, spherical; 2, oval.
Note: in Herennia spermathecae are adjacent, oval, with gland pores over their entire surface (Figs 2C–D, 18D, 31E).
(88) Spermathecae separation: 0, wide (separated more than two widths); 1, small or none (separated less than two widths).
(89) Epigynal sclerotised arch: 0, absent; 1, present (Figs 2C, 18D, 31E).
(90) Female copulatory aperture: 0, never plugged; 1, sometimes plugged with emboli a/o conductors (Fig. 11). See Discussion.
(91) Female copulatory plugs: 0, emboli; 1, emboli plus (embolic) conductors (Fig. 11).
(92) Cribellum: 0, present; 1, absent.
(93) ALS piriform gland spigot bases: 0, normal; 1, reduced.
Note: the spinneret spigot characters (93–99) are from Hormiga et al. (1995), characters 54–60, and follow the homology assessments of Coddington (1989). An almost uniform nephilid spinneret morphology is: ALS with ‘normal PI field’ where the PI spigot base is nearly as long or longer than the shaft (Griswold et al., 1998: character 69, fig. 48B), the major ampullate spigot and a nubbin, PMS with a sparse aciniform field, and a nubbin, PLS with the aggregate spigots embracing the flagelliform, and with the two cylindrical spigots of normal size, the mesal being peripheral (compare with Figs 7E–F, 21D–F).
(94) PMS nubbin: 0, absent; 1, present.
(95) PMS aciniform field: 0, extensive; 1, sparse.
(96) PLS mesal cylindrical gland spigot base: 0, subequal to other PLS cylindrical spigot; 1, larger.
(97) PLS mesal cylindrical gland spigot position: 0, central; 1, peripheral.
(98) PLS aggregate-flagelliform relation: 0, aggregates apart from flagelliform; 1, distal aggregate spigots embrace flagelliform.
(99) PLS aggregate gland spigot: 0, normal; 1, large.
(100) Male size: 0, more than half the size of female; 1, less than 0.4 female.
Note: corresponds to character 14 in Hormiga et al. (1995) arbitrarily quantifying the extreme sexual size dimorphism, within nephilids typical of Nephila, Nephilengys and Herennia (Fig. 1).
(101) Male dorsal abdomen: 0, cuticle soft; 1, with scutum (Figs 3A–C, 24A–B, F).
(102) Male lateral eyes: 0, separate (Figs 3A–B, 23A–E); 1, juxtaposed.
(103) Male cephalic region: 0, narrower than in female (Fig. 1C, F); 1, same proportion to cephalothorax as in female.
(104) Male clypeus: 0, as in female; 1, more horizontal (Fig. 1A, E).
(105) Male v. female cheliceral size: 0, same; 1, larger; 2, smaller (Fig. 1A, E).
(106) Male paturon posteriorly: 0, smooth; 1, with a tubercle.
(107) Male leg II tibial macrosetae: 0, similar to those on tibia I; 1, stronger and more robust; 2, absent.
(108) Male endite depression: 0, absent; 1, present.
(109) Male palpal trochanter: 0, short (twice the width or less); 1, long (more than twice the width).
(110) Male palpal femoral tubercle: 0, absent; 1, present.
Note: see character 3 of Scharff and Coddington (1997: Fig. 4).
(111) Male palpal patella macrosetae: 0, none; 1, one (Figs 4A, 18A, 30A); 2, two.
Note: modified from character 19 in Hormiga et al. (1995).
(112) Male palpal tibia length: 0, short (not exceeding 1.5 times its width); 1, long (exceeding 1.5 times its width).
(113) Cymbium length: 0, short (less than 2 × width); 1, long (more than 2 × width).
(114) Cymbial ectal margin: 0, sclerotised as cymbium; 1, transparent.
(115) Paracymbium (P): 0, absent; 1, present.
(116) Paracymbial base sclerotisation: 0, like cymbium; 1, less sclerotised.
(117) Paracymbium morphology: 0, short basal structure, more or less hook-shaped; 1, longer than wide and finger-like; 2, flat and roughly rectangular; 3, U-shaped; 4, flat and roughly triangular; 5, Phonognatha condition.
