Detecting range shifts among Australian fishes in response to climate change
David J. Booth A D , Nick Bond B C and Peter Macreadie AA School of the Environment, University of Technology Sydney, Broadway, NSW 2007, Australia.
B School of Biological Sciences and eWater CRC, Monash University, Clayton, Vic. 3800, Australia.
C Present address: Australian Rivers Institute, Griffith University, Nathan, Qld 4111, Australia.
D Corresponding author. Email: David.Booth@uts.edu.au
Marine and Freshwater Research 62(9) 1027-1042 https://doi.org/10.1071/MF10270
Submitted: 30 October 2010 Accepted: 14 June 2011 Published: 21 September 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
One of the most obvious and expected impacts of climate change is a shift in the distributional range of organisms, which could have considerable ecological and economic consequences. Australian waters are hotspots for climate-induced environmental changes; here, we review these potential changes and their apparent and potential implications for freshwater, estuarine and marine fish. Our meta-analysis detected <300 papers globally on ‘fish’ and ‘range shifts’, with ~7% being from Australia. Of the Australian papers, only one study exhibited definitive evidence of climate-induced range shifts, with most studies focussing instead on future predictions. There was little consensus in the literature regarding the definition of ‘range’, largely because of populations having distributions that fluctuate regularly. For example, many marine populations have broad dispersal of offspring (causing vagrancy). Similarly, in freshwater and estuarine systems, regular environmental changes (e.g. seasonal, ENSO cycles – not related to climate change) cause expansion and contraction of populations, which confounds efforts to detect range ‘shifts’. We found that increases in water temperature, reduced freshwater flows and changes in ocean currents are likely to be the key drivers of climate-induced range shifts in Australian fishes. Although large-scale frequent and rigorous direct surveys of fishes across their entire distributional ranges, especially at range edges, will be essential to detect range shifts of fishes in response to climate change, we suggest careful co-opting of fisheries, museum and other regional databases as a potential, but imperfect alternative.
Additional keywords: catch databases, climate-change impacts, distributional patterns, distributional range, geographic limits, habitat loss, ocean acidification, range edge, sea-level rise.
Introduction
A central idea in the climate-change debate is that species distributions are generally shifted pole-ward as a result of climate change-induced temperature rises (e.g. Sorte et al. 2010). Across all aquatic systems – marine, estuarine and freshwater – a mix of high dispersal rates and potential vagrant populations, coupled with high levels of temporal environmental variability, present a challenging backdrop against which to clearly distinguish the effects of a changing climate. Climate-change drivers such as temperature and rainfall can operate directly to affect range or indirectly through effects on habitat or food resources of fishes (e.g. Morrongiello et al. 2011; Pratchett et al. 2011) or on their reproduction (Pankhurst and Munday 2011). Climate-change drivers can also interact with other factors such as overfishing, habitat removal, disease and pollution to reduce or spatially shift species. In the present review, we first develop definitions for fish ‘range’ and ‘range shift’, which have been poorly articulated.
Surprisingly few studies worldwide have attempted or successfully achieved tests of range shifts for fishes. Environmental drivers linked to climate change and fish distributions are briefly reviewed in the paper, to complement more extensive reviews in this Special Issue of Marine and Freshwater Research. We review the available evidence for range shifts among fishes generally, as well as more specifically within an Australian context. We also summarise the various approaches that have, or could be, used in the future to detect or predict such shifts. Finally, for the purpose of managing and adapting Australian fisheries resources to a changing climate, we recommend key approaches to detecting range shifts and their causes.
What is ‘range’ for marine, estuarine and freshwater fishes?
Defining key ecological phenomena is never a trivial exercise (e.g. competition, Tilman 1987) and defining distributional range, and range edge, is problematic for organisms in most natural systems. Despite the fundamental importance of understanding distribution and range edges of fishes for both ecological understanding and species management, especially in the light of climate change, there is no satisfactory definition of range (or range edge) for fishes in the literature. Simply defining distributional range has a history of conflict in the ecological literature. From early definitions such as from Elton (1924), which stated that range encompasses broad geographic limits inside which a species may be found more or less permanently distributed, it has been unclear how species can be captured in such a definition if their ranges fluctuate widely on a seasonal basis, or if their offspring disperse well beyond the core adult range (Andrewartha and Birch 1954). This poses a significant challenge in examining range shifts in fish across marine, estuarine and freshwater environments.
Marine reef fish species are characterised by a bipartite life cycle (Leis 2006), with larvae as the dispersive phase, sometimes moving thousands of kilometres after hatching. Some inland species will also move large distances during flood periods in particular (Unmack 2000). This makes range measurement especially difficult. Parmesan et al. (2005) and Sorte et al. (2010) defined ‘range shift’ as a change in the distribution of species boundaries from their previously recorded boundaries, which may include range contractions, range relocations and range extensions. In freshwater systems, range shifts can be heavily constrained by catchment boundaries. For many species that have highly dispersive larvae, distribution patterns are most appropriately described by using metapopulation models (Kritzer and Sale 2004), with adult populations connected by larval propagules. Therefore, the vagaries of larval dispersal as well as persistence in adult habitat are key ingredients to successful establishment. We here propose a layered model of species distribution that can be applied in marine, estuarine and freshwater systems (see Fig. 1), including the following:

|
Core adult range: distributional area over which breeding populations produce offspring that settle within the core area;
Non-viable breeding adult range: breeding populations within the core adult area that produce offspring that settle outside the core area;
Non-breeding adult range: non-breeding adult populations;
Recruitment periphery: consistent settlement of recruits that only occasionally mature into adults – storage effects not sufficient to form adult populations; and
Sporadic recruitment periphery: occasional settlement only.
An example of marine reef fish distribution (Fig. 1a) highlights the concept of core breeding range and layers extending to peripheral recruitment habitat. Which part of this constitutes range must be defined. For example, breeding range may be most important for species conservation, whereas full range, including recruitment periphery, may be required for disease- or parasite-transmission studies. Depth distributions may differ across latitudinal ranges (Kingsford and Carlson 2010; Malcolm et al. 2010) so surveying must incorporate this, and although usually quite sedentary and persistent, recruit densities may fluctuate widely within and among years (Booth et al. 2007).
