Distribution, habitat and biology of a rare and threatened eastern Australian endemic shark: Colclough’s shark, Brachaelurus colcloughi Ogilby, 1908
Peter M. Kyne A E , Leonard J. V. Compagno B , Joanna Stead C , Micha V. Jackson D and Michael B. Bennett CA Tropical Rivers and Coastal Knowledge, Charles Darwin University, Darwin, NT 0909, Australia.
B Shark Research Center, Iziko – South African Museum, P.O. Box 61, Cape Town 8000, South Africa.
C School of Biomedical Sciences, The University of Queensland, St Lucia, Qld 4072, Australia.
D North Australian Indigenous Land and Sea Management Alliance, Charles Darwin University, Darwin, NT 0909, Australia.
E Corresponding author. Email: peter.kyne@cdu.edu.au
Marine and Freshwater Research 62(6) 540-547 https://doi.org/10.1071/MF10160
Submitted: 18 June 2010 Accepted: 22 November 2010 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Despite increasing research effort and conservation focus on sharks, small species of little commercial value are often overlooked, although they make a considerable contribution to global diversity. The poorly known Colclough’s shark, Brachaelurus colcloughi, is naturally rare to uncommon and is encountered only irregularly. Collating all known records (n = 50), we review the species’ geographic and bathymetric distribution, habitat, reproductive biology and diet. All but four B. colcloughi records are from within a core distribution of <2° latitude on the Australian east coast. Bathymetric distribution is from less than 4 to 217 m depth, with all but three records from less than 100 m depth. The species shelters on rocky reefs during the day and is thought to forage nocturnally around reefs and adjacent substrates. B. colcloughi is viviparous, with litter sizes of 6–7. Mature males and females have been observed from 61.0- and 54.5-cm total length, respectively. Gravid females have been collected in austral winter months. Dietary analysis indicates a predominantly piscivorous diet. Our results are placed in the context of existing threats and future research and management directions, demonstrating that shark species with low abundances and restricted ranges, such as B. colcloughi, require a suite of management arrangements to ensure long-term population viability.
Additional keywords: bycatch, diet, Heteroscyllium, IUCN Red List, reproductive biology.
Introduction
Although there has been a general increase in the conservation and management focus on sharks (and their relatives the batoids, collectively constituting the Elasmobranchii) (e.g. Camhi et al. 1998; Stevens et al. 2000; Dulvy et al. 2008; Simpfendorfer and Kyne 2009), small, poorly known species are often overlooked. These species, particularly if they are rare, inconspicuous and have no or little commercial or recreational value, are often of lower priority for research and conservation planning. Colclough’s shark, Brachaelurus colcloughi Ogilby, 1908, also commonly referred to as the blue-grey carpetshark, is an example of such a species. It is also of interest in being one of the few elasmobranchs whose occurrence is centred primarily around a rapidly expanding urban area (south-east Queensland (SEQ), Australia, including the conurbations of the Gold Coast, Greater Brisbane and the Sunshine Coast) (Stimson and Taylor 1999). A large and increasing proportion of the world’s human population is concentrated within coastal regions, and heavily populated coastal zones, such as SEQ, can focus industrial, commercial and recreational activities (Suchanek 1994; Small and Nicholls 2003). The cumulative effects of multiple stresses exerted by increased anthropogenic activity place heavy pressure on the coastal and marine environment, and elasmobranchs occurring within nearshore environments may be susceptible to these impacts (Jennings et al. 2008; Knip et al. 2010). In light of these concentrated impacts, it is valuable to understand the basics of elasmobranch distribution, habitat and ecology to assist with their conservation and management.
Brachaelurus colcloughi is a small benthic elasmobranch with a maximum recorded size of 85-cm total length (TL) (Queensland Museum record) (Fig. 1). It is sometimes placed within the genus Heteroscyllium (i.e. Compagno 2001), and is one of only two species within the carpet shark (Orectolobiformes) family Brachaeluridae (blind sharks) (Compagno 2001). Both B. colcloughi and the blind shark Brachaelurus waddi (Bloch & Schneider, 1801) are endemic to the east coast of Australia in the western Pacific. Although B. waddi is common across its range from southern Queensland to southern New South Wales, B. colcloughi is a little-known species and is considered rare (Compagno et al. 2005); despite decades of survey work and ongoing fishing activities across and adjacent to, its known range, it is not encountered with any regularity. Prior to 2001, only ~20 records of this shark were known. Its apparent rarity and the occurrence of threatening processes across its limited geographic range prompted its listing as Vulnerable C2a(ii) on the IUCN Red List of Threatened Species (Compagno et al. 2005).

|
Despite the concern raised for the status of B. colcloughi by Compagno et al. (2005), there is in fact little supporting information to accurately evaluate its population status, trends, capture in fisheries or the effects of identified threatening processes on the species, and thus its management requirements. Uncertainty regarding the population status of B. colcloughi is related to a lack of systematic data collection on the species and its likely misidentification as the common and morphologically similar B. waddi or grey carpetshark Chiloscyllium punctatum Müller & Henle, 1838.
