Distribution, reproduction and population structure of three gulper sharks (Centrophorus, Centrophoridae) in south-east Australian waters
K. J. Graham A C and R. K. Daley BA Cronulla Fisheries Research Centre of Excellence, PO Box 21, Cronulla, NSW 2230, Australia.
B CSIRO Marine and Atmospheric Research, GPO Box 1538, Hobart, Tas. 7001, Australia.
C Corresponding author. Email: Ken.Graham@industry.nsw.gov.au
Marine and Freshwater Research 62(6) 583-595 https://doi.org/10.1071/MF10158
Submitted: 18 June 2010 Accepted: 4 January 2011 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Gulper sharks (Centrophorus spp.) are commercially fished in all oceans but the taxonomy and biology of many species are not clearly defined, and stocks are extremely vulnerable to over-exploitation. We present distributional, size-frequency and reproductive data for three species (Centrophorus harrissoni, C. moluccensis and C. zeehaani) that fishing has largely extirpated from the south-east Australian upper continental slope. Trawl-survey catches in 1976–77 from lightly exploited stocks comprised mostly mature males with few mature females or juveniles; a 2009 long-line survey caught higher proportions of mature females and, for C. harrissoni, more juveniles. Females of the three species grew larger and reached maturity at a greater size than males and, for both sexes, maturity sizes were more than 80% of their maximum observed lengths. Reproduction was continuous but data were insufficient to determine seasonality. Ovarian and uterine fecundity were singular for C. zeehaani whereas C. harrissoni and C. moluccensis developed two oocytes and produced one or two embryos; evidence suggests that the left-side uterus is less functional than the right-side. In response to these species’ inherent low productivity and continuing reduced numbers, managers have introduced landing restrictions and area closures to enhance Centrophorus stocks in southern Australian waters.
Additional keywords: dogfishes, fecundity, management.
Introduction
Since about 1990, the genus Centrophorus has been under taxonomic scrutiny both globally and within Australia, facilitated by the increased availability of specimens from more widespread deepwater fishing and the advances in DNA analyses. While problems still remain with the correct identification of Centrophorus sharks in many parts of the world (e.g. McLaughlin and Morrissey 2005; Bañón et al. 2008), the taxonomy of Australian species has been largely resolved. Seven species of Centrophorus are currently recognised from Australian waters (Last and Stevens 2009), including the three species, C. harrissoni, C. moluccensis, and C. zeehaani, discussed in this paper. The newly described C. zeehaani (White et al. 2008), which is endemic to southern Australia, is referable to C. uyato in Last and Stevens (1994), Graham et al. (2001) and Daley et al. (2002).
Gulper sharks are medium-sized demersal sharks (adult lengths between ~0.7 and 1.7 m) that are commercially exploited for both human consumption and the high squalene content of their livers (Compagno 1984; Gordon 1999; Daley et al. 2002). Significant landings of Centrophorus squamosus and C. granulosus are reported from several areas of the north-east Atlantic Ocean (Girard and Du Buit 1999; Clarke et al. 2001; Correia and Smith 2003) and, for C. granulosus, from the Mediterranean Sea (Guallart and Vicent 2001). While Centroscymnus spp. (Somniosidae) contribute the most to Japan’s deepsea shark fishery (Yano and Tanaka 1988), several species of Centrophorus (e.g. C. atromarginatus, C. lusitanicus) are also landed from Japanese and/or Taiwanese waters (Compagno et al. 2005), and five species of gulper sharks were found at various fish landing sites in Indonesia by White and Dharmadi (2010).
In Australia, the lack of species-specific data for sharks in fishery statistics and market sales make it difficult to estimate historical gulper shark catches or landings. The three species in this study were abundant on the New South Wales (NSW) and eastern Bass Strait upper continental slope at the advent of deepwater trawling in the 1970s (Graham et al. 2001) and large quantities were caught during the early years of this fishery (1975–85). However, a significant proportion of the catch was discarded, with preference given to the more marketable teleosts and because of market restrictions on shark meat with perceived high mercury levels (Daley et al. 2002). While gulper shark landings in south-eastern Australia were probably in the order of several hundred tonnes per annum during the 1980s and early 1990s, catches thereafter quickly declined. This trend was consistent with the results of stratified trawling surveys off NSW in 1976–77 and 1996–97 that revealed a greater than 95% reduction in the mean catch rates of Centrophorus between the two survey periods (Graham et al. 2001), and for other southern Australian areas where there were also rapid population reductions of Centrophorus with associated declines in market sales (Daley et al. 2002). Owing to the severely depleted stocks and the subsequent introduction of catch restrictions, reported landings of gulper shark from southern Australia since 2002 have been less than 20 t per annum (Wilson et al. 2009).
Previous studies into the biology of deepwater squaloid sharks have mainly focussed on the essentially mid-slope species of Centroscyllium, Centroscymnus, Deania and Etmopterus from the North Atlantic Ocean (Yano 1995; Jakobsdóttir 2001; Clarke et al. 2002), Japan (Yano and Tanaka 1984, 1988) and New Zealand (Wetherbee 1996), while most studies into the biology of Centrophorus dealt with C. squamosus from the north-west Atlantic, for example Girard and Du Buit (1999), Girard et al. (2000), Clarke et al. (2001), Bañón et al. (2008), Figueiredo et al. (2008) and Severino et al. (2009). Of the shallower upper-slope species, Bass et al. (1976) gave brief details of size at maturity for ‘C. scalpratus’ (C. cf. moluccensis) off South Africa while, more recently, Guallart and Vicent (2001) studied embryo development in C. granulosus from the Mediterranean, McLaughlin and Morrissey (2005) described the reproductive biology of C. cf. uyato from Jamaica, and White and Dharmadi (2010) presented biological information for five Indonesian species including C. moluccensis.
There have been no prior studies into the biology of Australian centrophorid sharks, although some data presented here were included in the earlier appraisal of the southern Australian deepwater shark fishery (Daley et al. 2002). The majority of data were recorded between 1976 and 1997 during demersal-trawling surveys off central and southern NSW by FRV Kapala and, particularly for C. moluccensis, during the 2009 FV Diana deepwater long-line survey for gulper sharks between northern NSW and north-eastern Tasmania. Supplementary data were collected between 1995 and 2005 from commercial trawling and long-line operations off eastern Bass Strait, Tasmania and western Victoria. Most reproductive data were collected opportunistically, mainly after shark numbers had declined, and some results are derived from relatively small numbers of observations. Considering the parlous state of gulper shark stocks off southern Australia, coupled with the regional endemism of two of the species, it is important to formally document all available information on these vulnerable sharks. For the three species, we map their geographic and depth distributions off south-east Australia, present biological and demographic data, and describe management actions taken to conserve gulper sharks in Australian waters.
Methods
Geographic and depth distributions
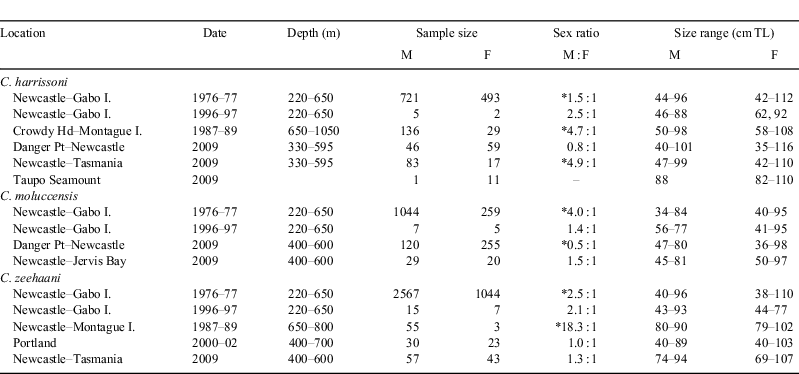
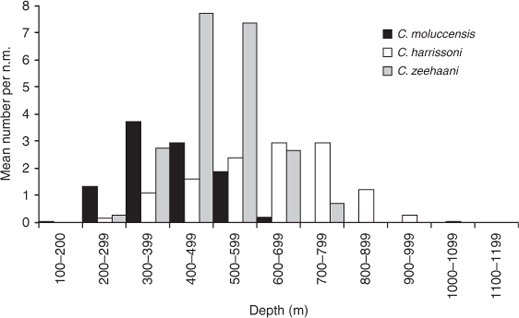
Distributions were derived mainly from catch records of ~1400 outer continental shelf and slope trawl stations off NSW and north-eastern Bass Strait (FRV Kapala) and almost 90 long-line and dropline stations fished during the 2009 FV Diana east coast survey. Further information was collected during research cruises by FRV Soela (CSIRO) and from observations of commercial vessel catches around south-eastern Australia. Characteristic depth distributions were determined from 817 Kapala survey stations between latitudes 32°S and 36°S during 1976–89 (i.e. on grounds where stations were distributed across all depths between 100 and 1200 m, and during the period when the three species were regularly caught). To make the data comparable, trawl catches during 1976–89 were pooled across years and for each species, the mean catch number per nautical mile trawled was calculated for each of 10 100-m depth strata between 100 and 1100 m.