In contrast, many estuarine and freshwater fish populations are not at spatial equilibrium; that is, their ranges and occupancy of suitable habitat expand and contract. These expansion and contraction events reflect the dynamic nature of each of these environments. Estuaries occupy relatively small, transitional zones between rivers and the sea, and are subjected to marine influences (e.g. waves, tides and saltwater incursion) and freshwater influences (e.g. freshwater runoff, which brings influxes of nutrients and sediments), all of which create physical barriers that influence dispersal, feeding, reproduction and other life-history parameters of estuarine fish. Further, these influences change over short (e.g. seasonal) and long (e.g. ENSO cycles) time scales, and at local (within estuaries) and regional (between estuaries) spatial scales. A key challenge for defining range in estuarine fish then becomes separating temporary vagrancy, which relates to tolerance, from actual range shifts, which requires long-term persistence (Davis and Shaw 2001). For this review, we define a fish as having an ‘estuarine-range’ if it spends the majority of its life cycle in a semi-enclosed body of water where freshwater and saltwater meet, although we note that there are other ‘estuarine-dependent’ fish species (e.g. diadromous fish species) that may show range shifts in response to climate change.
In freshwater systems, and arid-zone rivers in particular, inter-annual variation in rainfall and runoff generate highly dynamic patterns of habitat persistence, with periodic contractions to isolated refugia (Fig. 1b), resulting in highly variable patterns of occurrence through time (Unmack 2001; Arthington et al. 2005; Bunn et al. 2006). In such systems, detection of range shifts becomes difficult because vagrant and previously unsurveyed populations are likely to be encountered relatively often. Thus, even where, for example, a more southerly occurrence is recorded in a particular survey, without very extensive records it becomes difficult to demonstrate that a range ‘shift’ has occurred as distinct from the existing range being poorly defined.
Meta-analysis of worldwide climate-change range-shift studies in fishes
Surprisingly, very few fish studies worldwide adequately show range shifts, and fewer demonstrate indisputable links to climate change. Meta-analysis of papers drawn from the ISI database Web of Science (Fig. 2) showed several trends by using appropriate keywords. Worldwide, there are fewer than 300 studies on fish range linked to climate change. Most are marine, few are estuarine or freshwater, although numbers have increased rapidly in the past decade, partly fuelled by the climate-change debate. About 7% of these studies have been Australian. Only one-quarter of the studies involve extensive field sampling of ranges, and a further one-quarter incorporate some field data into predictive or descriptive models. Given the importance of evaluating range shifts to understanding the impacts of climate change on the environment and its economic value, this paucity of empirical data is worrying.
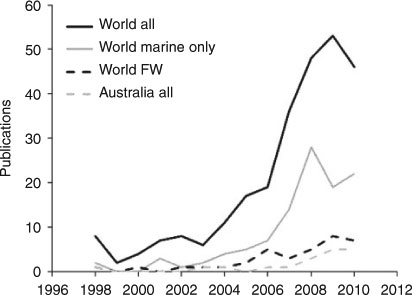
|
The best available evidence of climate-related range-shifts has made opportunistic use of rigorous historical data on fish catches, which are then compared with contemporary fish catches and linked with changing water temperatures. For example, Perry et al. (2005) used a commercial fishery dataset from the North Sea to show that two-thirds of exploited and non-exploited species have shifted in mean latitude or depth during 1977–2001. Similarly, Nye et al. (2009) analysed temporal trends in fish stocks in the north-eastern USA continental shelf during 1968–2007 and identified pole-ward shifts of many stocks in relation large-scale temperature increases and changes in ocean circulation.
Such examples from estuarine systems are rare; however, Fodrie et al. (2010) quantified changes in fish assemblages within seagrass meadows of estuaries (albeit a rather loose definition of estuary; Able 2005) within the Gulf of Mexico between the 1970s and 2006–2007, and identified a suite of tropical and subtropical species that were present in the latter sampling period, but not in the former, which they attributed to regional increases in air and sea-surface temperatures (>3°C) during the 30-year period.
In freshwater systems, there are several long-term studies linking patterns of climate variability to periodic shifts in demographic processes. For example, in a 30-year study of brown trout (Salmo trutta) populations in north-western England, Elliott et al. (1997) showed a clear impact of drought years on survivorship and recruitment, and many more studies demonstrated the influence of climatic variables in explaining historic distributional patterns (e.g. Minns and Moore 1995). Depending on the way in which a species range is characterised, there is also evidence that periodic droughts will extirpate local populations, particularly at the margins of a species’ distribution (e.g. Closs and Lake 1996). Fragmentation of populations during protracted droughts or wet–dry cycles may also last sufficiently long to promote genetic divergence (Douglas et al. 2003; Faulks et al. 2010a). Evidence for recent range shifts, however, is limited, although several studies implicated climate shifts in causing changes in species distributions. For example, Daufresne and Boët (2007) analysed trends in the distribution of fish in the Rhone River in France, and observed longitudinal changes in the distribution of thermophilic fish, namely chub (Leuciscus cephalus) and barbell (Barbus barbus), which began to replace dace (Leuciscus leuciscus) in more northerly upstream habitats. These shifts were correlated with changes in water and atmospheric temperatures over the same period, and appeared independent of changes linked to more localised aspects of river hydrology and thermal pollution. Similarly, Boughton et al. (2005) implicated climatic factors in the northward range shift of rainbow trout (Oncorhynchus mykiss) in California, although anthropogenic barriers to migration are seen as the primary driver of population declines.
Key climate-change impacts that can affect life history and range
Many of the environmental drivers that would be expected to be correlated with shifts in range or large-scale abundance of fish species are climate-change related, and each of these may impinge differently depending on life-history stage or habitat. These drivers, and their links to life-history events that shape range, are summarised in Table 1 and briefly described below. We note, however, that this discussion is by no means exhaustive and we direct readers to other papers in this Special Issue for a more detailed discussion of the direct and indirect pathways of climate-change impacts (e.g. Lough et al. 2011).
Water temperature
For ectotherms such as fishes, water temperature and its links to metabolism and swimming ability is a key environmental variable. For marine fish larvae, swimming speed and consequent dispersal are positively affected by temperature (Munday et al. 2009) but opposing this is the inverse relationship between water temperature and days to metamorphosis (e.g. O’Connor et al. 2007), by which time larval reef fish must have located suitable adult habitat. The sum of these temperature influences, along with ocean current speed, will determine the dispersal envelope. Pearce and Hutchins (2009) presented a 25-year dataset on recruitment of the tropical damselfish (Abudefduf vaigiensis) to Rottnest Island, south-eastern Western Australia, which correlates well with temporal patterns of sea-surface temperature (SST), implying a net dispersal increase with rise in SST.