Over the last decade, further sampling of the shark fauna of SEQ (Taylor 2007; Kyne 2008; Stead 2010) has yielded additional records and specimens of B. colcloughi, which provide an opportunity to document additional information on the species. We review all museum collection data and literature records and combine these with data from specimens collected in the field during 2001–2007 to provide baseline information on the species’ geographic and bathymetric occurrence, habitat requirements, reproductive biology and diet, and to identify knowledge gaps and information required for effective management and conservation.
Materials and methods
The present study reviewed museum collections and the literature for records of B. colcloughi, and examined material collected from bycatch of commercial fisheries in SEQ. Museum records were obtained from the Australian Museum, Sydney (AMS), the Australian National Fish Collection, Hobart (CSIRO) and the Queensland Museum, Brisbane (QM). Museum records (n = 28), as well as fisheries observer records provided to the QM (J. Johnson, pers. comm.) (n = 2) provided data on the geographic and bathymetric occurrence and habitat of the species. Additional information on these attributes, together with data on reproductive biology and diet, were obtained from bycatch specimens (n = 19) (three bycatch specimens were accessioned into museum collections and three were released alive at sea; therefore, not all data were available from all bycatch specimens). Literature searches provided a further site record (i.e. Parker 1999). Geographic records were plotted using ArcGIS and analysed by regions defined as northern New South Wales (NSW) (south of the NSW/Queensland (Qld) border; 28°10′S), Moreton Bay, SEQ (central point: 27°15′S, 153°15′E; the area bounded to the east by Moreton, North Stradbroke and South Stradbroke Islands), SEQ excluding Moreton Bay (north of 28°10′S and south of 24°42′S), and the Capricorn region, Central Qld (north of 24°42′S).
Bycatch specimens were collected by trawl (n = 6) in the Qld East Coast Trawl Fishery (ECTF) or by gill-net (n = 11) and tunnel net (n = 2) in the Qld East Coast Inshore Fin Fish Fishery (ECIFFF). Trawl specimens were collected in September and October 2001 by commercial otter-board trawlers fitted with three 2-seam Florida Flyer nets (net body mesh size 50.8 mm, codend mesh size 44.5 mm, headrope length 12.8 m). Gill-net specimens were collected between May 2005 and May 2006 by monofilament bottom-set gill-net (length 700–800 m, drop 2 m, mesh size 152, 178 or 203 mm). Tunnel net specimens were collected in June 2007; most gear specifications are unavailable with the exception of the mesh size of the ‘tunnel’, which was 38 mm.
For bycatch specimens, total length (TL, the distance from the tip of the snout to the distant margin of the caudal fin) was measured on the ventral surface to ±1 mm. Maturity stages (immature or mature; adolescent individuals were not observed for either sex) were assessed for males and females. Males were classed as immature if they possessed short, uncalcified claspers, and the testes and the remainder of the reproductive tract were undeveloped, or mature if they had calcified and elongated claspers, the testes were developed and lobular, and the epididymides highly coiled. Females were classed as immature if they possessed undeveloped ovaries, undifferentiated oviducal glands and thin uteri, or mature if they possessed developed ovaries with yellow vitellogenic follicles, and fully developed oviducal glands and uteri; embryos were also sometimes present in mature females. Reproductive systems were removed, with seminal vesicles examined macroscopically in mature males for the presence of sperm, and ovaries in females examined for the presence of follicles, with follicle diameter measured to ±1 mm to determine maximum follicle diameter (MFD), and the uteri inspected for ovulated ova or embryos. When possible, embryos were sexed by external examination for the presence or absence of claspers. The TL of embryos was measured to ±0.1 mm and mass was measured to ±0.1 g. If present, the diameter of external yolk sacs was measured to ±0.1 mm. The literature was reviewed for a comparison of reproductive parameters between B. colcloughi and other viviparous orectoloboid sharks.
Bycatch specimens were examined for dietary analyses (n =13). For each specimen, the stomach was excised, opened and all stomach contents removed. Prey items were identified to the lowest possible taxonomic level and counted and weighed to ±0.1 g. Unidentified prey items were categorised as ‘unknown’ within their broad taxonomic grouping. The incidence of empty stomachs was recorded and expressed as a percentage of the total number of stomachs examined. To analyse dietary composition, the percentage frequency of occurrence (%Fo, percentage of stomachs containing a specific prey item or category), percentage numerical composition (%Nc, number of a specific prey item or category as a percentage of the total number of prey items found) and percentage mass composition (%Mc, mass of a specific prey item or category as a percentage of the total mass of prey ingested) were determined. These three indices were combined to calculate the index of relative importance (IRI), a standardised index of dietary composition (Pinkas et al. 1971). The IRI was calculated for the major prey categories as IRI = (Nc + Mc)Fo and was expressed on a percentage basis (%IRI), allowing easier comparison of IRI values between prey categories (Cortés 1997). The average number of prey items per stomach containing prey was calculated.