Length–frequency and length–weight data
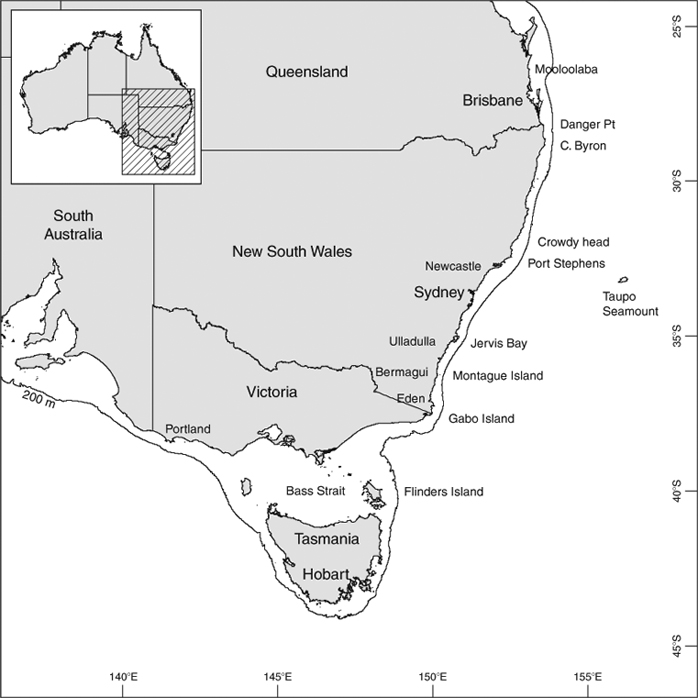
Total length (TL) was the distance from the snout tip to the posterior tip of the caudal fin with the shark lying in a natural position; measurements of each species by sex were recorded to the nearest cm below total length and, unless otherwise stated, all shark sizes in the text and tables are for TL. Length data were routinely collected from three locations on the NSW-eastern Victoria upper slope (Sydney–Newcastle, Ulladulla–Montague Island and Eden–Gabo Island; Fig. 1) during comparable stratified trawl surveys in 1976–77 and 1996–97 (details of gear, survey methods and overall results are in Andrew et al. 1997 and Graham et al. 2001). All sharks from small catches, or subsamples of large catches, were measured. Figures showing length distributions are for total catch (total numbers for subsampled catches were calculated by simple proportioning) pooled for all survey areas during 1976–77; the proportion of each size class for each sex is the percentage of the total catch (male plus female). Length data for the Diana long-line catches are presented as total numbers for each 2-cm size class. Length–weight data were mostly collected during the 1976–77 surveys, but were supplemented by data from later research trawling off Bermagui and Portland. Total weight (Wt) was recorded to the nearest 100 g, and length–weight (TL-Wt) regressions were obtained by fitting a power curve to the data in the form of Wt = aTLb for each sex and species. The curves for different sexes were compared for each species using an F-test based on the sums of squares from the fits for individual sexes and the sum of squares for the fit based on males and females combined. The test statistic was:
|
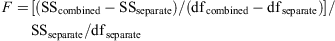
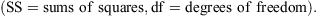
Reproduction
Maturity criteria were based on indices developed for deepwater sharks by Yano (1995), Girard and Du Buit (1999), Stehmann (2002) and Walker (2005). Outer clasper length (CLO; Compagno 1984) was the distance from the clasper tip to its insertion into the pelvic fin, measured with Vernier callipers to the nearest mm. Mature males were those with calcified claspers and spermatozoa able to be expressed when the base of the pelvic fins was pressed; those with partially or non-calcified claspers were classed as immature. Females were examined internally; developing ovarian eggs (oocytes) were counted and measured for oocyte diameter (OD), and the condition of the uteri was recorded, i.e. whether threadlike (immature), or expanded/flaccid or gravid (mature). For pregnant females, the number of uterine eggs or embryos was recorded, and embryos were measured (TL cm). Females classified as immature were those with undeveloped ovaries (oocytes white, <2 mm diameter) and thread-like uteri, and subadult or adolescent females with small developing (pale yellow) ovarian oocytes (~2–10 mm diameter) and uteri showing no expansion. Mature females had developing (yellow) ovarian oocytes >10 mm diameter and uteri ranging from minimal to full expansion, or were gravid. To determine the length at 50% maturity (L50) for each sex, the proportion of mature males and females at each 1-cm size class was calculated. These data were then fitted to a 3-parameter logistic function [y = a/(1 – exp(–(x – x0)/b))] using SigmaPlot 2002 for Windows (version 10.0; Systat Software Inc., Chicago, IL) to generate the L50 maturity estimate; the model’s goodness of fit was assessed using the R2 statistic.
Results
Geographic and depth distributions
Both C. harrissoni (350 records) and C. moluccensis (233 records) were caught in Kapala stations distributed across the full latitudinal range of sampling from east of the NSW–Queensland border (latitude 28°03′S) to south-east of Gabo Island (38°15′), whereas C. zeehaani (310 records) were only taken south of Crowdy Head from latitude 32°15′S (Fig. 1). During the 2009 long-line survey, C. harrissoni was caught at 33 stations between northern NSW and Flinders Island off north-eastern Tasmania, and at the Taupo Seamount (~33°06′S, 156°15′E) ~200 nm east of Newcastle. In contrast, C. moluccensis was caught only as far south as Jervis Bay (35°10′S) while C. zeehaani was first taken off the central NSW coast at latitude 33°35′S, followed by captures further south including off eastern Tasmania. During other research projects, C. zeehaani was the only species of the three recorded from the west coast of Tasmania, western Victoria and South Australia.
Overall depth ranges were, for C. harrissoni 275–1050 m, for C. moluccensis 145–615 m, and for C. zeehaani 220–745 m (Fig. 2). The three species were caught sympatrically at 69 trawl stations (~10% of stations between 200 and 650 m) and two long-line stations east of Sydney, in depths between 275 and 615 m. The few records of C. harrissoni and C. zeehaani in depths shallower than 300 m were from night-time trawls and, while no C. zeehaani were caught deeper than 750 m, C. harrissoni was recorded from 17 of 153 stations in 900–1050 m but none in 54 deeper trawls. C. moluccensis was the only member of the genus caught on the outer shelf (100–200 m), with seven records in 145–165 m, all from night-time trawls conducted during various continental shelf trawling activities (e.g. Graham et al. 1992).
|
Reproduction
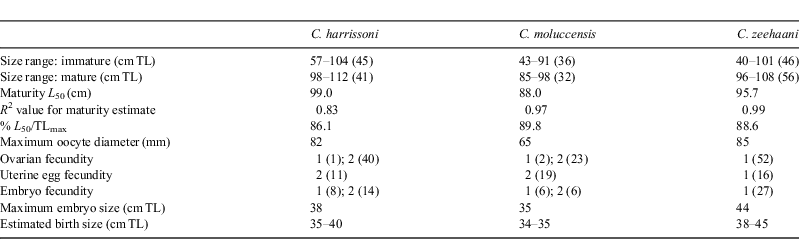
Most reproductive data were from specimens caught off NSW, eastern Bass Strait and north-eastern Tasmania, with additional data for C. zeehaani from catches off Portland, western Victoria (Table 1).