For fishes advected well outside their adult ranges, an ‘overwintering bottleneck’ may occur where low winter water temperatures appear to have a severe modifying effect on densities (e.g. Figueira and Booth 2010). Projections of an average 2°C rise in winter water temperatures off the south-eastern Australian coast by 2080 suggest that overwintering may be a regular occurrence for a suite of coral reef fishes by then, with consequent range shifts likely. Differences in general temperature tolerances among fish species may also indicate shifts in range and assemblage structures under future climate change (Nilsson et al. 2009). In contrast, a suite of reef fishes restricted to the southern Great Barrier Reef has little scope for southern range shift because of unsuitable habitat, and may drastically contract in range (Munday et al. 2007). Species with shifting distributions in relation to changes in water temperature can have faster life cycles and smaller body sizes than non-shifting species (Perry et al. 2005).
For estuarine fish, temperature (along with salinity) is one of the key determinants influencing both their distribution (e.g. Harrison and Whitfield 2006) and abundance (e.g. Power and Attrill 2003), especially for those that live in shallow estuaries with irregular riverine flow or exchange with the sea (e.g. coastal lagoons). Temperature affects physiological processes in estuarine fish, including maturation, feeding and growth (Gill et al. 1996; Power and Attrill 2002; Power and Attrill 2007). There is also evidence that temperature indirectly determines the distribution of fish within estuaries through its influence on resource distribution (Attrill and Power 2002; Attrill and Power 2004), although links of temperature-driven range shifts in estuarine fish are lacking.
Thermal constraints are equally important in freshwater environments, and the range of most species is probably limited to some extent by either upper or lower temperature tolerances, although these can be extreme. For example, the upper and lower LD50 temperatures for spangled perch (Leiopotherapon unicolor), one of Australia’s most widespread fish species, span a range from 5°C to 39°C, and it is only in the very south that the lower temperature bounds have an impact on the species range (Llewellyn 1973). Together with species such as golden perch (Macquaria ambigua), rainbowfish (Melanotaenia fluviatilis) and some gudgeon species (e.g. Hypseleotris and Philypnodon), spangled perch may therefore expand southward under a warming climate, although such trends may be constrained by reduced streamflows, particularly in smaller rivers (Bond et al. 2011). In contrast, several cool-water native species, including river blackfish (Gadopsis marmoratus) and two-spined blackfish (G. bispinosus), will likely contract in range towards more southerly or higher altitude streams (e.g. Bond et al. 2011).
Effects of increasing temperature will also be exacerbated by reduced flow volumes, and in some lower-altitude catchments, a lack of suitable upstream refuges may lead to local extinctions. Thus, increasing temperatures may extirpate some introduced species such as brown trout (Salmo trutta) from some streams, potentially reducing their competitive and predatory impacts on small native fish such as Galaxias olidus, which (in some populations) has a higher thermal tolerance than do salmonids (e.g. see Closs and Lake 1996). The effects of temperature on growth rates may also have indirect impacts by altering reproductive output and competitive interactions (e.g. Morrongiello et al. 2011).
Freshwater flow
Estuarine fishes
Estuarine fish abundances are linked to annual fluctuations in freshwater discharge (Cyrus and Blaber 1992). Levels of freshwater runoff are determined by climate (e.g. rainfall), hydrological characteristics in a given area (e.g. dynamics of drainage channels) and anthropogenic activities (water consumption and runoff generation). Salinity is the main physical parameter that influences demographic processes in fish and is linked with freshwater input; however, freshwater flow also influences turbidity, pH and temperature. In contrast to northern hemisphere estuaries, Australian estuaries are characterised by irregular freshwater flow regimes (Roy et al. 2001). This has become particularly noticeable in south-eastern Australia, with recent drought conditions (which have been linked to climate change) causing declining abundances of estuarine fish (Gillson et al. 2009).
Freshwater input affects a range of life-history parameters of estuarine fish, including the timing of spawning (e.g. Newton 1996), the buoyancy of eggs and early stages (e.g. Mackenzie et al. 2007), shifts in the amount and location of suitable spawning grounds (Nicholson et al. 2008) and the movement patterns of fish (e.g. Childs et al. 2008). Freshwater inputs can also affect survival, particularly during major pulse events or low-rainfall periods, which can cause mass mortality of fish (Chuwen et al. 2007). There is good empirical data to support a link between freshwater inflows and estuarine fish production (e.g. Meynecke et al. 2006; Balston 2009), and thus long-term changes in inflows associated with climate change may increase production of some species. Most research on the effects of freshwater inputs has come from descriptive and small-scale experiments (Gillanders and Kingsford 2002); however, increasing use of acoustic tracking technology is allowing better coupling of patterns of fish movements with freshwater flow into estuaries (e.g. Hindell 2007; Hindell Jenkins et al. 2008).
Freshwater species
Spatial patterns of runoff are a major determinant of fish distributions in rivers and streams, especially river size (mean discharge), patterns of flow variability (from daily to interannual time-scales) and the presence or absence of particular flow events such as overbank floods and periods of cessation of flow (Poff and Allan 1995; Bunn and Arthington 2002). These various components of the flow regime strongly influence habitat availability and water quality, and can influence a range of life-history processes (Balcombe et al. 2011). Floods in particular are an important trigger for spawning in some species and deliver a pulse of energy to rivers that drives productivity in the channel. Several studies have demonstrated the importance of cease-to-flow events in excluding certain species from some rivers (e.g. Dodds et al. 2004; Bond et al. 2010, 2011). Such patterns are likely to arise from both direct habitat loss (drying) and the rapid changes in water quality (increasing temperatures and decreased dissolved oxygen) when flows cease (Boulton and Lake 1990). Researchers are now beginning to consider the impacts of climate change on ecologically important aspects of flow regimes, as well as runoff per se (Sanborn and Bledsoe 2006; Gibson et al. 2005), and incorporation of this information into species-distribution models highlights the importance of hydrology in predicting range shifts (e.g. Lyons et al. 2010; Bond et al. 2011).
Habitat loss
In coastal reefs, despite some climatic buffering, large-scale habitat changes have occurred and are expected to occur under climate change, largely as a result of water-temperature rises. For example, coral bleaching is now widespread, and can result in dramatic loss in reef fishes (Booth and Beretta 2002; Pratchett et al. 2008). So far, this has led to drastic local reductions in the range of some species, but not yet in a biogeographically significant way (i.e. widespread range shifts; see discussion in Munday et al. (2007) for coral reef fishes). Key temperate marine habitats are shifting in response to climate change-related SST shifts. Ling et al. (2009) demonstrated that urchin (mainly Centrostephanus rodgersii) barrens, a key coastal reef habitat in south-eastern Australia, are expanding pole-ward into south-eastern Tasmania, at the expense of kelp (e.g. Ecklonia sp., Phyllospora sp., Wernberg et al. 2011). Reef fish assemblages differ greatly among these habitats. In New South Wales (NSW), Gillanders and Kingsford (1998) found small blue groper (Achoerodus viridis) in greater numbers in kelp beds than in adjacent urchin barrens, although the species appeared to be flexible in its use of habitats on reefs, suggesting that populations would persist if urchin barrens expanded in an area. In north-eastern New Zealand, some species of reef fish have shown similar patterns, whereas others were more closely linked to kelp or urchin barrens (Anderson and Millar 2004). Parma microlepsis in NSW has been shown to depend on barrens habitat (Holbrook et al. 1994). Therefore, range shifts of habitat-specific species of fish can be expected (see Last et al. 2011).