Results
Distribution
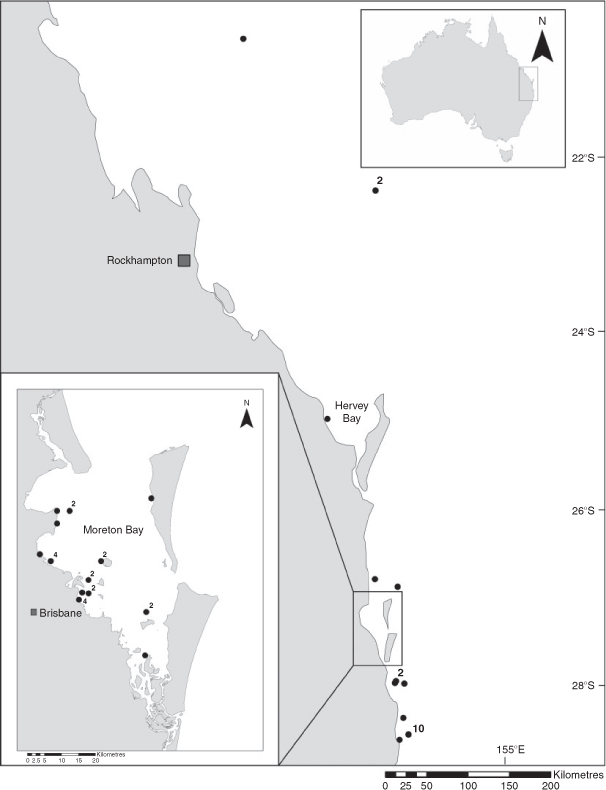
Brachaelurus colcloughi is endemic to the east coast of Australia, with confirmed records from Julian Rocks, off Byron Bay, northern NSW (28°36.8′S, 153°37.6′E) to the Hard Line Reefs off the Capricorn Coast of Central Qld (20°41′S, 151°21′E) (Fig. 2). Of 50 available records, 56% (n = 28) are from Moreton Bay, SEQ, 24% (n = 12) are from northern NSW, 14% (n = 7) are from SEQ outside of Moreton Bay, and 6% (n = 3) are from the Capricorn region of Qld Records range across ~8° of latitude, however, all but four are south of ~27°48′S, highlighting a core distribution of <2° latitude within SEQ and northern NSW. There are further reports of B. colcloughi from off North Stradbroke Island and the Gold Coast, SEQ, in a dive magazine (Marsh 2003), which have not been included in our analysis.
|
Habitat
Brachaelurus colcloughi has a recorded bathymetric range of <4–217 m depth; however, all but three records for which depth information was available (n = 38) were collected in water of depths ≤98 m. The deeper-water records (one individual at 217 m and two at 130–160 m) were trawled at the northern extent of the species' occurrence on the southern Great Barrier Reef system. All gill-net and tunnel-net bycatch specimens (n = 13) were taken in shallow inshore waters <4 m deep and museum records from Moreton Bay for which there are depth data available (n = 4) are from ≤10 m deep. Queensland trawl bycatch specimens (n = 8) were taken on the continental shelf at 26–160 m depth, whereas museum records from NSW (n = 11) were trawled at depths of 54–71 m. Parker (1999) reported the species from depths of 10–22 m at Julian Rocks in northern NSW.
All gill-net bycatch specimens were collected on seagrass (eel grass Zostera capricorni) beds with a mud–sand–shell substrate, and nearly always where there was a ledge or drop-off nearby. All were collected during the night. Both tunnel net bycatch specimens were collected adjacent to coral reefs. Specific details of habitat are available for three QM records: (1) a 516-mm-TL female collected at night from coarse silty sand and shell substrate, ~40 m away from a submerged rocky reef with numerous overhangs and small caves; (2) a 700-mm-TL female collected during the day from under a coral ledge where it was sheltering at the back of a narrow cave under Acropora spp. coral with a coarse sand substrate; and (3) a 755-mm-TL female collected during the day from deep under a wreck where it was sheltering between the wreck hull and fine sand substrate (J. Johnson, pers. comm.).