(118) Paracymbium edge: 0, glabrous; 1, with setae.
(119) Anterior paracymbial apophysis (APA): 0, absent; 1, present.
(120) Paracymbial margin fold: 0, absent; 1, present.
(121) Paracymbium apically: 0, rounded; 1, with a prong.
(122) Tegulum in ectal view: 0, same size as or larger than subtegulum (Fig. 4A); 1, smaller than subtegulum.
(123) Reservoir course: 0, spiralled; 1, with a switchback (Fig. 4E).
(124) Ventral tegular switchback: 0, single; 1, double.
(125) Ejaculatory duct: 0, within the entire length of embolus; 1, joins distal embolus.
(126) Median apophysis (MA): 0, absent; 1, present.
(127) Median apophysis: 0, without sperm duct; 1, with a loop of the sperm duct.
(128) Median apophysis thread-like spur: 0, absent; 1, present.
(129) Apical tegular apophysis (ATA): 0, absent; 1, present (Figs 4A, 12A–B, E, 13B–E, 18A–B, 30A, C).
(130) Ventral tegular apophysis (VTA): 0, absent; 1, present.
(131) Mesal tegular apophysis (MTA): 0, absent; 1, present.
(132) Theridiid tegular apophysis (TTA): 0, absent; 1, present.
(133) Conductor (C): 0, present; 1, absent.
(134) Conductor size: 0, small (less than half bulb volume); 1, large (more than half bulb volume).
(135) Conductor form: 0, rounded; 1, grooved for embolus.
(136) Embolic conductor (EC): 0, absent; 1, present.
Note: Nephila, Nephilengys, Herennia, Clitaetra, Phonognatha and Deliochus possess one or more sclerites of the embolic division, connected to EB and T via membrane. The sclerite(s) function(s) as conductor, enclosing the embolus. In Nephila and Clitaetra (Kuntner, in press) the sclerite is simple, long and finger-like, with a groove in which the embolus sits fully wrapped. In Nephilengys and Herennia (Figs 4A–C, 12–13, 18A–B, 30, 17B–C) the sclerite is complex, wide and sigmoidal, with membranous and sclerotised parts (distally with ridged edges), though functioning as one large sclerite.
(137) EC membrane: 0, absent; 1, present.
(138) EC shape: 0, complex; 1, finger-like.
(139) Finger EC: 0, short; 1, long.
(140) EC (division): 0, not subdivided; 1, subdivided into more sclerites.
(141) Distal EC flap: 0, absent; 1, present.
(142) EC edge: 0, smooth; 1, ridged.
(143) EC curvature: 0, more or less straight; 1, sigmoidal; 2, bent distally.
(144) EC tip: 0, straight; 1, with a hook.
(145) Embolus (E) length: 0, long (> 2 × CB); 1, medium (0.5–1.5 CB length); 2, short (< 1/2 cymbium).
(146) Embolus form: 0, thin (Figs 4C–E, 30C–E); 1, thick; 2, filiform.
(147) Embolus–tegulum orientation: 0, parallel; 1, 90 degrees.
(148) Embolus–tegulum membrane: 0, absent; 1, present.
(149) Embolus base: 0, thin; 1, enlarged ( = radix) (Figs 4B–C, 12C–D, 13B, F, 18B, 30B–E).
(150) Embolus base distal part: 0, smooth; 1, denticulated.
(151) Embolic apophysis: 0, absent; 1, present.
(152) Radical membrane: 0, absent; 1, present.
(153) Stipes: 0, absent; 1, present.
(154) Embolus constriction: 0, absent; 1, present.
(155) Embolus: 0, smooth; 1, hooked.
Note: embolus has a single hook in Argiope and Herennia (Figs 4C–E, 30C–E) and a row of hooks in Deliochus.
(156) Embolus distal apophysis: 0, present; 1, absent.
(157) Embolus tip: 0, flat (Fig. 30C–E); 1, cylindrical (Fig. 4C–D).