Urbanisation of estuarine shorelines has caused widespread habitat loss worldwide, including Australia (Edgar et al. 2000). Climate change will exacerbate further losses through a variety of environmental drivers (e.g. increases in water temperature, sea level rise) discussed herein (Kennish 2002). Physical impacts associated with coastal development (e.g. installation and construction of docks, piers and boat ramps), and dredging, pollution and destructive fishing all negatively affect aquatic habitat. Loss of estuarine habitats has far-reaching ecological ramifications. For estuarine fish, the most obvious outcome is a loss of available habitat. This is especially consequential for fish that use estuarine habitats as a nursery ground during their juvenile stages of development – of which there are many Australian species (Gillanders et al. 2003; Meynecke et al. 2008). Estuarine fish may also be indirectly affected through changes in trophic cascades. For example, Edgar and Barrett (2000) showed that urbanisation of Tasmanian estuaries caused changes in sediment composition from sandy to muddy beds, and a concomitant shift in benthic community assemblages (which are an important food source for estuarine fish).
In many parts of Australia, existing hydrologic stress due to human water use is already high, and these impacts are likely to intensify under expected climate scenarios (CSIRO 2008; Pratchett et al. 2011). Together with land-use change and other anthropogenic impacts, hydrologic alteration has already led to substantial range contractions for many freshwater fish in Australia (Mallen-Cooper 1992). Modelling suggests that in areas with significant agricultural development, the pre-existing impacts of human water use on stream flows far exceed the magnitude of changes resulting from climate change alone (CSIRO 2008; Pratchett et al. 2011). Nonetheless, impacts from climate change will increase periods of cease to flow in low-rainfall areas, further decreasing habitat availability in many rivers during dry periods (Bond et al. 2010). Such shifts and associated impacts on fish populations have been well documented during recent drought in south-eastern Australia (Bond and Lake 2005). Other potential changes to habitat include, at a very fine scale, the loss of aquatic plants, which provide important structural habitat in rivers and wetlands (Balcombe et al. 2011), and at much larger scales, levels of connection among habitat patches containing local populations (Labbe and Fausch 2000). Thus, at larger spatial scales, changes in connectivity among local populations may increase overall extinction risk of local and regional populations, as has been previously predicted and documented for fragmented fish populations in desert streams (see Fagan 2002; Fagan et al. 2002).
Sea-level rise
Sea-level rise is likely to have strong indirect effects on fish production through its effects on key estuarine fish habitats, namely mangroves, seagrass and saltmarsh (Kennish 2002; Bond and Lake 2005). Theoretically, these habitats have the potential to respond to sea-level rise by slowly migrating up the shore (Haslett et al. 2001); however, their ability to do this depends on land being available for a landward progression, i.e. sediment accretion rates need to keep up with rising sea levels (Kennish 2001). In addition, there should not be physical barriers (e.g. bulkheads, rock walls) to block habitat migration up the shoreline.
In Australia, mangrove stands are increasing in area at the expense of saltmarsh in the south-east, and key saltmarsh habitats are shrinking (Saintilan and Williams 1999; Ellison 2005). Saltmarsh is a nursery for crab zoea (larvae) production that, in turn, acts as a key food source for estuarine fishes (Mazumder et al. 2006). Sea-level rise in Australia may also directly affect estuarine and marine fish during their pre- and post-settlement phases. For example, Jenkins et al. (1997) showed that spatial and temporal variability in recruitment of a temperate, seagrass-associated fish, King George whiting (Sillaginodes punctata), was largely determined by physical processes, including the residual sea level (caused by changes in barometric pressure). Other Australian commercial species that might be similarly affected include pink snapper (Pagurus auratus), southern sea garfish (Hyporhamphus melanochir), Australian herring (Arripis georgianus), whiting and mullet (Fletcher and Head 2006).
Ocean currents and wind patterns
Western (EAC) and Eastern (LC: Leeuwin) Boundary currents bracket Australia and are expected to alter under climate-change scenarios. The strength of the LC is influenced by ENSO-related thermocline anomalies, and may be weakening, whereas the EAC is projected to strengthen and drive further pole-ward into Tasmanian waters by late this century (Ridgway 2007). The LC and its interannual variability have profound impacts on marine ecosystems off the western and southern coasts of Australia. For example, high recruitment of the western rock lobster (Panulirus cygnus) fishery of Western Australia is influenced by a stronger LC and the associated warmer water temperatures (Caputi et al. 2009). The strengthening EAC will lead to higher SSTs in south-eastern Australian waters, but also advect tropical and subtropical fauna pole-ward. Especially for the EAC, strengthening and increasing southward extent is likely and have already occurred as a result of climate change. This, of course, interacts with water temperature (see above) and also increases potential for warmer-water species to move south through advantages to larval dispersal. Conversely, southern species may find it difficult to disperse north or even maintain range, possibly precipitating a shift south.
Changes in wind strength and direction are predicted under climate-change scenarios, which can affect mixing and circulation of water. In Port Phillip Bay (Victoria, Australia), post-larval abundances of King George whiting (Sillaginodes punctata) at seagrass sites are strongly correlated with zonal westerly winds, the main westerly wind belt over Tasmania, and are likely to be influenced by climate change (Jenkins et al. 1997; Jenkins 2005).