Reproductive biology
Males at 475–555-mm TL were immature (n = 4) and males at 613–731-mm TL were mature (n = 5). Sperm was present in the seminal vesicles of all mature males; these individuals were collected in the austral winter months of June, July and August. Females at 398–474-mm TL were immature (n = 2) and at 545–685-mm TL were mature (n = 4). Brachaelurus colcloughi displays a lecithotrophic reproductive mode referred to as aplacental yolksac viviparity, where embryos rely on yolk from a single ovulated ovum for development. Two gravid females were recorded. One collected in early June (620-mm TL) was carrying seven embryos with a mean (±s.d.) size of 162.9 ± 6.7-mm TL (range 149.9–169.9-mm TL) and mass of 24.7 ± 2.8 g (see Accessory Publication). External yolk sacs were present, with a mean diameter of 18.9 ± 3.1 mm. Four embryos were in the right uterus (2♀, 2♂) and three in the left uterus (2♀, 1♂). The second gravid female collected in late August (653-mm TL) was carrying two embryos in the right uterus that were at sizes of 185.2-mm and 185.4-mm TL, and weighed 35.3 and 34.8 g, respectively (see Accessory Publication). External yolk sacs had been completely absorbed, suggesting that they were close to parturition. The right uterus was loose around the embryos, and the left uterus was expanded, suggesting that the female had recently given birth to additional pups (an alternative explanation is that additional pups were aborted upon capture). Two non-gravid adult females had large ovarian follicles of 36-mm diameter. One of these was collected in early June; a collection date is not available for the other.
Diet
Of 13 specimens examined for dietary analyses, six stomachs (46.2%) were empty. The limited dietary analysis of the remaining seven specimens indicated that sampled B. colcloughi were predominantly piscivorous, with teleost fishes comprising the majority (85.7% Fo; 93.6% Nc; 99.8% Mc; 99.4% IRI) of prey ingested (Table 1). Cephalopoda constituted the remaining prey (0.6% IRI). The average number of prey items per stomach was 2.3.
Discussion
Distribution, habitat and population status
Last and Stevens (2009) reported B. colcloughi from off the tip of Cape York Peninsula and in the area south of Princes Charlotte Bay in northern Qld However, there are no specimens to support the species occurring off northern Qld (Pogonoski et al. 2002), and these records may be erroneous (possibly based on misidentifications of the similar C. punctatum). These records are therefore not included in the description of distribution presented here, although future surveys in those areas should be mindful of the possible occurrence of the species. The distribution map presented in Compagno (2001) also indicates that the species is more wide-ranging across the entire east coast of Qld Again, there are no records to support such a range, and B. colcloughi, as presently known, should be considered to be restricted to the waters of central to southern Qld and northern NSW.
Details of habitat occupancy available for individual records and information accompanying bycatch specimens indicate that the species shelters on rocky reefs during the day and is thought to forage nocturnally around reefs and over adjacent seagrass beds and soft substrates. Carraro and Gladstone (2006) showed that ornate wobbegong Orectolobus ornatus (De Vis, 1883) selected topographically complex daytime refugia that were characterised by a high volume of crevices, with individuals showing site fidelity to specific resting positions. It is unknown if B. colcloughi displays similar fidelity.
The threatened species listing of B. colcloughi by the IUCN is based partially on the species’ suspected low population size (estimated to be <10 000 mature individuals based on the IUCN Red List Category and Criteria applied by Compagno et al. 2005). However, there are no robust population assessments to test the validity of this assertion. The species has hitherto been considered very rare, with only ~20 records known before 2001 (Compagno et al. 2005). Records compiled here suggest that it may not be as rare as previously thought, but despite decades of survey work and ongoing fishing activities across its range, as well as areas adjacent to its known range, and across its entire depth range and beyond, it is not encountered with any regularity. The low number of specimens sampled during recent intensive survey work and fishery-dependent sampling, with a focus on Moreton Bay and SEQ, and including considerable coverage of available habitat (Taylor 2007; Kyne 2008; Stead 2010), support the rare status of this shark. In surveys of the bycatch of the Qld ECTF between 26°42′S and 27°59′S in depths of 19–86 m, B. colcloughi had a catch rate of 0.005 ± 0.003 ha–1 (the overall catch rate of chondrichthyan fishes was 0.958 ± 0.175 ha–1) (Kyne 2008). Anecdotal evidence from commercial fishers suggests that the species was trawled more regularly (albeit in small numbers) in the 1970s when a large fleet of small trawlers operated in Moreton Bay’s inshore waters (the number of small trawlers operating in the area has since decreased substantially) (J. Johnson, pers. comm.). It is, however, impossible to determine actual historic catch rates.
Johnson (1999) reported B. colcloughi as being ‘common’ inside Moreton Bay, but ‘rare’ outside the Moreton Bay region. The ‘common’ status afforded the species is based on Johnson’s (1999) relative abundance indicator of 10–100 records from Moreton Bay. The QM holds the most specimens of the species of any museum collection (n = 16), with the majority of these (n = 14) from within Moreton Bay. Parker (1999) reported the species as ‘occasional’ in a survey of Julian Rocks and adjacent waters in northern NSW (of four survey sites, the species was recorded only at Julian Rocks). There are no quantitative data of the species’ occurrence in Parker (1999), although ‘occasional’ is defined as ‘recorded at some habitats although uncommon at others’.