Centrophorus harrissoni
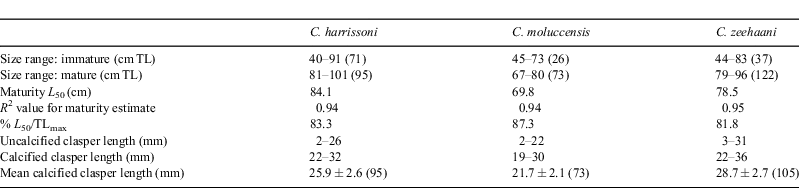
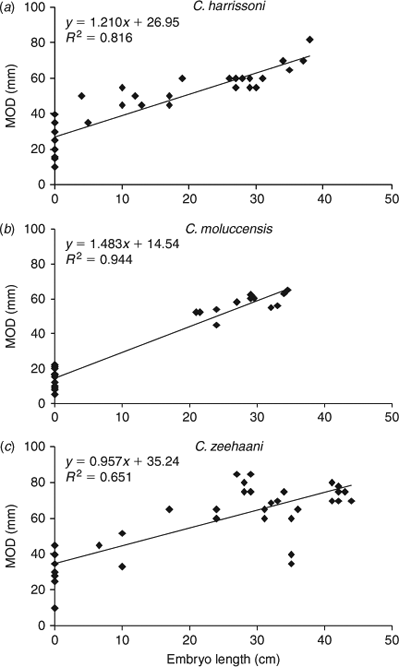
Male maturation (clasper elongation and calcification) occurred mainly across the size range of 75 to 85 cm (Fig. 3a), with the smallest mature male at 81 cm and, apart from a single subadult of 91 cm, all larger than 86 cm being mature. Estimated maturity L50 was 84 cm, ~83% of the maximum length recorded for C. harrissoni males (Table 2). Some females were immature to 104 cm, while the two smallest mature females were 98 cm, including one that was pregnant; the resulting L50 maturity estimate was 99.0 cm (Table 3). Two females (101, 102-cm TL) had immature uteri but mature ovaries, one containing a single oocyte of 30-mm diameter and the other with two 60-mm oocytes. There were 11 females pregnant with two uterine eggs, and had ovaries with two developing oocytes (OD range 10–40 mm, 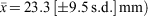 . Two females each had a single uterine egg, a single embryo in the other uterus (data not included in Table 3), and two developing ovarian oocytes. The embryos measured 13 and 27 cm, and the respective oocyte diameters were 45 and 55 mm, but it was not recorded whether both oocytes of the respective ovaries were of similar diameter. Eight females had a single embryo, while 14 had an embryo in each uterus, and all but one of these 22 pregnant females had two developing ovarian oocytes. The one female (107-cm TL) with a single growing oocyte also had a single embryo, suggesting a hereditary breeding pattern for that individual. Oocyte diameters of females with embryos ranged from 35 to 82 mm and were correlated (r2 = 0.81, P < 0.0001, n = 33) with embryo length (Fig. 4a), consistent with continuous reproduction. Embryos were between 5 and 38-cm TL and the 37–38 cm pups had fully absorbed their external yolk sacs. With full-term embryos to 38 cm and the smallest capture (by long-line) being 35 cm (Table 1), birth size is between 35 and 40 cm. As all female reproductive data were collected in July–September, any breeding seasonality could not be determined.
. Two females each had a single uterine egg, a single embryo in the other uterus (data not included in Table 3), and two developing ovarian oocytes. The embryos measured 13 and 27 cm, and the respective oocyte diameters were 45 and 55 mm, but it was not recorded whether both oocytes of the respective ovaries were of similar diameter. Eight females had a single embryo, while 14 had an embryo in each uterus, and all but one of these 22 pregnant females had two developing ovarian oocytes. The one female (107-cm TL) with a single growing oocyte also had a single embryo, suggesting a hereditary breeding pattern for that individual. Oocyte diameters of females with embryos ranged from 35 to 82 mm and were correlated (r2 = 0.81, P < 0.0001, n = 33) with embryo length (Fig. 4a), consistent with continuous reproduction. Embryos were between 5 and 38-cm TL and the 37–38 cm pups had fully absorbed their external yolk sacs. With full-term embryos to 38 cm and the smallest capture (by long-line) being 35 cm (Table 1), birth size is between 35 and 40 cm. As all female reproductive data were collected in July–September, any breeding seasonality could not be determined.
|
|
|
|
Centrophorus moluccensis
Reproductive data for C. moluccensis came mainly from specimens caught off northern NSW during the September 2009 long-line survey (Table 1). In males, claspers developed between 60 and 70-cm TL and all males larger than 73 cm were mature with outer clasper lengths greater than 19 mm (Fig. 3b). The estimated male L50 size at maturity was 70 cm, ~87% of their maximum recorded size (Table 2). The largest immature and smallest mature females were 91 and 85 cm respectively, resulting in a L50 maturity size of 88 cm, which was 90% of maximum total length (Table 3). Oocyte and uteri diameters of five mature but non-pregnant females (85–91-cm TL) were 27–42 and 6–10 mm. Of 31 pregnant females (88–98-cm TL), 19 had a candled egg in each uterus, six had a single embryo (always in the right uterus) and six carried an embryo in each uterus; one female with a single embryo had an atrophied egg in the left uterus. The size range of single embryos (21–34 cm) was similar to twin embryos (21–35 cm). Maximum oocyte diameter was strongly correlated with embryo size (r2 = 0.94, P < 0.0001, n = 31) with ODs of 5–22 mm 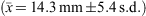 for recently ovulated females, and 45–65 mm for those with embryos (Fig. 4b), indicating continuous reproduction. Birth size of C. moluccensis is around 34–35 cm, with the largest embryo (35 cm) and smallest captured neonate (34 cm; Table 1) in this range. Three of the pregnant females were sampled in December and the remainder in August–September, precluding determination of any breeding seasonality.
for recently ovulated females, and 45–65 mm for those with embryos (Fig. 4b), indicating continuous reproduction. Birth size of C. moluccensis is around 34–35 cm, with the largest embryo (35 cm) and smallest captured neonate (34 cm; Table 1) in this range. Three of the pregnant females were sampled in December and the remainder in August–September, precluding determination of any breeding seasonality.
Centrophorus zeehaani
Clasper development mainly occurred in males mostly between 70 and 80-cm TL (Fig. 3c), and all males longer than 84 cm were mature; estimated maturity L50 was 79 cm, ~82% of maximum observed male length (Table 2). The largest immature female was 101 cm, whilst the smallest mature and pregnant specimens were 96 and 97 cm respectively; estimated maturity L50 (from few observations) was 96 cm, 89% of maximum female length (Table 3). Seven mature but non-pregnant females (96–103 cm) had developing oocytes with diameters of 40–70 mm, and a further two (103, 106-cm TL) were close to ovulation, with oocyte diameters of 80 mm. There were 43 pregnant females, each with a single embryo or uterine egg, and a single developing ovarian oocyte. The OD range of 13 females with uterine eggs was 10–45 mm (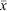 = 31.0 mm ±10.9 s.d.), whereas the OD range for those carrying an embryo was 35–85 mm (
= 31.0 mm ±10.9 s.d.), whereas the OD range for those carrying an embryo was 35–85 mm (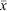 = 67.0 mm ± 14.2 s.d.). The relationship between oocyte diameter and embryo size (Fig. 4c) was significantly correlated (r2 = 0.65, P < 0.0001, n = 40), consistent with continuous reproduction. Embryos measured 6–44-cm TL and all pups larger than 41 cm had fully absorbed the external yolk sacs. The smallest captured neonate was 38 cm, indicating a birth size range of 38–45 cm. Although observations were made across 10 months, the data did not indicate any breeding seasonality.
= 67.0 mm ± 14.2 s.d.). The relationship between oocyte diameter and embryo size (Fig. 4c) was significantly correlated (r2 = 0.65, P < 0.0001, n = 40), consistent with continuous reproduction. Embryos measured 6–44-cm TL and all pups larger than 41 cm had fully absorbed the external yolk sacs. The smallest captured neonate was 38 cm, indicating a birth size range of 38–45 cm. Although observations were made across 10 months, the data did not indicate any breeding seasonality.