Acidification
Reductions in ocean pH, and the linked increase in CO2 concentration, have been shown to affect homing in coral reef fishes (Munday et al. 2010); however, the generalisation of these results to marine fishes and the implications for range shifts are unknown at present. Relative to ocean and freshwater systems, the effects of acidification on estuarine fishes have been largely ignored. However, recent data from the Puget Sound, a large estuary complex in the USA Pacific North-west, suggest that ocean acidification accounts for 24–49% of the pH decrease relative to pre-industrial values, and up to 49–82% of atmospheric CO2 increase (Feely et al. 2010). It is difficult to know whether estuarine fishes will respond in similar ways to freshwater and marine fishes, given that most of the literature has focussed on responses (e.g. reproduction, early development, growth and behaviour) of marine and freshwater fishes to prolonged CO2 exposure (Ishimatsu et al. 2008), whereas estuarine fishes are more likely to be exposed to pulses in CO2 exposure because of the highly dynamic nature of estuarine environments. Acidification of rivers in Australia will most likely arise only in localised areas as a result of decreasing river flows exposing acid sulfate soils (ASS) rather than from increased CO2 absorption. The extent and magnitude of such impacts are demonstrated by impacts from drought in parts of the Lower Murray River in Victoria and South Australia (Fitzpatrick et al. 2009), where reinundation of ASS led to rapid oxidation, resulting in low oxygen concentrations and low pH, thus causing severe fish kills (Lamontagne et al. 2004). Fish species such as the already threatened Murray hardyhead (Craterocephalus fluviatilis) that inhabit shallow floodplain wetlands along the lower Murray River, and other areas with ASS, are particularly at risk.
Approaches to range-shift detection in Australian fishes
Our review has uncovered little in the way of direct evidence for climate-induced range shifts in Australian fish species, but has considered a range of likely climate-induced changes in aquatic ecosystems that may affect species distributions in the future. Much anecdotal evidence of climate-driven local-species extinctions exists; however, the lack of scientifically rigorous monitoring is apparent. Nonetheless, there are numerous clearly identified mechanisms by which climate change is expected to induce range shifts across marine, estuarine and freshwater environments. The challenge will be in determining whether these do occur in the future. Here, we review the few reliable studies linking non-stationary climate drivers to range shifts, before discussing a range of indirect approaches to inferring range shifts or predicting likely range shifts in response to future climate change.
Direct field census v. recorded change in environmental variables
One of the rare examples of direct evidence of a relationship between faunal changes and climate-change drivers in Australia is that of Stuart-Smith et al. (2010) who used over 100 underwater surveys to compare assemblages on Tasmanian rocky reefs between the early 1990s and 2005. Long-term SST monitoring at Maria Island showed SST rises of up to 1°C over the course of the study, however, the authors concluded that reef communities had remained relatively stable over that time. Significantly, however, ranges of several key eastern marine fishes had shifted south. For instance, the southern-range edges of the weed whiting (Siphonognathus attenuatus) and luderick (Girella tricuspidate) shifted over two degrees of latitude off eastern Tasmania, and two previously absent eastern species, the crimson-band wrasse (Notolabrus gymnogenis) and the marblefish (Aplodactylus lophodon) were observed in 2005.
Probably the most compelling and comprehensive example of demonstrated climate-induced range shifts of fishes in Australia was recently reported in Last et al. (2011). This study monitored Tasmanian coastal fish distributions and included data since the late 1800s using multiple methods (e.g. spearfisher records, commercial-fisheries data, scientific surveys, REDMAP data) to quantify perceived changes in ichthyofauna. In total, 61 of 300 fish species off the coast of Tasmania appeared to have undergone significant shifts in range and/or abundance.
Some losses (e.g. 5 predatory fish species) were attributed to exploitation; however, 45 species (in 27 families) showed pole-ward range shifts, possibly likely to be related to climate change (SST increases, strengthening East Australian Current).
Existing databases
Commercial-catch databases
Given the generational nature of fishing fleets, it is likely that commercial fishers can provide anecdotal accounts of climate-related range shifts that may serve to trigger further investigation of changes in fish ranges. Of course, comparisons of past and present fish catches are susceptible to sampling artefacts, especially in commercial-fishing records, whereby fishing gears change through time, or where earlier records failed to report fish lacking commercial value (Byrkjedal et al. 2004). An example of the use and limitations of such datasets comes from the NSW commercial catch database housed and maintained at Industry and Investment NSW. This database is divided by fishery, such as estuarine haul, ocean line and trap, and by latitude. From 1940 to 1960, no effort data were recorded, whereas from 1961 to 1997, effort data were not reliable in most cases. For the whole database, catch was not only related to availability but also to market preferences, circumstances of fishers and changes in legislation. Also, privacy requirements prevent reporting of datasets from groups of under five fishers, which can in some cases severely restrict the spatial resolution (e.g. latitudinal precision) of the data.
Despite these issues, Gillson et al. (2009) were able to use the estuary database to explore how catches of key commercial fish species caught by gill-net varied with river flow. D. Booth (unpubl. data) has used the database, in consultation with Industry and Investment NSW specialists, to follow catch and CPUE changes across time in key species that may be expected to alter distribution with climate change along the NSW coast, a key global hotspot for climate-change increases in SST and ocean currents (Ridgway 2007). For example, Fig. 3a shows a systematic increase in overall CPUE of the northern mudcrab (Scylla serrata), which may be interpreted as a response to climate-change SST increases over that period, although other explanations such as change in capture efficiency are plausible.
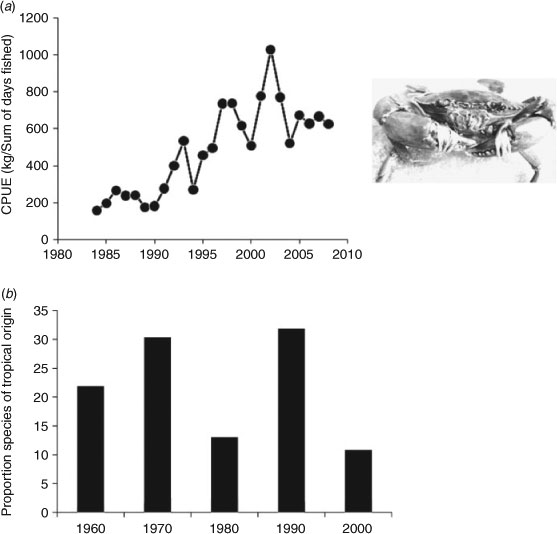
|
Recreational catch and ‘citizen-science’ databases
Where scientific databases are lacking, and provided data can be cross-referenced and calibrated, various recreational-fishing databases can be useful to determine species range shifts, which can then be related to climate drivers. First, records from fishing competitions, where catches are carefully recorded, including weights and identifications, can be valuable despite obvious biases (e.g. fish samples are biased towards larger individuals or desirable species). Such competitions are held regularly and in the same place, including spearfisher, game fishing and other marine competitions. Provided the researcher is allowed access and understands limitations, these can be useful (Steffe et al. 2005).