The records presented here do not represent all interactions that have occurred with the species, only those available in museum collections, recent bycatch sampling and the scientific literature. The overall extent to which the species is encountered by divers and fishers is unknown, although encounters are likely to be irregular. However, its documented geographic and bathymetric range is not explained by limited sampling; its known distribution, as well as areas to the north and south, is well-surveyed and consistently sampled.
Reproductive biology and diet
Previous suggestions of maturity in females at ~650-mm TL (i.e. Last and Stevens 2009) need to be readjusted, as mature females were observed from 545-mm TL in the present study. Compagno (2001) reports adolescent males at sizes to 516-mm TL, whereas we observed immature males to 555-mm TL, but with no mature males observed at <613-mm TL, a more accurate estimate of male size at maturity cannot be made. Although there are records in the literature of fecundity for B. colcloughi of 6–8 pups (Compagno et al. 2005; Last and Stevens 2009), there are only two previously examined gravid females, both held in the QM. These two gravid females were 658-mm and 755-mm TL, with litter sizes of 6 and 7 pups, respectively (also documented in Compagno 2001). Of the two gravid females examined from bycatch specimens, it is difficult to speculate on the litter size of one that possessed only two near-term embryos, and a uterine condition suggesting recent birthing (or possible abortion of pups). External yolk sacs were absent in these embryos (185-mm TL), which were observed in August, but were still attached to embryos (averaging 163-mm TL) recorded in June. Similarly, Compagno (2001) reported external yolk sacs attached to embryos at 164–168-mm TL, but not on embryos at 174–186-mm TL. Combining these observations, a size at birth of ~170–190-mm TL is likely. The observation of gravid females in austral winter months and near-term embryos in late August suggests a winter parturition period. However, the extent of the reproductive season and length of gestation remain unknown for B. colcloughi.
Of the order Orectolobiformes, the families Brachaeluridae (blind sharks), Orectolobidae (wobbegongs), Rhincodontidae (whale shark) and Ginglymostomatidae (nurse sharks) are viviparous; all of these show aplacental yolksac viviparity, although the tawny nurse shark, Nebrius ferrugineus (Lesson, 1831), is also reported to be viviparous with oophagous embryos (Compagno 2001). The remaining families in the order, Parascylliidae (collared carpetsharks), Hemiscylliidae (longtailed carpetsharks) and Stegostomatidae (zebra shark), are oviparous (Compagno 2001). Table 2 provides a summary of reproductive parameters documented for viviparous orectoloboid sharks (except N. ferrugineus). Brachaelurids have some of the smallest documented litter sizes of viviparous species in the order, but as their reproductive periodicity is unknown, their annual reproductive output is also unknown. If they have an annual reproductive cycle, then their fecundity (up to eight pups per year) may be similar to that of some Orectolobus spp., which have larger litter sizes, but a triennial reproductive cycle (see Huveneers et al. 2007b). The large follicles (MFD 36 mm) observed in two non-gravid female B. colcloughi suggests a lengthy ovarian cycle. Smaller follicle sizes of <30 mm are associated with annual ovarian cycles, whereas sizes reaching >40 mm suggest biennial or triennial ovarian cycles (Huveneers et al. 2007b; Walker 2007). For B. colcloughi, observed follicle sizes may point to a biennial reproductive cycle for the species. Elucidating the reproductive cycle as either annual or biennial will have considerable consequences for our understanding of the species’ productivity. Doing so, however, is difficult for such a rare and irregularly encountered species.

|
Compagno (2001) presumed that this shark fed on benthic invertebrates, but noted that the diet had not been recorded. Dietary analyses presented here, the first for the species, suggests a piscivorous diet, although the importance of invertebrate prey cannot be ruled out with the small sample size of stomachs examined. Most of the teleost fish genera and species identified in the stomach contents of B. colcloughi were benthic or benthopelagic, supporting a demersal feeding strategy for the species. Although not described quantitatively, the diet of B. waddi is reported to be a variety of invertebrates as well as small fishes (Whitley 1940; Compagno 2001). Of the limited number of quantitative dietary studies on carpetsharks, the small reef-associated epaulette shark Hemiscyllium ocellatum (Bonnaterre, 1788) has been shown to feed predominantly on benthic invertebrates (Heupel and Bennett 1998), C. punctatum is an opportunistic predator feeding on both benthic invertebrates and teleost fishes (Stead 2010), whereas wobbegongs Orectolobus spp. are largely piscivorous (Huveneers et al. 2007a). The nurse shark Ginglymostoma cirratum (Bonnaterre, 1788) is also primarily piscivorous (Castro 2000). Where several species of carpetsharks co-exist in the waters of eastern Australia (in particular northern NSW, where B. waddi, Orectolobus spp. and C. punctatum are common and B. colcloughi also occurs), resource partitioning may occur with different prey groups or prey sizes being selected by the different species, facilitating their co-existence on rocky reef environments.