Population structure
High numbers of each species were caught during the 1976–77 upper-slope survey off central and southern NSW (Table 1), with different trawl operations (each of 60 min duration) catching as many as 600 C. zeehaani, 130 C. moluccensis or 100 C. harrissoni specimens. For each of the species, the total catch (pooled from all trawls) comprised significantly greater numbers of males (χ2 test, P < 0.01) with sex ratios (males : females) ranging between 1.5 : 1 and 4 : 1 (Table 1). However, within species, sex ratios varied among the survey grounds (Andrew et al. 1997, for more details). The Sydney–Newcastle catch of C. moluccensis (n = 1048) was composed almost wholly of males (22 : 1) compared with the smaller number from Ulladulla–Montague Island (n = 253) where the sex ratio was more than five females to each male. Catches of C. harrissoni off Sydney–Newcastle (n = 132) and Eden–Gabo Island (n = 656) contained similar numbers of males and females, but off Ulladulla–Montague Island (n = 426), males outnumbered females by almost 4 : 1. The relatively small catches of C. zeehaani from the Sydney–Newcastle area (n = 193) were mostly females (9 : 1); in contrast, catches off Ulladulla–Montague Island (n = 2129) and Eden–Gabo Island (n = 1288) were predominately males, with respective sex ratios of 2.2 : 1 and 5.5 : 1. By size, all species were dominated by adult males, with much smaller proportions of adult females (Fig. 5). About 88% of C. harrissoni males and 77% of C. moluccensis and C. zeehaani males were larger than their respective L50 maturity sizes, while only 35%, 15% and 40% of females of the three species (respectively) were mature. In 1987–89, 165 C. harrissoni and 58 C. zeehaani specimens were caught during midslope trawling off Sydney and Ulladulla (Table 1); 87% and 95% of these samples comprised mature males. Across all grounds, juveniles comprised about one-third (33–35%) of total numbers of each species. The 1996–97 repeat upper-slope survey caught so few of any species (Table 1) that no meaningful size comparisons could be made with the earlier data.
|
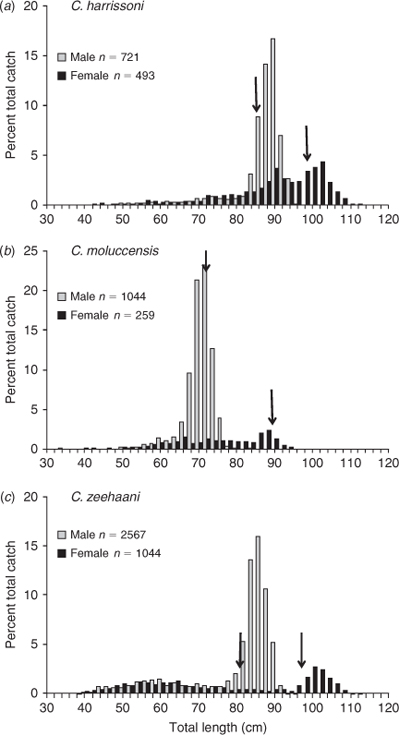
Sex ratios and length distributions of catches taken during the 2009 long-line survey also varied regionally. Like the trawl catches, the pooled size composition of long-lined C. harrissoni (Fig. 6a) showed a dominant mode of mature males and a smaller mode of adult females. The 12 C. harrissoni specimens caught on the Taupo Seamount were all larger than 80-cm TL (Table 1) and included one mature male and six females of mature size (>100 cm). Coastally, similar numbers of C. harrissoni were caught north and south of Newcastle (Table 1) but southern catches were dominated by mature males (76% of the total of 83 males) while only one of 17 females was mature. In contrast, more females than males were taken north of Newcastle (Table 1) and most were immature with only 40% of females and 30% of males being larger than their respective L50 maturity sizes. Catches of C. moluccensis north of Newcastle contained significantly more females than males (χ2 test, P < 0.01) whereas the smaller numbers taken between Newcastle and Jervis Bay on or adjacent to trawling grounds were mainly males (Table 1). Of the total catch, 84% of males and 43% of females were larger than their respective estimated L50 maturity lengths of 70 and 88 cm (Fig. 6b). About 89% of C. zeehaani specimens were caught at stations between Newcastle and Jervis Bay, with most in an area east of Sydney protected from trawling. The total catch comprised similar numbers of males and females, and almost all (of both sexes) were adult-sized (Table 1, Fig. 6c).
|
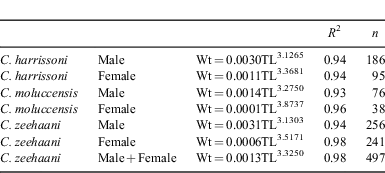
Length–weight relationships for each sex of the three species are listed in Table 4. The male and female fitted curves for C. harrissoni and C. moluccensis differed significantly (F-test, P < 0.01), but not for C. zeehaani (P = 0.14).
|
Discussion
Distribution
The distributions in Last and Stevens (2009) incorporate observations made during the earlier years of this study. The northern record for C. harrissoni on the east coast was the Clarence River (northern NSW) but its coastal distribution can now be extended further north to Cape Byron (Diana survey) and to Mooloolaba (~26°40′S) in southern Queensland (J. Rowley, pers. comm. and photographs to K. Graham, March 2010). This more northern distribution is consistent with the collection in 2004 of three C. harrissoni juveniles (39–60-cm TL) from 440–670 m on the Fraser Seamount (24°25′S, 155°17′E) east of Bundaberg (J. Johnson, Queensland Museum, pers. comm.), extending its known range to Coral Sea waters off central Queensland. The capture of C. harrissoni at the Taupo Seamount ~230 nm east of mainland Australia (Diana survey) and records from the Norfolk, Three Kings and Kermadec Ridges (Duffy 2007) suggest that this species is more widespread across the south-west Pacific than previously thought. However, DNA samples are needed from these widely separated localities to check for possible cryptic speciation within the genus as exemplified by the recent description of C. westraliensis, which was formerly considered a population of C. harrissoni (White et al. 2008).
The data for C. moluccensis showed evidence of a contraction of its southern range on Australia’s east coast. During the 1976–77 Kapala surveys, before upper-slope trawling had impacted on shark numbers, the species was relatively abundant between Sydney and Newcastle, moderate numbers were caught off Ulladulla, and three specimens were taken as far south as 38°12′S on the Gabo Island grounds (Andrew et al. 1997). However, during the 1980s, fewer than 10 C. moluccensis specimens were caught in numerous Kapala trawls between Jervis Bay and Gabo Island and, in the repeat 1997 survey, none was caught on the two southern NSW grounds. Consistent with this trend, the most southern capture of C. moluccensis during the 2009 Diana long-line survey was off Jervis Bay. There are no verified records of C. moluccensis from Tasmania or along the south coast of the continent; the report of Endeavour dogfish (C. moluccensis) from trawl survey catches in the Great Australian Bight (Newton and Klaer 1991) almost certainly relates to C. zeehaani. That species is distributed around the southern half of Australia from Forster (~32°S) in central NSW to Bunbury (~33°S) in Western Australia (White et al. 2008). On the east coast, the most northerly trawl capture of C. zeehaani by Kapala was in 1987 off Crowdy Head (31°52′S). Because of rough terrain, deepwater trawling north of this latitude was confined to relatively small areas off Yamba (29°30′S) and the NSW–Queensland border but there were no C. zeehaani captures on either ground. Several long-line stations were fished between 28°00′S and 33°00′S during the 2009 survey, but the most northern capture of C. zeehaani was south-east of Newcastle (33°35′S) possibly indicating a southward contraction of its distribution of more than 100 nautical miles (185 km) during the past 20 years. The highest catch rate of C. zeehaani during the long-line survey was in a trawling closure east of Sydney, suggesting that the protected area was effective in protecting gulper shark numbers.
Drawing on data from a large number of trawl and long-line stations distributed across a wide depth range, the core depths and overall ranges for the three species off eastern Australia were well defined, although the relative abundances shown in Fig. 2 should be interpreted as indicative only because the amount of sampling across years and depths was uneven and no attempt was made to allow for different gear sizes. However, it is clear that these species prefer upper-slope depths in the 300–800 m range and with the trawl fishery targeting these (and occasionally deeper) depths over the last 30 years, it is not surprising that gulper sharks have been largely eliminated from these grounds (Graham et al. 2001). It is likely that the apparently reduced latitudinal ranges of both C. moluccensis and C. zeehaani are also attributable to continual trawling.
The depth ranges for Centrophorus moluccensis and C. zeehaani spanned 470 and 525 m respectively, while C. harrissoni had a slightly greater depth span of 775 m. Depth ranges recorded for 12 other upper and mid slope dogsharks from south-east Australia also spanned less than ~800 m (Daley et al. 2002). However, reported depths for many species of Centrophorus show ranges with spans in excess of 1000 m (e.g. 50–1440 m for C. granulosus, 300–1440 m for C. lusitanicus and 230–2400 m for C. squamosus; Compagno et al. 2005). It is unusual for demersal species to inhabit such great depth ranges and it is likely that the extremes of these ranges were originally derived from station data where capture depths could not be precisely defined, or were from stations sampled before the advent of electronic depth sounders and actual depths were under or overestimated. Such an example was apparent for fishes collected off Sydney in 1906 where the documented depth was ‘800 fathoms’ (1460 m) and this depth attribute was subsequently applied to all species from that station (McCulloch 1907); recent intensive sampling in the area has shown that almost all species from that station only inhabit the upper slope in depths less than ~600 m (Iwamoto and Graham 2001).
