
Australian Journal of Chemistry
Volume 69 Number 9 2016
RESEARCH FRONT: Seventh Asia-Pacific Conference of Theoretical and Computational Chemistry (APCTCC7)
CHv69n9_FOSeventh Asia-Pacific Conference of Theoretical and Computational Chemistry (APCTCC7)

The Seventh Asia-Pacific Conference of Theoretical and Computational Chemistry (APCTCC7) was held in Kaohsiung, Taiwan, from 25 to 28 January 2016. The papers in this Research Front showcase the latest research in theoretical and computational chemistry, including cutting edge applications in drug discovery, materials science, sustainable resources, and nanotechnology.
CH16093Theoretical Investigation of Oxidative Cleavage of Cholesterol by Dual O2 Activation and Sulfide Reduction
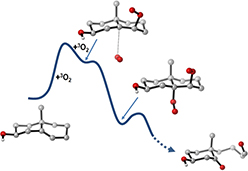
Theoretical calculations are used to explore a plausible mechanism for oxidative cleavage of cholesterol mediated by two ground-state O2 molecules and methionine.
CH16206Mechanistic Insights into Water-Catalyzed Formation of Levoglucosenone from Anhydrosugar Intermediates by Means of High-Level Theoretical Procedures
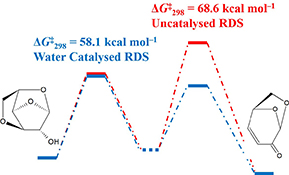
This work uses high-level thermochemical procedures to investigate the reaction mechanism for the conversion of important pyrolysis intermediates into levoglucosenone. This process is of importance in biomass conversion. We consider both the uncatalyzed and water-catalyzed reaction mechanisms. We find that a water catalyst leads to a significant reduction in the reaction barrier height for the entire process and to a change in the reaction mechanism.
CH16134Asymptotic Functional Form Preservation Method with Supersymmetric Quantum Mechanics for Anharmonic Oscillators
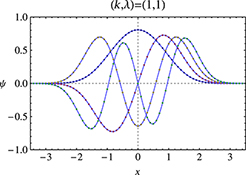
The asymptotic functional form preservation method is developed in the framework of supersymmetric quantum mechanics to obtain the energies and wave functions of anharmonic oscillators. Several ansatz functional forms are proposed for the superpotential to obtain the ground and excited state energies with high accuracy.
CH16187Electronic and Optical Properties of the Narrowest Armchair Graphene Nanoribbons Studied by Density Functional Methods
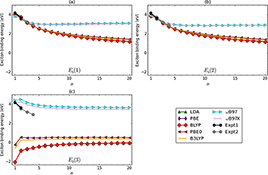
The electronic and optical properties of the narrowest armchair graphene nanoribbons (NAGNRs) are studied by various density functional methods. While NAGNRs are found to have stable singlet ground states, the lowest triplet states and the ground states of the cations and anions of NAGNRs exhibit some multi-reference character.
CH16208Guest–Host Interaction of Coinage Metals in π-Rich Cavities
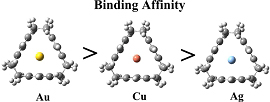
It is demonstrated that the reason for the peripheral bonding mode of the coinage metal atoms with [2.2.2]paracyclophane and deltaphane is not an increase of steric repulsion on the inside of the molecular cavity, but rather the decrease of the electrostatic and orbital contributions to the bonding and that the interaction between the cyclophanes and coinage metal cations is non-covalent.
CH16226Molecular Electrostatic Potential-Based Atoms in Molecules: Shielding Effects and Reactivity Patterns
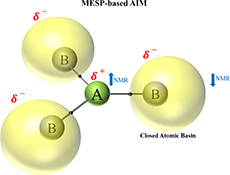
Salient chemical applications of the recently proposed Atoms in Molecules (AIM) concept based on molecular electrostatic potential (MESP) have been explored. The present work clearly demonstrates the connection of MESP-based AIM with reactivity as well as shielding and deshielding of atoms in NMR studies.
CH16225Pentanidium-Catalyzed Asymmetric Phase-Transfer Conjugate Addition: Prediction of Stereoselectivity via DFT Calculations and Docking Sampling of Transition States, and Origin of Stereoselectivity
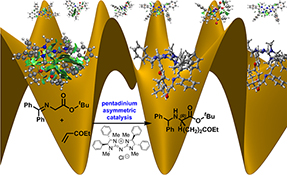
The catalytic mechanism and origin of stereoselectivity of pentanidium-catalyzed asymmetric phase-transfer conjugate addition were studied by DFT calculations. A docking approach was used to sample the conformational space of the diastereomeric C–C bond-forming transition states. The calculated enantioselectivity is in excellent accord with experiment result.
CH16248Energetics of the Proton Transfer Pathway for Tyrosine D in Photosystem II
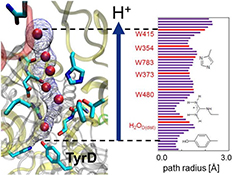
The proton transfer pathway for redox active tyrosine D (TyrD) in photosystem II is a hydrogen-bond network that involves D2-Arg180 and a series of water molecules. The potential-energy profile of all hydrogen bonds along the proton transfer pathway indicates that the overall proton transfer from TyrD is energetically downhill.