Threats
The area of occupancy of B. colcloughi faces anthropogenic pressures in the form of fisheries, both commercial and recreational, and the alteration and loss of habitat – its rarity, limited reproductive capacity (as evidenced by its small litter size) and restricted distribution make it inherently vulnerable to population depletion (Compagno 2001). A considerable proportion of its range is subject to trawling effort by the Qld ECTF, both by beam-trawl vessels operating in shallow nearshore waters (rivers and estuaries) and by larger otter-trawl vessels operating in Moreton Bay and on the continental shelf (QDPI&F 2009). Inshore areas are also fished by the ECIFFF, and SEQ is a recreational fishing hotspot, with Moreton Bay alone accounting for one-third of the recreational fishing effort in Qld (Williams 2002). The species is also apparently exploited at low levels for the marine aquarium trade (Pogonoski et al. 2002; Compagno et al. 2005), but specific details are not available.
The compulsory use of turtle exclusion devices (TEDs) by otter-trawlers in the ECTF may reduce the bycatch of larger individuals, but may not be effective at excluding smaller sharks (Kyne 2008). However, a female B. colcloughi of 67-cm TL was captured in a trawl net fitted with a TED during a bycatch reduction survey, highlighting that even relatively large individuals may not be excluded (Kyne 2008). This is likely a result of the size of space between the bars of the TEDs; in Qld, bar spacing cannot be greater than 120 mm. However, to deal with periodical aggregations of jellyfish (Catostylus mosaicus), otter-trawl operators in Moreton Bay sometimes utilise a second grid with the standard bar spacing of 120 mm offset by bars spaced at 60 mm (A. Courtney, pers. comm.). The resultant 60-mm bar spacing is likely to be more effective at excluding smaller shark bycatch, although specific data are lacking. Beam-trawlers, which are restricted to rivers and estuaries, are not required to use TEDs, but will do so on occasion to exclude jellyfish and large debris, particularly after flooding (A. Courtney, pers. comm.).
Although inner coastal reefs have been identified as critical habitat for the species (Pogonoski et al. 2002), it would appear that inshore seagrass beds are also critical habitat for foraging, as suggested by the collection of net bycatch specimens in this habitat. The apparent behaviour of B. colcloughi of taking refuge in caves and under ledges on rocky reefs during the day, and emerging nocturnally to forage around reefs and over adjacent seagrass beds and soft substrates, exposes it to fishing activities that operate at night, including trawling. The continued loss or alteration of important inshore habitats will further threaten to reduce the already small area of occupancy of this shark (Pogonoski et al. 2002; Compagno et al. 2005). For example, current pressures on Moreton Bay, a core area of the species’ distribution, include large-scale developments that impact on nearshore marine habitats (e.g. the Port of Brisbane and Brisbane Airport land ‘reclamation’ projects), runoff from terrestrial sources, and pollution from marine and terrestrial sources, as well as the aforementioned fishing activities. These impacts combine to threaten the species’ main population, but at present there is insufficient information to determine the extent of impact from threatening processes.
Future research and management directions
The lack of basic ecological knowledge of rare, inconspicuous marine species can hinder effective fisheries management and conservation planning. Rare elasmobranchs with a restricted range and limited productivity may require precautionary management to counter the lack of detailed data. Marine Protected Areas, fisheries management, public awareness and education, and research effort all have a role to play in conserving these rare species. For B. colcloughi, an immediate research objective that could direct conservation planning towards adequate habitat protection is to accurately examine the species’ habitat use (including site fidelity and home ranges) and movement patterns (both short and long-term). Essential to implementing management strategies is knowledge of how widely individuals forage at night away from critical daytime resting habitat, and how these movements may overlap with fishing activities. An investigation of the species’ use of existing Marine Protected Areas (e.g. protected zones within the Moreton Bay Marine Park), to determine if these adequately protect critical habitat, is also warranted.
Limiting mortality from recreational and commercial fishing is an obvious beneficial conservation goal for rare marine species. In the specific case of B. colcloughi, it is recommended that the species be designated a ‘no-take’ species under Qld and NSW fisheries legislation. Additionally, the incorporation of B. colcloughi into commercial fishing logbooks would assist in obtaining information on occurrence and interactions with commercial fisheries, and educating fishers on correct identification and safe handling and release would assist in recording that information and potentially increase post-release survival. Together, these management measures could have considerable benefits for the long-term population viability of this rare species, and may serve as a case study to securing a rare, poorly known, threatened shark, that may in future be applied to other species.
Acknowledgements
Thanks to Jeff Johnson (QM) for curatorial assistance and for providing information on habitat, to Mark McGrouther (AMS) and Alastair Graham (CSIRO) for curatorial assistance, to Charlie Huveneers for assistance with the literature and to John Pogonoski for providing an image of the species. For the collection of material from the ECTF, we thank Anthony Courtney, Matthew Campbell, Shane Gaddes, Peter Gaddes, Phil Pearson and Tom Robertson, and from Moreton Bay, John Page and Steve Taylor. Specimens were collected under Queensland Fisheries Service General Fisheries Permits PRM02360D, PRM02030C and PRM03951I. Collection of material from the ECTF was part of a broader Fisheries Research and Development Corporation project (FRDC 2000/170). Finally, we thank Charlie Huveneers, John Pogonoski, Andrew Boulton and the Guest Editors of this Special Issue for their comments on the manuscript.