The overlapping core depth ranges (Fig. 2) and frequent sympatric capture off NSW of these gulper sharks of similar size and morphology suggest subtly different ecological niches for each species. While the distributions of C. moluccensis and C. zeehaani (on the east coast) only overlap off central NSW, with the core of their ranges respectively to the north and south, C. harrissoni is distributed across large areas inhabited by both species. Records of stomach contents (K. Graham, unpubl. data) show that C. moluccensis and particularly C. harrissoni feed primarily on mesopelagic prey such as lantern fishes (Myctophidae) and squids whereas the diet of C. zeehaani, although also including myctophids and squids, contained a higher proportion of demersal fishes and crustaceans such as the teleosts Helicolenus barathri (Sebastidae), Hoplichthys haswelli (Hoplichthyidae), Benthodesmus elongatus and Lepidopus caudatus (Trichiuridae), and prawns Haliporoides sibogae (Solenoceridae) and Plesionika martia (Pandalidae). Thus, there appears to be some ecological separation among the three species in that C. zeehaani is more reliant on demersal prey than the other two species and, although having overlapping depth ranges, C. moluccensis mainly lives shallower than C. harrissoni (Fig. 2).
Reproduction
As is typical of many sharks, sizes at maturity and maximum lengths of the three study species were sexually dimorphic, with females of each growing ~12% longer and attaining approximately twice the weight of their respective males. This sexual dimorphism was reflected in the length–weight relationships for C. moluccensis and C. harrissoni males and females, which, for each species, differed significantly between the sexes. Maturation occurred across a relatively narrow size range of each sex and species (3–11 cm) with these transition ranges incorporating between 15 and 90 observations, thus providing sufficient data to give adequate L50 maturity estimates (R2 values >0.8). Following Walker (2005), who argued that female sharks with developing oocytes should be treated as mature, those females with immature uteri but clearly maturing oocytes (>10 mm diameter) were classed as mature. While other studies required both oocytes and uteri to be developed for females to be classed as mature (e.g. Bañón et al. 2008), in this study, female L50 estimates using either criteria varied by less than 1-cm TL. The L50 values, when expressed as a percentage of maximum recorded length, showed males (82–87%) and females (86–90%) reached maturity when almost fully grown. Similarly, minimum breeding sizes in excess of 80% of observed maximums were reported for C. granulosus (Guallart and Vicent 2001), C. squamosus (Compagno et al. 2005), C. cf. uyato (McLaughlin and Morrissey 2005) and the Indonesian species C. atromarginatus, C. cf. lusitanicus and C. isodon (White and Dharmadi 2010). Late onset of breeding, relative to size, was also found in other south-east Australian deepwater dogsharks including Centroscyllium kamoharai (male L50 = 76% of maximum size, female 81%), Centroscymnus spp. (70–80, 78–83%), Deania spp. (77–80, 78–83%) and Squalus spp. (75–82, 77–86%) (Daley et al. 2002; Graham 2005).
The direct relationship between ovarian oocyte size and embryo size in the three study species was consistent with a continuous breeding cycle where parturition is quickly followed by ovulation – a strategy also determined for C. cf. uyato (McLaughlin and Morrissey 2005) and shared by Squalus, the other main squaliform genus inhabiting outer-shelf and upper-slope depths off south-east Australia and elsewhere (Chen et al. 1981; Hanchet 1988; Watson and Smale 1998; Graham 2005). This contrasts with the non-continuous cycles of dogshark genera such as Centroscyllium, Centroscymnus, Deania and Etmopterus which are more commonly found in midslope depths (e.g. Clark and King 1989; Yano 1995; Wetherbee 1996; Daley et al. 2002).
There was no explicit evidence of breeding seasonality in any species although the data for C. moluccensis from the September 2009 long-line survey showed that 61% of pregnant females had recently ovulated, suggesting some synchronicity of ovulation. This inferred seasonality was supported by the absence of embryos smaller than 21 cm, which may be interpreted as the approximate size to which embryos grow in their first year of gestation. With such a scenario, and with birth size at ~35 cm, year 1+ embryos may be expected to be in the 20–25-cm size range near the start of the second year of gestation. However, such a deduction was confounded by the remaining pregnant females that contained embryos ranging from 21 to 35 cm, more consistent with non-seasonal parturition. Nevertheless, the observations did not conflict with the likelihood of a 2-year pregnancy as was determined for C. granulosus (Guallart and Vicent 2001), or possibly even a 3-year gestation, which was postulated by McLaughlin and Morrissey (2005) for C. cf. uyato off Jamaica. Among other squaliform sharks, a similar period of almost 2 years has been estimated for Squalus acanthias (Ketchen 1972; Hanchet 1988) and S. mitsukurii (Wilson and Seki 1994), while Clarke et al. (2002) speculated that gestation in Deania calcea may be as long as or longer than for S. acanthias.
Fecundity was singular in C. zeehaani, while in C. harrissoni and C. moluccensis there were either one or two offspring per pregnancy. In C. zeehaani, fecundity did not vary, with more than 50 females containing a single developing oocyte and 43 carrying a single candle or embryo. Apart from two specimens, all C. moluccensis ovaries had two developing oocytes; the exceptions had recently ovulated and the single yellow oocytes were relatively small (16–20 mm diameter) and it is likely that a second oocyte would have developed during pregnancy. All recently ovulated C. moluccensis had two uterine eggs but only 50% of later-term females were carrying two embryos. Single embryo sizes ranged between 21 and 35 cm and there was no evidence that second embryos had been aborted during capture, indicating that a proportion of ovulated eggs or small embryos became unviable during pregnancy and were aborted. A similar pattern was found in C. harrissoni, where all except one female were observed with two developing oocytes and all but two that had recently ovulated were carrying two uterine ova; however, only 64% with discernable embryos had two pups. The two C. harrissoni females with single uterine eggs also had a well developed embryo (13 and 27-cm TL) in their second uterus, and the ovaries of each had two large developing oocytes (45 and 55 mm respectively). It was not recorded whether both oocytes in each of the ovaries were of the same size but, if such was the case, it is possible that ovulation would be synchronised after the eventual birth of both embryos; conversely, oocytes of unequal size would suggest a continual alternating breeding cycle with a developing oocyte and one uterus functioning independently of the other pair. Again, in the 36% of C. harrissoni females with single embryos (19–37-cm TL), there was no evidence of any being aborted and it appears that this less-than-optimal embryo fecundity observed in C. harrissoni and C. moluccensis reflects their natural breeding success off NSW.
It was not recorded for C. harrissoni which uterus was active in females with single embryos. In C. moluccensis, however, all singles were found in the right-side uterus, including one female with an atrophied egg in the left uterus. These observations indicated that the right uterus was favoured over the left, and the non-development or loss of an egg or embryo did not occur randomly from either uterus. Assuming C. moluccensis and other species of Centrophorus are lecithotrophic species, as was established for C. granulosus by Guallart and Vicent (2001), the loss of an ovulated egg or small embryo should be largely independent of any physiological stress in the mother. The evidence from C. moluccensis suggests that fertilisation is less successful on the left side and this may be an evolutionary trait in Centrophorus. McLaughlin and Morrissey (2005) found almost 70% of 16 pregnant C. cf. uyato females with two embryos but only one of five single embryos was in the left uterus. In eight C. zeehaani females, where the side containing the embryo or candled ova was recorded, all were in the right-side uterus. It can be hypothesised that in a species such as C. zeehaani (and possibly other Centrophorus with singular fecundity), the left-side uterus has become non-functional, giving rise to the production of a single pup larger in proportion to maternal size than those of species normally producing two pups. The lengths of fully developed pups in C. zeehaani were up to 45% of female L50, appreciably longer and almost double the weight (estimated from length–weight relationships) of the birth sizes of C. harrissoni and C. moluccensis (Table 3).