New online public websites (e.g. REDMAP, available at www.REDMAP.org.au, accessed 15 June 2010) coordinate widespread fish observations across a range of amateur fishing, diving and scientific observers, and have the potential to capture new appearances and range shifts of fishes in coastal marine habitats. ‘Reeflife’ surveys (Edgar et al. 2009) have combined rigorous experimental design and data manipulation and storage with amateur diver training to survey habitat and key fish and invertebrate species around southern (and more recently northern) Australia. The consistent methodology and frequent surveys will make it an excellent vehicle to detect range shifts over the coming years.
Museum and government collection databases
Museums sponsor collecting trips around the Australian coast and in freshwater habitats, and the archived specimens and data collected could be of value in mapping ranges and range shifts of fishes. On the plus side, the collections are often quantitative (e.g. rotenone stations), specimens are usually accurately identified, locations are often revisited over decades, and specimens are usually stored in collections that can be accessed for measurement later. Similarly, most government agencies maintain databases of fish survey records from government and university research programs. Historically, these have been an underutilised resource; however, increasingly they are being used as a source of data for examining species distribution patterns via more quantitative modelling approaches, incorporating GIS to link species records to spatial information layers on climate, runoff and other environmental variables (e.g. Growns 2008; Bond et al. 2011). Shortcomings in relying on these sources of data include the range of sampling approaches used, varied sampling objectives and variable collection intensity, all of which may render them inadequate for detection of subtle or early beginnings of range shifting. However, range shifts are largely going to be reflected in changes in the presence or absence of species, and government databases are mostly quite reliable in the way they capture this type of binary data. Nonetheless, in some cases even cursory examinations of these sorts of databases can reveal interesting trends. For example, latitudinal changes in the proportion of tropical species over decadal time scales, on the basis of data from Australian Museum rotenone collections stations from coastal NSW sites from the 1960s to the present (Fig. 3b), do not show expected steady increase in species of tropical origin.
Physiological models
Another indirect approach to monitoring range shifts is to overlay known physiological limits of the taxon in question on current and/or historical distribution maps and to extrapolate distribution changes on the basis of projections of how the environmental variable in question is predicted to change (e.g. cane-toad invasions; Shine 2010). Elith et al. (2010) and Cheung et al. (2009) used a dynamic bioclimate envelope model to project range shifts of over 1000 exploited fishes and invertebrates, using past data and future models of values for key ocean environment variables such as SST, sea ice cover, salinity and upwelling. That is, they coupled past distribution change with these environmental variables to project future changes.
Laboratory data on physiological tolerances to key environmental drivers can supplement and refine these models. For example, Hofmann (2005) used biochemical and molecular techniques to gain insight into the role of temperature in setting species distribution patterns in the marine environment, and Barnes et al. (2009) inferred range limits based on physiological tolerances in benthic Arctic marine invertebrates. However, these laboratory-based approaches should be used with care. For instance, vagrant damselfishes (Abudefduf vaigiensis) can survive under laboratory conditions down to at least 15°C (Figueira et al. 2009), whereas the same species disappears in the field below 17°C (D. Booth, unpubl. data; see Fig. 4). Predation pressure appears to account for losses of this species off Sydney below 17°C, at which temperature they have slowed escape responses (D. Booth, pers. comm.). The overall problem with this approach is that information on physiological tolerances and other relevant traits is lacking for many Australian species (e.g. see Crook et al. 2010 in relation to uncertainty around tolerances for freshwater fish).
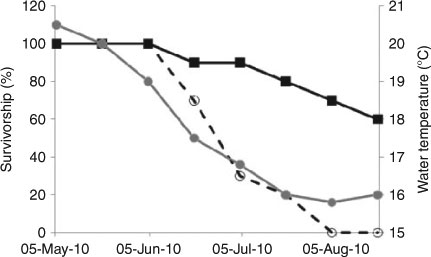
|
Limitations of physiological approaches to predicting range shifts include no recognition of the capabilities or constraints of larval dispersal and the effects of key resource requirements (habitat and food). For example, pole-ward shifts in distribution of fishes may partly depend on changes in local environmental conditions as a result of climate change (e.g. Lough 2008).
Other indirect approaches
Demographic models
Although correlative approaches have been widely used to infer potential range shifts in fish, changes in physiological costs and demographic processes such as survivorship or reproductive output may also lead to eventual population losses and hence range contractions (e.g. Van Winkle et al. 1997). Relatively data-intensive, demographic models may be more likely to reveal ecological surprises than are simple bioclimatic modelling approaches, because of their potential to capture dynamic feedbacks, which can give rise to strong non-linearities and identify thresholds of collapse in local populations (Perry and Bond 2009).
Paleoecology
Interrogating palaeoecological data to understand contemporary and future biotic responses to climate change offers an increasingly promising approach, especially when data records are extracted from lake and marine sediments because of their ability to log climate change and concomitant biological responses (Willis et al. 2010). The obvious advantage of paleoecological datasets is that they have the capacity to record ecological and evolutionary processes over time scales that far exceed most observational records. For example, Newbrey et al. (2009) used fossil fish material from the Cretaceous Dinosaur Park Formation of Alberta (Canada) to document the presence of members of the Characiformes (relatives of the piranha and neon tetras) in North America in the Late Cretaceous, a time of significantly warmer global temperatures than the present time. Fish otoliths, which can act as records of growth, chemical environment and life-history events are well preserved in sediments, aboriginal middens and other archival sources, and show great promise as paleogeological tools to detect climate change and range shifts. In addition to help understand the geographical range of fish, paleoecological data can be used to make inferences about range shifts over time (e.g. Read et al. 2006; Murray and Wilson 2009). This approach has not been successfully used for Australian fishes as yet (B. Gillanders, pers. comm.).
Genetics
The genetic structure of populations can provide information on the potential biogeographic history of organisms. In aquatic environments, range expansion and contraction events can be inferred by matching information on the phylogenetic separation of taxa with information on oceanic circulation and physical barriers that might limit dispersal (e.g. Barber et al. 2000; Dawson 2001; Ayre et al. 2009; Faulks et al. 2010b). Such approaches are now widespread; however, there is also an increasing move towards relying not only on the use of neutral markers to assess historical patterns of gene flow, but towards the detection of genes that are actively being selected for along environmental gradients. This is an area that offers considerable promise in terms of understanding variation in the adaptation potential of not only different taxa but also different populations distributed in different geographic areas (Hoffmann and Willi 2008).
Conclusions and recommendations
Whereas the resolution and availability of past and predicted data on climate-change environmental variables are rapidly increasing with advances in technology, (e.g. satellite SST and ocean-colour data), biological data such as fish distributions are much sparser and more field labour-intensive and have not kept pace. Matching the temporal and spatial resolution of the ‘independent’ (physical) and ‘response’ (biological) variables is problematic. We conclude that the present knowledge of the actual fish ranges in all three ecosystems, especially that of the non-commercial species, is poor. Key guidebooks (e.g. Gomon et al. 1994) and distribution websites (e.g. FishBase (www.fishbase.org) and Biomaps (www.biomaps.net.au)) provide information on regional distributions and range edges of marine species; however, it is unclear how accurate and recent all of these sources are in their field monitoring of range edge.