CH16248 Abstract | CH16248 Full Text | CH16248PDF (944 KB) | CH16248Supplementary Material (10 KB) Open Access Article
CH16183Computation of Electron Delocalization for Extended Cyclic Conjugated Molecules
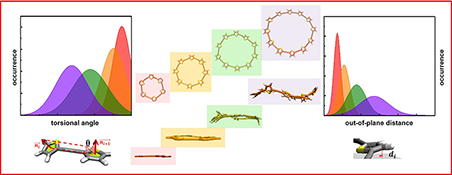
We present a simple yet systematic way to investigate the degree of electron delocalization and structural heterogeneity of cyclic conjugated molecules based on molecular dynamics simulations. π-Electron delocalization is strongly correlated with the planarity of the system. Torsional angle and out-of-plane distance distributions were calculated for the degree of electron delocalization.
CH16232An In Silico Approach of Coumarin-Derived Inhibitors for Human DNA Topoisomerase I
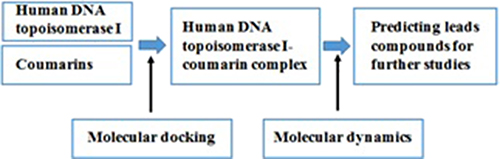
Human DNA topoisomerase I (Htopo I) is a validated target for anti-cancer agents; however, available anti-cancer agents are linked with several limitations. Therefore, discovering novel inhibitors for Htopo I is essential. Recent studies have demonstrated that coumarins exhibit anti-cancer activity against several targets. Thus, the current study focussed on predicting coumarin-derived inhibitors as lead compounds for further drug design.
CH15796Electroanalytical Opportunities Derived from Ion Transfer at Interfaces between Immiscible Electrolyte Solutions
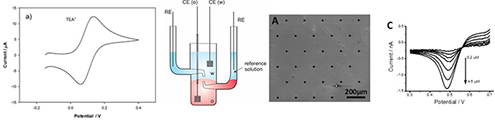
An introduction to electrochemistry at interfaces between immiscible electrolyte solutions and recent advances in the electroanalytical chemistry based on ion-transfer reactions at these interfaces are presented.
CH16044Heteronuclear NMR Spectroscopic Investigations of Gallium Complexes of Substituted Thiosemicarbazones Including X-Ray Crystal Structure, a New Halogen Exchange Strategy, and 18F Radiolabelling
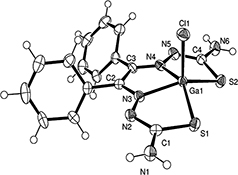
Five thiosemicarbazone ligands have been synthesized and their coordination chemistry with gallium investigated. All compounds have been fully characterized by NMR and X-ray crystal structures of two of the complexes are reported. Additionally, the positron emitting isotope 18F was introduced in the structure of the diphenyl gallium thiosemicarbazone complex using a halogen exchange method.
CH16014A Green Approach for the Synthesis of Novel 7,11-Dihydro-6H-chromeno[3,4-e]isoxazolo[5,4-b]pyridin-6-one Derivatives Using Acidic Ionic Liquid [C4mim][HSO4]
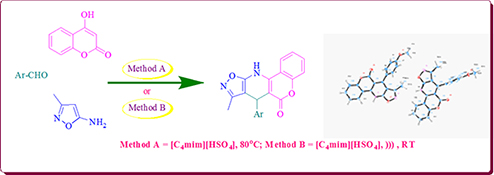
A multicomponent reaction capable of affording a wide range of novel 7,11-dihydro-6H-chromeno[3,4-e]isoxazolo[5,4-b]pyridin-6-one derivatives via one-pot three-component condensation of 4-hydroxycoumarin, aromatic aldehydes, and 5-amino-3-methylisoxazole in ionic liquid [C4mim][HSO4] is reported.
CH15794The Influence of Amino Group Position on Aryl Moiety of SarAr on Metal Complexation and Protein Labelling
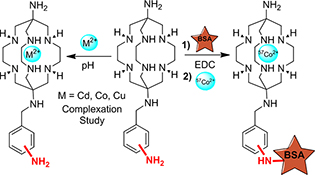
New hexaazamacrobicyclic cage bi-functional chelators (BFCs), 1-N-(3-aminobenzyl)-3,6,10,13,16,19-hexaazabicyclo[6.6.6]eicosane-1,8-diamine (m-SarAr) and 1-N-(2-aminobenzyl)-3,6,10,13,16,19-hexaazabicyclo[6.6.6]eicosane-1,8-diamine (o-SarAr), were synthesised and their metal complexation and conjugation to proteins were compared with p-SarAr.
CH160083D Ln–Organic Frameworks Featuring Lantern-Shaped Dihelicate Chains: Synthesis and Magnetic and Photophysical Properties
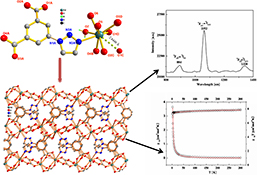
A series of novel 3D Ln–organic frameworks ([Ln(tia)(C2O4)0.5(H2O)]) with excellent magnetic and luminescent properties have been designed and synthesised.