The breeding characteristics of C. zeehaani females (mature size 96–108-cm TL, singular fecundity, oocytes to 85 mm diameter and birth size of 38–44 cm) compare closely to those of C. granulosus from the Mediterranean Sea, in which the largest oocyte found was 87 mm diameter and pregnant females were 94–106-cm TL, producing single embryos with estimated birth size of 34–46-cm TL (Guallart and Vicent 2001). These authors described the fecundity of C. granulosus with its single embryo and gestation of about 2 years as one of the lowest recorded. Assuming similar gestation times, C. zeehaani joins C. granulosus, along with C. atromarginatus and C. cf. lusitanicus, which also produce single offspring (White and Dharmadi 2010), in a group of sharks with extremely low fecundity. However, the birth size of these species (where it could be ascertained from data in the literature) were all around 45% of maternal length, compared with smaller-sized eggs and birth sizes (less than 40% of maternal length) of Centrophorus species that usually have twin ovarian eggs and embryos. If it can be assumed that larger newborns have a better chance of survival, the evolution of a single offspring strategy with increased birth size may compensate for the ultra-low fecundity.
Results from this and other studies (see references cited above) suggest that Centrophorus can be divided into three natural groups based on their size and reproductive strategies, and we suggest that these attributes could be taken into account during further taxonomic revision of the genus. A group including C. acus, C. niaukang and C. squamosus are large species with mature males and females attaining 100–130-cm and 140–170-cm TL respectively, and having comparatively high ovarian (5–11) and uterine (4–8) fecundities. The size and biology of ‘C. granulosus’ from the north-eastern Atlantic Ocean reported by Bañón et al. (2008) also fits these criteria but, as discussed by those authors, their specimens were more likely to be a form of C. niaukang. Other species of Centrophorus are substantially smaller (males and females less than 100 and 115 cm respectively) and these can be grouped either into species with seemingly obligatory singular ovarian and uterine fecundity (C. atromarginatus, C. granulosus, C. cf. lusitanicus and C. zeehaani), or those that usually produce two ovarian and uterine eggs and embryos (C. harrissoni, C. isodon, C. moluccensis and C. cf. uyato). No biological information was found for C. seychellorum, C. tessellatus or C. westraliensis.
Population structure
The 1976–77 surveys were completed before the commercial trawl fishery became established on the NSW upper slope (Graham et al. 2001). Consequently, the 1976–77 size compositions are historically important as the data reflect the stock structure of each species on trawling grounds before the impact of commercial fishing. The most important feature for all three species was the dominance of large, mostly adult, males, relatively small numbers of mature females and few juveniles of either sex. Biased sex ratios in dogshark catches are commonly reported and although segregation of the sexes by depth has been observed in several midslope dogsharks (e.g. Yano and Tanaka 1988; Yano 1995; Clarke et al. 2001; Jakobsdóttir 2001), segregation by depth was unlikely for Centrophorus off NSW. Sampling during the 1976–77 survey encompassed most of the preferred depth range (300–650 m) of these species, and subsequent trawling in depths between 600 and 1000 m also caught predominantly males (Table 1). There was also no indication of any seasonal migrations of males or females that may have affected the sex ratios of catches.
The relatively small proportion of juveniles in catches was consistent for all species across all grounds and depths. With the large birth sizes for the three species (35–44-cm TL), it was unlikely that many juveniles escaped through the 90-mm codend meshes of the trawl, particularly as high numbers of the small dogshark Squalus megalops (30–60-cm TL) were caught during the same survey (Graham 2005). The greatest number of juveniles was caught on the Ulladulla ground, where almost 40% of C. zeehaani specimens were less than 70 cm (Andrew et al. 1997). These small juveniles were distributed almost evenly across the depth zones sampled and were associated with similar numbers (in proportion to total catch in each depth zone) of adult sharks, thus giving no suggestion of maturity-level segregation by depth, as has been shown for the brier shark Deania calcea (Clark and King 1989). A similar lack of Squalus megalops juveniles in South African trawl catches led Compagno et al. (1991) to suggest that the young may be pelagic. However, there was no evidence for such behaviour in Centrophorus off NSW as no juveniles were caught during several near-bottom pelagic trawls made over the upper slope off Sydney in 1976–77 (K. Graham, pers. obs.).
The capture of small juveniles (including neonates) by long-line indicates that, as with trawling, all size classes of gulper sharks are vulnerable to this fishing method. Relatively high numbers of C. harrissoni juveniles (<80-cm TL) were caught during the 2009 long-line survey (Fig. 6a) and most were taken off central NSW at stations off Port Stephens, an area that was continually trawled during the first 20 years of the NSW upper-slope fishery (Graham et al. 2001). However, there has been much less trawling on this ground over the last 10 years and the long-line captures of numerous C. harrissoni juveniles (including neonates), along with similar numbers of mainly large C. moluccensis specimens, suggest that gulper sharks are becoming re-established on this part of the NSW coast.
Management implications and responses
There have been long-held concerns about the sustainability of shark fisheries (e.g. Holden 1974; Compagno 1990; Walker 1998; Gordon 1999; Stevens et al. 2000) and the results of this study confirm that the life-history characteristics of Centrophorus species make them extremely vulnerable to fishing pressure. Kyne and Simpfendorfer (2007) reported that the intrinsic rebound potential of deepwater chondrichthyans was very low and limited their ability to sustain fishing pressure or recover from over-fishing, and further stated that Centrophorus species are possibly the least productive of any chondrichthyan fish. Because of these attributes, several gulper sharks are included in the IUCN Red List of threatened species, with widespread species such as C. granulosus and C. squamosus being assessed as ‘Vulnerable’, and C. acus and C. niaukang as ‘Near Threatened’ (www.iucnredlist.org). Where they are targeted in the north-east Atlantic, catches of C. granulosus and C. squamosus have steadily declined (Correia and Smith 2003; White 2003; Guallart et al. 2006) but the only fishery-independent data quantifying the effects of commercial exploitation on gulper sharks is the Australian study by Graham et al. (2001), who found a greater than 95% reduction in the relative abundances of Centrophorus species on NSW and adjacent trawl grounds between 1976 and 1997. Acting on these results, the IUCN Red List process assessed C. harrissoni and C. zeehaani (then designated as ‘C. uyato Australian subpopulation’) as ‘Critically Endangered’ and the Australian subpopulation of C. moluccensis as ‘Endangered’ (Pogonoski and Pollard 2003a, 2003b, 2003c). The three species are also currently under consideration for listing as ‘threatened’ under Australia’s Environment Protection and Biodiversity Conservation Act 1999 (Wilson et al. 2009).
In response to these conservation issues, the Australian Fisheries Management Authority (AFMA) has implemented gulper shark catch limits (15 kg per day) with the objective of preventing targeted fishing in Australian Commonwealth managed waters. In addition, some offshore seamounts and areas of deep water off Sydney, eastern Bass Strait and South Australia have been closed to trawling, long-lining and gill-netting (AFMA 2010). It was encouraging that during the 2009 long-line survey for gulper sharks, higher than average catch rates of Centrophorus were achieved in two of these protected areas. Therefore, taking into account the recent survey data and conservation actions, there may be grounds for lessening the IUCN Red List threat status of the three species. However, gulper sharks off south-east Australia remain assessed as ‘overfished’ and ‘subject to overfishing’ (Stobutzki et al. 2010) based on the expectation that that the harvest rate in any fished area will exceed Maximum Sustainable Yield (MSY), estimated by Forrest and Walters (2009) to be ~1%. It is clear that the productivity of our study species is so low that even if stocks are not targeted and gulper sharks are only taken as incidental by-catch, populations are unlikely to recover in those areas where numbers have been greatly reduced but fishing continues. Given their intrinsic low productivity, the continuing exploitation of gulper sharks in other parts of the world almost certainly places those species at similar risk.
Acknowledgements
We would like to thank Terry Gorman for initiating deepwater fisheries research off NSW in the 1970s, and John Stevens for coordinating the cooperative research that synthesised the existing information and led to a series of new studies into Australia’s deepwater dogsharks. Special thanks go to the masters and crews of the FRV Kapala and FV Diana for their willing cooperation and assistance during the various surveys. We would also like to acknowledge the CSIRO Wealth from Oceans Flagship and the Fisheries Research and Development Corporation for support of these studies and ongoing surveys. John Pogonoski is thanked for his rigorous critique of the initial draft of the manuscript, while Alan Williams, the Guest Editors and two anonymous referees provided constructive suggestions and improvements to the paper. Judy Upston and Dennis Reid are also thanked for their assistance with data analyses.