References
Camhi, M., Fowler, S., Musick, J., Bräutigam, A., and Fordham, S. (1998). Sharks and their relatives – ecology and conservation. Occasional Paper of the IUCN Species Survival Commission No. 20, Gland, Switzerland. Available at http://www.flmnh.ufl.edu/fish/organizations/ssg/shark2.pdf [Verified 14 March 2011].Carraro, R., and Gladstone, W. (2006). Habitat preferences and site fidelity of the ornate wobbegong shark (Orectolobus ornatus) on rocky reefs of New South Wales. Pacific Science 60, 207–223.
| Habitat preferences and site fidelity of the ornate wobbegong shark (Orectolobus ornatus) on rocky reefs of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Castro, J. I. (2000). The biology of the nurse shark, Ginglymostoma cirratum, off the Florida east coast and the Bahama Islands. Environmental Biology of Fishes 58, 1–22.
| The biology of the nurse shark, Ginglymostoma cirratum, off the Florida east coast and the Bahama Islands.Crossref | GoogleScholarGoogle Scholar |
Chidlow, J. (2003). The biology of wobbegong sharks (Family Orectolobidae) from south-western Australian waters. Masters Thesis, James Cook University, Townsville.
Compagno, L. J. V. (2001). ‘Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date. Volume 2. Bullhead, Mackerel and Carpet Sharks (Heterodontiformes, Lamniformes and Orectolobiformes).’ FAO Species Catalogue for Fishery Purposes. No. 1, Vol. 2. (FAO: Rome.)
Compagno, L. J. V., Last, P., and Stevens, J. (2005). Heteroscyllium colcloughi. In IUCN 2010. ‘IUCN Red List of Threatened Species (Version 2010.4).’ Available at http://www.iucnredlist.org [Verified 14 March 2011].
Cortés, E. (1997). A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Sciences 54, 726–738.
| A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes.Crossref | GoogleScholarGoogle Scholar |
Dulvy, N. K., Baum, J. K., Clarke, S., Compagno, L. J. V., Cortés, E., et al. (2008). You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquatic Conservation: Marine and Freshwater Ecosystems 18, 459–482.
| You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays.Crossref | GoogleScholarGoogle Scholar |
Heupel, M. R., and Bennett, M. B. (1998). Observations on the diet and feeding habits of the epaulette shark, Hemiscyllium ocellatum (Bonnaterre), on Heron Island Reef, Great Barrier Reef, Australia. Marine and Freshwater Research 49, 753–756.
| Observations on the diet and feeding habits of the epaulette shark, Hemiscyllium ocellatum (Bonnaterre), on Heron Island Reef, Great Barrier Reef, Australia.Crossref | GoogleScholarGoogle Scholar |
Huveneers, C., Otway, N. M., Gibbs, S. E., and Harcourt, R. G. (2007). Quantitative diet assessment of wobbegong sharks (genus Orectolobus) in New South Wales, Australia. ICES Journal of Marine Science 64, 1272–1281..
Huveneers, C., Walker, T. I., Otway, N. M., and Harcourt, R. G. (2007). Reproductive synchrony of three sympatric species of wobbegong shark (genus Orectolobus) in New South Wales, Australia: reproductive parameter estimates necessary for population modelling. Marine and Freshwater Research 58, 765–777.
| Reproductive synchrony of three sympatric species of wobbegong shark (genus Orectolobus) in New South Wales, Australia: reproductive parameter estimates necessary for population modelling.Crossref | GoogleScholarGoogle Scholar |
Jennings, D. E., Gruber, S. H., Franks, B. R., Kessel, S. T., and Robertson, A. L. (2008). Effects of large-scale anthropogenic development on juvenile lemon shark (Negaprion brevirostris) populations of Bimini, Bahamas. Environmental Biology of Fishes 83, 369–377.
| Effects of large-scale anthropogenic development on juvenile lemon shark (Negaprion brevirostris) populations of Bimini, Bahamas.Crossref | GoogleScholarGoogle Scholar |
Johnson, J. W. (1999). Annotated checklist of the fishes of Moreton Bay, Queensland, Australia. Memoirs of the Queensland Museum 43, 709–762..