We recommend the following:
-
Targeting key regions and habitats for intensive monitoring. Climate-change hotspots, such as coastal waters off south-eastern and south-western Australia would be areas of most change for marine and estuarine species and should be the focus for studies. In freshwater systems, less-heavily regulated river basins may provide independent measures of climate-change impacts, although in many cases, climate impacts will be greatest in modified river systems (Palmer et al. 2008). Threatened habitats such as urbanised estuaries vulnerable to sea-level rise and vanishing kelp habitats (eastern and western coast) could also be targeted. Locations, such as the south-eastern Tasmanian coast (Last et al. 2011), should be targeted because they represent places that are most likely to show actual loss of species and where range shifts may be most obvious.
-
Targeting key species. Key commercial fish species may be prone to shifting away from the economic zones of the states or even national waters, so research on predicting these changes could be targeted. Range-shifters have some of the attributes of invasive species and stronger collaboration with biosecurity researchers would be useful.
-
Improving value and cross-referencing of databases. Although many non-specific databases have problems with data accuracy and geo-referencing, they clearly have much to offer in terms of examining large-scale and long-term trends in fish-occurrence patterns. It is important that such databases are adequately maintained and we recommend a national approach to calibrating and/or cross-referencing databases in the future. The newly formed National Climate Change Adaptation Research Facility (NCCARF) would be an excellent vehicle to investigate such dataset consolidation.
-
Supporting field monitoring of fish distributions and key climate drivers. Undisputedly, the best evidence for change in fish distributions would come from intensive spatially structured and frequent fish censuses. These are rarely carried out at temporal or spatial scales sufficient to resolve range shifts. The development of the Integrated Marine Observing System (IMOS) and linked inshore ocean-climate measurements will assist in monitoring of climate-change drivers at appropriate scales in marine systems. It is possible that some of the auditing programs in freshwater systems will have similar value in the long term. These potential benefits should be considered in evaluating the wider usefulness of such programs.
-
Linking understanding of climate-induced range shifts in fishes to management responses. For instance, in NSW, the State Government’s Monitoring, Evaluation and Reporting Program seeks to support ongoing range monitoring of key terrestrial, freshwater and marine habitats and to interface them with appropriate practical responses.
Given Australia’s position as a world ‘hotspot’ for climate change and our relatively advanced knowledge of key species, we have an exciting opportunity to lead worldwide in understanding climate change-induced range shifts of marine and freshwater fishes.
Acknowledgements
We thank the referees and Guest Editors for comments that greatly improved this manuscript. We also thank Mark McGrouther (Australian Museum Fish section) and Jim Craig and Dr Charles Gray (NSW Department of Industry and Investment) for generously supplying databases and associated advice. Nick Bond was supported by eWater Cooperative Research Centre. PM was supported by a Paddy Pallin Marine Science Grant and a Brian Robinson Fellowship. This is Contribution 44 of Sydney Institute of Marine Sciences. This paper arose from the Australian Society for Fish Biology 2010 symposium ‘Climate change and the aquatic environment: the future for fish and fisheries’ presented at the meeting sponsored by the Museum of Victoria, the Department of Sustainability and Environment, Victoria, and the Fisheries Research and Development Corporation.
References
Able, K. W. (2005). A re-examination of fish estuarine dependence: evidence for connectivity between estuarine and ocean habitats. Estuarine, Coastal and Shelf Science 64, 5–17.| A re-examination of fish estuarine dependence: evidence for connectivity between estuarine and ocean habitats.Crossref | GoogleScholarGoogle Scholar |
Anderson, M. J., and Millar, R. B. (2004). Spatial variation and effects of habitat on temperate reef fish assemblages in northeastern New Zealand. Journal of Experimental Marine Biology and Ecology 305, 191–221.
| Spatial variation and effects of habitat on temperate reef fish assemblages in northeastern New Zealand.Crossref | GoogleScholarGoogle Scholar |
Andrewartha, H. G., and Birch, L. C. (1954). ‘The Distribution and Abundance of Animals.’ (University of Chicago Press: Chicago, IL.)
Arthington, A. H., Balcombe, S. R., Wilson, G. A., Thoms, M. C., and Marshall, J. (2005). Spatial and temporal variation in fish-assemblage structure in isolated waterholes during the 2001 dry season of an arid-zone floodplain river, Cooper Creek, Australia. Marine and Freshwater Research 56, 25–35.
| Spatial and temporal variation in fish-assemblage structure in isolated waterholes during the 2001 dry season of an arid-zone floodplain river, Cooper Creek, Australia.Crossref | GoogleScholarGoogle Scholar |
Attrill, M. J., and Power, M. (2002). Climatic influence on a marine fish assemblage. Nature 417, 275–278.
| Climatic influence on a marine fish assemblage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xjs1agt70%3D&md5=26ca080dfd1f942076eadf80fd5bad97CAS |
Attrill, M. J., and Power, M. (2004). Partitioning of temperature resources amongst an estuarine fish assemblage. Estuarine, Coastal and Shelf Science 61, 725–738.
| Partitioning of temperature resources amongst an estuarine fish assemblage.Crossref | GoogleScholarGoogle Scholar |
Ayre, D. J., Minchinton, T. E., and Perrin, C. (2009). Does life history predict past and current connectivity for rocky intertidal invertebrates across a marine biogeographic barrier? Molecular Ecology 18, 1887–1903.
| Does life history predict past and current connectivity for rocky intertidal invertebrates across a marine biogeographic barrier?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmsVekt7s%3D&md5=d8b3e500dca36e114e6f0ba6485ad795CAS |
Balcombe, S. R., Sheldon, F., Capon, S. J., Bond, N. R., Hadwen, W. L., Marsh, N., and Bernays, S. J. (2011). Climate-change threats to native fish in degraded rivers and floodplains of the Murray–Darling Basin, Australia. Marine and Freshwater Research 62, 1099–1114.
| Climate-change threats to native fish in degraded rivers and floodplains of the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Balston, J. (2009). An analysis of the impacts of long-term climate variability on the commercial barramundi (Lates calcarifer) fishery of north-east Queensland, Australia. Fisheries Research 99, 83–89.