References
AFMA (2010). Southern and Eastern Scalefish and Shark Fishery Management Arrangements Booklet. Australian Fisheries Management Authority, Canberra. Available at http://www.afma.gov.au/fisheries/sess/sess/publications/default.htm [accessed 17 June 2010].Andrew, N. L., Graham, K. J., Hodgson, K. E., and Gordon, G. N. G. (1997). Changes after twenty years in relative abundance and size composition of commercial fishes caught during fishery independent surveys on SEF trawl grounds. FRDC Project No. 96/139. NSW Fisheries Final Report Series No. 1. NSW Fisheries Research Institute, Sydney.
Bañón, R., Pineiro, C., and Casas, J. M. (2008). Biological observations on the gulper shark Centrophorus granulosus (Chondrichthyes: Centrophoridae) off the coast of Galicia (north-western Spain, eastern Atlantic). Journal of the Marine Biological Association of the United Kingdom 88, 411–414.
| Biological observations on the gulper shark Centrophorus granulosus (Chondrichthyes: Centrophoridae) off the coast of Galicia (north-western Spain, eastern Atlantic).Crossref | GoogleScholarGoogle Scholar |
Bass, A. J., D’Aubrey, J. D., and Kistnasamy, N. (1976). Sharks of the east coast of southern Africa. VI. The families Oxynotidae, Squalidae, Dalatiidae and Echinorhinidae. Investigational Report No. 45. South African Association for Marine Biological Research, Oceanographic Research Institute, Durban.
Chen, C., Taniuchi, T., and Nose, Y. (1981). Some aspects of reproduction in the pointed-snout dogfish Squalus japonicus taken off Nagasaki and Chosi. Bulletin of the Japanese Society of Scientific Fisheries 47, 1157–1164.
Clark, M. R., and King, K. J. (1989). Deepwater fish resources off the North Island, New Zealand: results of a trawl survey, May 1985 to June 1986. New Zealand Fisheries Technical Report No. 11. MAFFish, Wellington.
Clarke, M. W., Connolly, P. L., and Bracken, J. J. (2001). Aspects of the reproduction of the deep water sharks Centroscymnus coelolepis and Centrophorus squamosus from west of Ireland and Scotland. Journal of the Marine Biological Association of the United Kingdom 81, 1019–1029.
Clarke, M. W., Connolly, P. L., and Bracken, J. J. (2002). Catch, discarding, age estimation, growth and maturity of the squalid shark Deania calceus west and north of Ireland. Fisheries Research 56, 139–153.
| Catch, discarding, age estimation, growth and maturity of the squalid shark Deania calceus west and north of Ireland.Crossref | GoogleScholarGoogle Scholar |
Compagno, L. J. V. (1984). FAO species catalogue, Vol. 4. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 1. Hexanchiformes to Lamniformes: viii, 1–250. FAO Fisheries Synopsis 125. Food and Agriculture Organization, Rome.
Compagno, L. J. V. (1990). Shark exploitation and conservation. In ‘Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and Status of the Fisheries’. (Eds H. L. Pratt Jr, S. H. Gruber and T. Taniuchi.) NOAA Technical Report NMFS 90. pp. 391–414. US Department of Commerce, Seattle, WA.
Compagno, L. J. V., Ebert, D. A., and Cowley, P. D. (1991). Distribution of offshore demersal cartilaginous fish (class Chondrichthyes) off the west coast of southern Africa, with notes on their systematics. South African Journal of Marine Science 11, 43–139.
Compagno, L., Dando, M., and Fowler, S. (2005). ‘Sharks of the World.’ (Princeton University Press: Princeton, NJ.)
Correia, J. P. S., and Smith, M. F. L. (2003). Elasmobranch landings for the Portuguese commercial fishery from 1986 to 2001. Marine Fisheries Review 65, 32–40.
Daley, R., Stevens, J. D., and Graham, K. (2002). Catch analysis and productivity of the deepwater dogfish resource in southern Australia. FRDC Project 1998/108. CSIRO Marine Research, Fisheries Research and Development Corporation and NSW Fisheries, Australia.
Duffy, C. A. J. (2007). First record of Centrophorus harrissoni from New Zealand, with observations on squamation in Centrophoridae (Squaliformes). New Zealand Journal of Marine and Freshwater Research 41, 163–173.
| First record of Centrophorus harrissoni from New Zealand, with observations on squamation in Centrophoridae (Squaliformes).Crossref | GoogleScholarGoogle Scholar |
Figueiredo, I., Moura, T., Neves, A., and Gordo, L. S. (2008). Reproductive strategy of leafscale gulper shark Centrophorus squamosus and the Portuguese dogfish Centroscymnus coelolepis on the Portuguese continental slope. Journal of Fish Biology 73, 206–225.
| Reproductive strategy of leafscale gulper shark Centrophorus squamosus and the Portuguese dogfish Centroscymnus coelolepis on the Portuguese continental slope.Crossref | GoogleScholarGoogle Scholar |
Forrest, R. E., and Walters, C. J. (2009). Estimating thresholds to optimal harvest rate for long-lived, low-fecundity sharks accounting for selectivity and density dependence in recruitment. Canadian Journal of Fisheries and Aquatic Sciences 66, 2062–2080.
| Estimating thresholds to optimal harvest rate for long-lived, low-fecundity sharks accounting for selectivity and density dependence in recruitment.Crossref | GoogleScholarGoogle Scholar |
Girard, M., and Du Buit, M. H. (1999). Reproductive biology of two deep-water sharks from the British Isles, Centroscymnus coelolepis and Centrophorus squamosus. Journal of the Marine Biological Association of the United Kingdom 79, 923–931.
| Reproductive biology of two deep-water sharks from the British Isles, Centroscymnus coelolepis and Centrophorus squamosus.Crossref | GoogleScholarGoogle Scholar |
Girard, M., Rivalan, P., and Sinquin, G. (2000). Testis and sperm morphology in two deep-waters squaloid sharks, Centroscymnus coelolepis and Centrophorus squamosus. Journal of Fish Biology 57, 1575–1589.
| Testis and sperm morphology in two deep-waters squaloid sharks, Centroscymnus coelolepis and Centrophorus squamosus.Crossref | GoogleScholarGoogle Scholar |
Gordon, J. D. M. (1999). Management considerations of deep-water shark fisheries. In ‘Case Studies of the Management of Elasmobranch Fisheries’. (Ed. R. Shotten.) pp. 774–818. FAO Fisheries Technical Paper 378/2, Food and Agriculture Organization, Rome.
Graham, K. J. (2005). Distribution, population structure and biological aspects of Squalus spp. (Chondrichthyes, Squaliformes) from New South Wales and adjacent Australian waters. Marine and Freshwater Research 56, 405–416.
| Distribution, population structure and biological aspects of Squalus spp. (Chondrichthyes, Squaliformes) from New South Wales and adjacent Australian waters.Crossref | GoogleScholarGoogle Scholar |
Graham, K. J., Kennelly, S. J., Andrew, N. L., and Montgomery, S. S. (1992). Report for Cruises 90–03 to 90–07 conducted during February–April 1990. Kapala Cruise Report No. 109. Fisheries Research Institute, Sydney.
Graham, K. J., Andrew, N. J., and Hodgson, K. E. (2001). Changes in relative abundance of sharks and rays on Australian South East Fishery trawl grounds after twenty years of fishing. Marine and Freshwater Research 52, 549–561.
| Changes in relative abundance of sharks and rays on Australian South East Fishery trawl grounds after twenty years of fishing.Crossref | GoogleScholarGoogle Scholar |
Guallart, J., and Vicent, J. J. (2001). Changes in composition during embryo development of the gulper shark Centrophorus granulosus (Elasmobranchii, Centrophoridae): an assessment of maternal-embryonic nutritional relationships. Environmental Biology of Fishes 61, 135–150.
| Changes in composition during embryo development of the gulper shark Centrophorus granulosus (Elasmobranchii, Centrophoridae): an assessment of maternal-embryonic nutritional relationships.Crossref | GoogleScholarGoogle Scholar |
Guallart, J., Serena, F., Mancusi, C., Casper, B. M., Burgess, G. H., et al. 2006. Centrophorus granulosus. In ‘IUCN 2010’. IUCN Red List of Threatened Species. Version 2010.4. Available at www.iucnredlist.org [accessed 29 November 2010].
