
Australian Journal of Chemistry
Volume 70 Number 4 2017
7th Heron Island Conference
CH170657th Heron Island Conference on Reactive Intermediates and Unusual Molecules

This special issue of the Journal contains a collection of papers authored by participants at the 7th Heron Island Conference on Reactive Intermediates and Unusual Molecules, which was held from 9 to 15 July 2016 at the Heron Island Resort.

The HERON reaction, named at the Second Heron Island Conference on Reactive Intermediates and Unusual Molecules, is a modern ‘named reaction’. HERON is an acronym for Heteroatom Rearrangements on Nitrogen, a unique chemical transformation.
CH16504The Variediene-Forming Carbocation Cyclization/Rearrangement Cascade
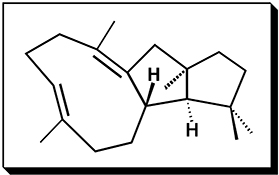
An energetically viable pathway to the diterpene variediene is described. Only one of the three secondary carbocations along this pathway is predicted to be a minimum on the potential energy surface.
CH16579Directionality and the Role of Polarization in Electric Field Effects on Radical Stability
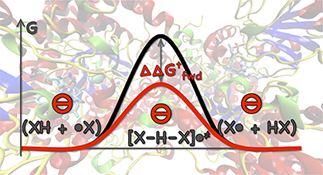
Electric field effects on the kinetics and thermodynamics of radical reactions have a significant, non-directional component arising in the increased polarizability of resonance-stabilized species.
CH16587Syntheses, Structural Studies, and Copper Iodide Complexes of Macrocycles Derived from Williamson Ether Syntheses Involving 2,9-Bis(4-hydroxyphenyl)-1,10-phenanthroline, α,ω-Dibromides, and Resorcinol or 2,7-Dihydroxynaphthalene

The title macrocycles were assembled by first combining the grey and maroon building blocks, followed by a condensation with 1. The CuI moieties were markedly removed from the 1,10-phenanthroline planes, as represented by the basketball above the basket rim, and voids in the crystals were analyzed in the context of rotaxane formation.
CH16621Interaction of Triplet Excited States of Ketones with Nucleophilic Groups: (π,π*) and (n,π*) versus (σ*,π*) States. Substituent-Induced State Switching in Triplet Ketones
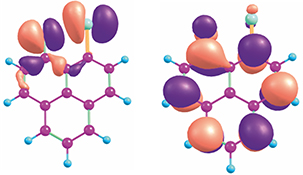
The presence of nucleophilic substituents neighbouring a ketone functional group switches the character of the lowest triplet excited states from (π,π*) or (n,π*) to (σ*,π*).
CH16602Hydroxyl Radicals via Collision-Induced Dissociation of Trimethylammonium Benzyl Alcohols
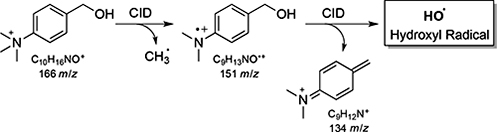
Arguably the most famous of all small molecule reactive intermediates is the hydroxyl radical. Methods to generate hydroxyl radicals from organic precursors are rare, with only one known example for the solution phase. Mass spectrometry is a key identifying technique is this area, and reported herein are new HO and DO radical precursors based on the trimethylammonium benzyl alcohol motif.
CH16622Nitrosonium-Mediated Phenol–Arene Cross-Coupling Involving Direct C–H Activation
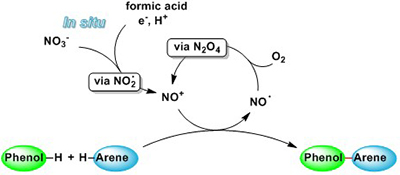
An environmentally benign and mild method for the in situ formation of NO+ from readily available nitrate salts by treatment with formic acid and MeOH is described. The synthetic applicability of the method was demonstrated for the oxidative phenol–arene C–C cross-coupling by direct C–H activation.
CH16604Singlet Photoreactivity of 3-Methyl-2-phenyl-2H-azirine
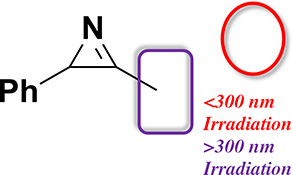
Laser flash photolysis of 3-methyl-2-phenyl-2H-azirine (1) in acetonitrile (irradiation wavelength = 266 nm) and in cryogenic argon matrices (irradiation wavelength = 254 nm) results in the corresponding ylide.
CH17026Full Cleavage of C≡C Bond in Electron-Deficient Alkynes via Reaction with Ethylenediamine
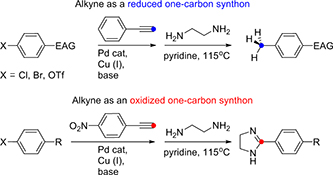
This work uses the electronic and spatial features of alkynes, enhanced by conjugated electron acceptors, to achieve complete triple-bond fragmentation. Sonogashira coupling, then ethylenediamine-mediated triple-bond scission allows selective halogen substitution in aryl halides by a methyl group or a 4,5-dihydroimidazol-2-yl moiety.
CH16580Brønsted Base-Mediated Aziridination of 2-Alkyl-Substituted-1,3-Dicarbonyl Compounds and 2-Acyl-Substituted-1,4-Dicarbonyl Compounds by Iminoiodanes
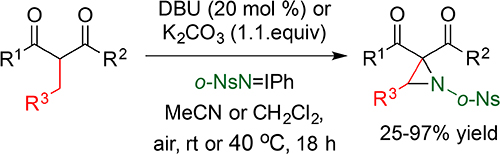
A Brønsted base-mediated method for the synthesis of α,α-diacylaziridines and α,α,β-triacylaziridines from reaction of 2-alkyl-substituted-1,3-dicarbonyl compounds and 2-acyl-substituted-1,4-dicarbonyl compounds with arylsulfonyliminoiodinanes (ArSO2N=IPh) under atmospheric conditions is described.
CH16566N-Heterocyclic Carbene-Catalysed Mukaiyama–Michael Reaction and Mukaiyama Aldol/Mukaiyama–Michael Three-Component Coupling Reaction
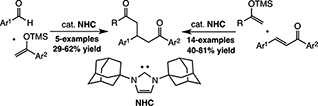
An N-heterocyclic carbene catalysed Mukaiyama–Michael addition between several TMS enol ethers and chalcone derivatives has been discovered. Also the related dehydrative Mukaiyama aldol followed by a Mukaiyama–Michael addition process has been developed. The enantioselective variant of these reactions has been examined with homochiral catalysts, which are active but fail to achieve enantioinduction.
CH16609Study on the Acid-Induced Ring-Expansion of an Oxaphosphirane Complex with an Electron-Withdrawing C-Substituent
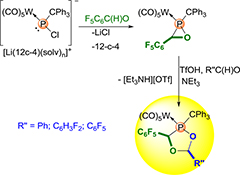
A study on the acid-induced ring-expansion reaction of the first oxaphosphirane complex bearing C6F5 as an electron-withdrawing C-substituent using trifluoromethanesulfonic acid and electronically different aldehydes is described. The stereoselectively obtained 1,3,4-dioxaphospholane complexes 4a–c were unambiguously characterized by elemental analysis, multinuclear NMR, mass spectrometry, and single crystal X-ray diffraction studies.