Joung, S.-J., Chen, C.-T., Clark, E., Uchida, S., and Huang, W. Y. P. (1996). The whale shark, Rhincodon typus, is a livebearer: 300 embryos found in one ‘megamamma’ supreme. Environmental Biology of Fishes 46, 219–223.
| The whale shark, Rhincodon typus, is a livebearer: 300 embryos found in one ‘megamamma’ supreme.Crossref | GoogleScholarGoogle Scholar |
Knip, D. M., Heupel, M. R., and Simpfendorfer, C. A. (2010). Sharks in nearshore environments: models, importance, and consequences. Marine Ecology Progress Series 402, 1–11.
| Sharks in nearshore environments: models, importance, and consequences.Crossref | GoogleScholarGoogle Scholar |
Kyne, P. M. (2008). Chondrichthyans and the Queensland East Coast Trawl Fishery: bycatch reduction, biology, conservation status and sustainability. Ph.D. Thesis, The University of Queensland, Brisbane.
Last, P. R., and Stevens, J. D. (2009). ‘Sharks and Rays of Australia’. 2nd edn. (CSIRO Publishing: Collingwood.)
Last, P. R., Pogonoski, J. J., and White, W. T. (2010). A new wobbegong shark, Orectolobus leptolineatus sp. nov. (Orectolobiformes : Orectolobidae), from the Western Central Pacific. In ‘Descriptions of New Sharks and Rays from Borneo’. (Eds P. R. Last, W. T. White and J. J. Pogonoski.) pp. 1–16. CSIRO Marine and Atmospheric Research Paper No. 032, Hobart. Available at http://www.cmar.csiro.au/docs/Descriptions-of-sharks-and-rays-from-Borneo-small.pdf [Verified 14 March 2011].
Marsh, N. (2003). The mysterious Colclough’s shark. Sportdiving Magazine 96, 28–30..
Parker, P. G. (1999). Fish assemblages at Julian Rocks and the adjacent waters of northern New South Wales, Australia. Australian Zoologist 31, 134–160..
Pinkas, L. M., Oliphant, S., and Iverson, I. L. K. (1971). Food habits of albacore, bluefin tuna and bonito in Californian waters. California Fish and Game 152, 1–105..
Pogonoski, J. J., Pollard, D. A., and Paxton, J. R. (2002). Conservation overview and action plan for Australian threatened and potentially threatened marine and estuarine fishes. Environment Australia, Canberra. Available at http://www.environment.gov.au/coasts/publications/marine-fish-action/pubs/marine-fish.pdf [Verified 14 March 2011].
QDPI&F (Queensland Department of Primary Industries and Fisheries) (2009). Annual status report 2008. East Coast Trawl Fishery. The State of Queensland, Department of Primary Industries and Fisheries, Brisbane.
Simpfendorfer, C. A., and Kyne, P. M. (2009). Limited potential to recover from overfishing raises concerns for deep-sea sharks, rays and chimaeras. Environmental Conservation 36, 97–103.
| Limited potential to recover from overfishing raises concerns for deep-sea sharks, rays and chimaeras.Crossref | GoogleScholarGoogle Scholar |
Small, C., and Nicholls, R. J. (2003). A global analysis of human settlement in coastal zones. Journal of Coastal Research 19, 584–599..
Stead, J. (2010). The biology and ecology of the brown-banded bamboo shark, Chiloscyllium punctatum and wobbegong sharks (Genus Orectolobus) in southeast Queensland, Australia. Ph.D. Thesis, The University of Queensland, Brisbane.
Stevens, J. D. (2007). Whale shark (Rhincodon typus) biology and ecology: a review of the primary literature. Fisheries Research 84, 4–9.
| Whale shark (Rhincodon typus) biology and ecology: a review of the primary literature.Crossref | GoogleScholarGoogle Scholar |
Stevens, J. D., Bonfil, R., Dulvy, N. K., and Walker, P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES Journal of Marine Science 57, 476–494.
| The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
Stimson, R. J., and Taylor, S. P. (1999). City profile: Brisbane. Cities 16, 285–295.
| City profile: Brisbane.Crossref | GoogleScholarGoogle Scholar |
Suchanek, T. H. (1994). Temperate coastal marine communities: biodiversity and threats. American Zoologist 34, 100–114..
Taylor, S. (2007). Population structure and resource partitioning among Carcharhiniform sharks in Moreton Bay, Southeast Queensland, Australia. Ph.D. Thesis, The University of Queensland, Brisbane.
Walker, T. I. (2007). Spatial and temporal variation in the reproductive biology of gummy shark Mustelus antarcticus (Chondrichthyes : Triakidae) harvested off southern Australia. Marine and Freshwater Research 58, 67–97.
| Spatial and temporal variation in the reproductive biology of gummy shark Mustelus antarcticus (Chondrichthyes : Triakidae) harvested off southern Australia.Crossref | GoogleScholarGoogle Scholar |
Whitley, G. P. (1940). ‘The Fishes of Australia. Part 1. The Sharks, Rays, Devil-fish and Other Primitive Fishes of Australia and New Zealand.’ (Royal Zoological Society of New South Wales: Sydney.)
Williams, L. E. (Ed.) (2002). Queensland’s fisheries resources: Current condition and recent trends 1988–2000. Queensland Department of Primary Industries, Brisbane.