Hanchet, S. (1988). Reproductive biology of Squalus acanthias from the east coast, South Island, New Zealand. New Zealand Journal of Marine and Freshwater Research 22, 537–549.
| Reproductive biology of Squalus acanthias from the east coast, South Island, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Holden, M. J. (1974). Problems in the rational exploitation of elasmobranch populations and some suggested solutions. In ‘Sea Fisheries Research’. (Ed. F. R. Harden-Jones.) pp. 117–137. (John Wiley: New York.)
Iwamoto, T., and Graham, K. J. (2001). Grenadiers (families Bathygadidae and Macrouridae, Gadiformes, Pisces) of New South Wales, Australia. Proceedings of the California Academy of Sciences 52, 407–509.
Jakobsdóttir, K. B. (2001). Biological aspects of two deep-water squalid sharks: Centroscyllium fabricii (Reinhardt, 1825) and Etmopterus princeps (Collett, 1904) in Icelandic waters. Fisheries Research 51, 247–265.
| Biological aspects of two deep-water squalid sharks: Centroscyllium fabricii (Reinhardt, 1825) and Etmopterus princeps (Collett, 1904) in Icelandic waters.Crossref | GoogleScholarGoogle Scholar |
Ketchen, K. S. (1972). Size at maturity, fecundity and embryonic growth of the spiny dogfish (Squalus acanthias) in British Columbian waters. Journal of the Fisheries Research Board of Canada 29, 1717–1723.
Kyne, P. M. and Simpfendorfer, C. A. (2007). A collation and summarization of available data on deepwater chondrichthyans: Biodiversity, life history and fisheries. A report prepared by the IUCN Shark Specialist Group for the Marine Conservation Biology Institute. Available at: http://www.flmnh.ufl.edu/fish/organizations/ssg/deepchondreport.pdf.
Last, P. R., and Stevens, J. D. (1994). ‘Sharks and Rays of Australia.’ (CSIRO Publishing: Melbourne.)
Last, P. R., and Stevens, J. D. (2009). ‘Sharks and Rays of Australia.’ 2nd edn. (CSIRO Publishing: Melbourne.)
McCulloch, A. R. (1907). The results of deep sea investigations in the Tasman Sea. II. The expedition of the Woy Woy. Fishes and crustaceans from eight hundred fathoms. Records of the Australian Museum 6, 345–355.
| The results of deep sea investigations in the Tasman Sea. II. The expedition of the Woy Woy. Fishes and crustaceans from eight hundred fathoms.Crossref | GoogleScholarGoogle Scholar |
McLaughlin, D. M., and Morrissey, J. M. (2005). Reproductive biology of Centrophorus cf. uyato from the Cayman Trench, Jamaica. Journal of the Marine Biological Association of the U.K. 85, 5027/1–8.
Newton, G., and Klaer, N. (1991). Deep-sea demersal fisheries resources of the Great Australian Bight: a multivessel trawl survey. Bureau of Rural Resources Bulletin No. 10. Australian Government Publishing Service, Canberra.
Pogonoski, J., and Pollard, D. (2003a). Centrophorus harrissoni. In ‘IUCN 2010’. IUCN Red List of Threatened Species. Version 2010.4. Available at www.iucnredlist.org [accessed 29 November 2010].
Pogonoski, J., and Pollard, D. (2003b). Centrophorus moluccensis. In ‘IUCN 2010’. IUCN Red List of Threatened Species. Version 2010.4. Available at www.iucnredlist.org [accessed 29 November 2010].
Pogonoski, J., and Pollard, D. (2003c). Centrophorus uyato. In ‘IUCN 2010’. IUCN Red List of Threatened Species. Version 2010.4. Available at www.iucnredlist.org [accessed 29 November 2010].
Severino, R. B., Afonso-Dias, I., Delgardo, J., and Afonso-Dias, M. (2009). Aspects of the biology of the leaf-scale gulper shark Centrophorus squamosus (Bonnaterre, 1788) off Madeira archipelago. Arquipelago: Life and Marine Sciences 26, 57–61.
Stehmann, F. W. (2002). Proposal of a maturity stages scale for oviparous and viviparous cartilaginous fishes (Pisces, Chondrichthyes). Archiv fuer Fischerei und Meeresforschung 50, 23–48.
Stevens, J. D., Bonfil, R., Dulvy, N. K., and Walker, P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES Journal of Marine Science 57, 476–494.
| The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
Stobutzki, I., Patterson, H., Ward, P., Sampaklis, A., Sahlqvist, P., et al. (2010). 9. Commonwealth trawl and scalefish hook sectors. In ‘Fishery Status Reports 2009: Status of Fish Stocks and Fisheries Managed by the Australian Government’. (Eds D. T. Wilson, R. Curtotti and G. A. Begg.) pp. 254–268. Australian Bureau of Agricultural and Resource Economics – Bureau of Rural Sciences, Canberra.
Walker, T. I. (1998). Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Marine and Freshwater Research 49, 553–572.
| Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries.Crossref | GoogleScholarGoogle Scholar |
Walker, T. I. (2005). Reproduction in fishery science. In ‘Reproductive Biology and Phylogeny of Chondrichthyans: Sharks, Rays and Chimaeras’. (Ed. W. C. Hamlett.) pp. 88–127. (Science Publishers: Enfield, NH.)
Watson, G., and Smale, M. J. (1998). Reproductive biology of the shortnose spiny dogfish, Squalus megalops, from the Agulhas Bank, South Africa. Marine and Freshwater Research 49, 695–703.
| Reproductive biology of the shortnose spiny dogfish, Squalus megalops, from the Agulhas Bank, South Africa.Crossref | GoogleScholarGoogle Scholar |
Wetherbee, B. M. (1996). Distribution and reproduction of the southern lantern shark from New Zealand. Journal of Fish Biology 49, 1186–1196.
| Distribution and reproduction of the southern lantern shark from New Zealand.Crossref | GoogleScholarGoogle Scholar |
White, W. T. (2003). Centrophorus squamosus. In ‘IUCN 2010’. IUCN Red List of Threatened Species. Version 2010.4. Available at www.iucnredlist.org [accessed 29 November 2010].
White, W.T., and Dharmadi, (2010). Aspects of maturation and reproduction in hexanchiform and squaliform sharks. Journal of Fish Biology 76, 1362–1378.
| Aspects of maturation and reproduction in hexanchiform and squaliform sharks.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvpsFSnsw%3D%3D&md5=16f962740090f18f4a8340b4b087b091CAS | 20537019PubMed |
White, W. T., Ebert, D. A., and Compagno, L. J. V. (2008). Description of two new species of gulper sharks, genus Centrophorus (Chondrichthyes: Squaliformes: Centrophoridae) from Australia. In ‘Descriptions of New Australian Chondrichthyans’. (Eds P. R. Last, W. T. White and J. J. Pogonoski.) pp. 1–21. (CSIRO Marine and Atmospheric Research: Hobart.)
Wilson, C. D., and Seki, M. P. (1994). Biology and population characteristics of Squalus mitsukurii from a seamount in the central North Pacific Ocean. Fishery Bulletin 92, 851–864.
Wilson, D. T., Patterson, H. M., Summerson, R., and Hobsbawn, P. I. (2009). Information to support management options for upper-slope gulper sharks (including Harrisson’s dogfish and southern dogfish). Final Report to the Fisheries Research and Development Corporation Project No. 2008/65. Bureau of Rural Sciences, Canberra.
Yano, K. (1995). Reproductive biology of the black dogfish, Centroscyllium fabricii, collected from waters off western Greenland. Journal of the Marine Biological Association of the United Kingdom 75, 285–310.
| Reproductive biology of the black dogfish, Centroscyllium fabricii, collected from waters off western Greenland.Crossref | GoogleScholarGoogle Scholar |
Yano, K., and Tanaka, S. (1984). Some biological aspects of the deepsea squaloid shark Centroscymnus from Suruga Bay, Japan. Bulletin of the Japanese Society of Scientific Fisheries 50, 249–256.
Yano, K., and Tanaka, S. (1988). Size at maturity, reproductive cycle, fecundity, and depth segregation of the deepsea squaloid sharks Centroscymnus owstoni and C. coelolepis in Suruga Bay, Japan. Bulletin of the Japanese Society of Scientific Fisheries 54, 167–174.