(158) Web architecture: 0, orb; 1, sheet; 2, gum foot.
Note: Herennia web architecture and known behaviours are summarised in text, see also Figs 14–15, 25–26.
(159) Orb–web angle: 0, horizontal (0–45 degrees); 1, vertical (46–90 degrees).
(160) Orb shape: 0, round; 1, rectangular.
(161) Silk color: 0, white; 1, golden.
(162) Stabilimentum: 0, absent; 1, present.
(163) Barrier (3D) web: 0, absent; 1, present.
(164) Hub position: 0, aerial; 1, against substrate.
(165) Hub relative position: 0, central; 1, displaced up; 2, displaced down.
(166) Hub bite-out: 0, present; 1, absent.
Note: Nephila, Nephilengys, Herennia, Clitaetra, Phonognatha and Uloborus leave the hub intact after completing the orb web, whereas araneids and tetragnathids remove it by biting it out. The character corresponds to character 45 in Hormiga et al. (1995), which was modified from the states G1 (hub left intact), G2 (hub centre removed) and G4 (entire hub removed) in Eberhard (1982).
(167) Hub: 0, closed; 1, open.
(168) Hub-cup: 0, absent; 1, present (Figs 14A–B – arrow, 25B – arrow).
Note: the hub of Herennia orbs is in the shape of a depression of dense silk, coming in touch with the substrate. Robinson and Lubin (1979) referred to the structure as the hub-cup.
(169) Hub loop – non-sticky spiral transition: 0, gradual; 1, abrupt.
(170) Radius construction: 0, cut and reeled; 1, doubled.
Note: corresponds to the characters 49 in Hormiga et al. (1995) and in part to 76 in Scharff and Coddington (1997), all modified from Eberhard’s (1982) character F (states F1, F2, F4). In araneids and tetragnathids the trip from hub to frame on a pre-existing radius attaches a new ‘temporary radius’, which is then cut and reeled on the way back to hub, while laying a new one behind (see Eberhard, 1982: fig. 5A–D, character state F1). Uloborids and nephilids do not cut and reel but spin a double radius (Eberhard 1982: figs 6, 8).
(171) Radius attachment on frame: 0, attached singly; 1, attached twice.
(172) Secondary (split) radii: 0, absent; 1, present.
(173) Tertiary (split) radii: 0, absent; 1, present.
(174) Pseudoradii: 0, absent; 1, present (Figs 15, 25A).
Note: a unique feature in Herennia webs (where known) are perpendicular ‘radii’ (which do not run through the hub) described and termed pseudoradii by Robinson and Lubin (1979: Fig. 2).
(175) Sticky spiral: 0, spiralling; 1, parallel.
(176) Non-sticky spiral (NSS): 0, removed; 1, persists in web.
(177) NSS form: 0, linear; 1, zig-zag Nephila form.
(178) First sticky spiral (SS) spiral–NSS contact: 0, NSS contacted; 1, no contact.
(179) Sticky spiral localisation: 0, oL1; 1, iL1; 2, oL4.
(180) Web posture: 0, flexed legs I, II; 1, extended legs I, II.
(181) Argiope posture: 0, absent; 1, present.
Note: a typical web pose of Argiope species is with first two and last two leg pairs close together, thus forming a ‘leg-cross’.
(182) Attack behaviour: 0, wrap-bite; 1, bite-wrap.
(183) Wrap-bite silk: 0, dry; 1, sticky.
(184) Cheliceral clasp: 0, absent; 1, present.
(185) Bulbus detachment (eunuchs): 0, absent; 1, present.
(186) Body shake: 0, absent; 1, present.
Note: in Clitaetra, Nephilengys and some Nephila a body shake is a common behavioural ritual in response to threat. A similar behaviour is known in Argiope.
(187) Side change: 0, absent; 1, when in danger, rushing on other side of orb.
(188) Partial web renewal: 0, absent; 1, present.
(189) Retreat: 0, absent; 1, off-web; 2, in web.
(190) Retreat form: 0, silken tube; 1, utilisation of a leaf.
|

|