| An analysis of the impacts of long-term climate variability on the commercial barramundi (Lates calcarifer) fishery of north-east Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Barber, P. H., Palumbi, S. R., Erdmann, M. V., and Moosa, M. K. (2000). Biogeography – A marine Wallace’s line? Nature 406, 692–693.
| Biogeography – A marine Wallace’s line?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmt1Cht7Y%3D&md5=0c51f7f716abb1b9d4dc3e4f28b45da6CAS |
Barnes, D. K. A., Griffiths, H. J., and Kaiser, S. (2009). Geographic range shift responses to climate change by Antarctic benthos: where we should look. Marine Ecology Progress Series 393, 13–26.
| Geographic range shift responses to climate change by Antarctic benthos: where we should look.Crossref | GoogleScholarGoogle Scholar |
Bond, N. R., and Lake, P. S. (2005). Ecological restoration and large-scale ecological disturbance: the effects of drought on the response by fish to a habitat restoration experiment. Restoration Ecology 13, 39–48.
| Ecological restoration and large-scale ecological disturbance: the effects of drought on the response by fish to a habitat restoration experiment.Crossref | GoogleScholarGoogle Scholar |
Bond, N. R., McMaster, D., Reich, P., Thomson, J., and Lake, P. S. (2010). Modelling the impacts of flow regulation on fish distributions in naturally intermittent lowland streams: an approach for predicting restoration responses. Freshwater Biology 55, 1997–2010.
| Modelling the impacts of flow regulation on fish distributions in naturally intermittent lowland streams: an approach for predicting restoration responses.Crossref | GoogleScholarGoogle Scholar |
Bond, N. R., Thomson, J., Reich, P., and Stein, J. A. (2011). Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Marine and Freshwater Research 62, 1043–1061.
| Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Booth, D. J., and Beretta, G. A. (2002). Changes in a fish assemblage after a coral bleaching event. Marine Ecology Progress Series 245, 205–212.
| Changes in a fish assemblage after a coral bleaching event.Crossref | GoogleScholarGoogle Scholar |
Booth, D. J., Figueira, W. F., Gregson, M. A., Brown, L., and Beretta, G. (2007). Occurrence of tropical fishes in temperate southeastern Australia: role of the East Australian Current. Estuarine, Coastal and Shelf Science 72, 102–114.
| Occurrence of tropical fishes in temperate southeastern Australia: role of the East Australian Current.Crossref | GoogleScholarGoogle Scholar |
Boughton, D., Fish, H., Pipal, K., Goin, J., Watson, F., Casagrande, J., Casagrande, J., and Stoecker, M. (2005). Contraction of the southern range limit for anadromous Oncorhynchus mykiss. National Oceanic and Atmospheric Administration (NOAA) Technical Memorandum, National Marine Fisheries Service (NMFS) – Southwest Fisheries Science Center (SWFSC) – 380.
Boulton, A. J., and Lake, P. S. (1990). The ecology of two intermittent streams in Victoria Australia I. Multivariate analysis of physicochemical features. Freshwater Biology 24, 123–141.
| The ecology of two intermittent streams in Victoria Australia I. Multivariate analysis of physicochemical features.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhtVyns74%3D&md5=e82840c58839f70d837c40b3101b50f1CAS |
Bunn, S., and Arthington, A. (2002). Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30, 492–507.
| Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity.Crossref | GoogleScholarGoogle Scholar |
Bunn, S. E., Thoms, M. C., Hamilton, S. K., and Capon, S. J. (2006). Flow variability in dryland rivers: boom, bust and the bits in between. River Research and Applications 22, 179–186.
| Flow variability in dryland rivers: boom, bust and the bits in between.Crossref | GoogleScholarGoogle Scholar |
Byrkjedal, I., Godo, O. R., and Heino, M. (2004). Northward range extensions of some mesopelagic fishes in the northeastern Atlantic. Sarsia 89, 484–489.
| Northward range extensions of some mesopelagic fishes in the northeastern Atlantic.Crossref | GoogleScholarGoogle Scholar |
Caputi, N., De Lestang, S., Feng, M., and Pearce, A. (2009). Seasonal variation in the long-term warming trend in water temperature off the Western Australian coast. Marine and Freshwater Research 60, 129–139.
| Seasonal variation in the long-term warming trend in water temperature off the Western Australian coast.Crossref | GoogleScholarGoogle Scholar |
Cheung, W. W., Lam, V. W., Sarmiento, J. L., Kearney, K., Watson, R., and Pauly, D. (2009). Projecting global marine biodiversity impacts under climate change scenarios. Fish and Fisheries 10, 235–251.
| Projecting global marine biodiversity impacts under climate change scenarios.Crossref | GoogleScholarGoogle Scholar |
Childs, A. R., Cowley, P. D., Naesje, T. F., Booth, A. J., Potts, W. M., Thorstad, E. B., and Økland, F. (2008). Do environmental factors influence the movement of estuarine fish? A case study using acoustic telemetry. Estuarine, Coastal and Shelf Science 78, 227–236.
| Do environmental factors influence the movement of estuarine fish? A case study using acoustic telemetry.Crossref | GoogleScholarGoogle Scholar |
Chuwen, B. M., Platell, M. E., and Potter, I. C. (2007). Dietary compositions of the sparid Acanthopagrus butcheri in three normally closed and variably hypersaline estuaries differ markedly. Environmental Biology of Fishes 80, 363–376.
| Dietary compositions of the sparid Acanthopagrus butcheri in three normally closed and variably hypersaline estuaries differ markedly.Crossref | GoogleScholarGoogle Scholar |
Closs, G., and Lake, P. (1996). Drought, differential mortality and the coexistence of a native and an introduced fish species in a south east Australian intermittent stream. Environmental Biology of Fishes 47, 17–26.
| Drought, differential mortality and the coexistence of a native and an introduced fish species in a south east Australian intermittent stream.Crossref | GoogleScholarGoogle Scholar |
Crook, D. A., Reich, P., Bond, N. R., Mcmaster, D., Koehn, J. D., and Lake, P. S. (2010). Using biological information to support proactive strategies for managing fish during drought. Marine and Freshwater Research 61, 379–387.
| Using biological information to support proactive strategies for managing fish during drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjvFSjs7g%3D&md5=80d269acaf21f989f18f33c88f071d64CAS |
CSIRO (2008). Water availability in the Murray–Darling Basin. A report to the Australian Government from the CSIRO Murray–Darling Basin Sustainable Yields Project. Commonwealth Scientific and Industrial Research Organisation, Canberra.
Cyrus, D. P., and Blaber, S. J. M. (1992). Turbidity and salinity in a tropical northen Australian estuary and their infuence on fish distribution. Estuarine, Coastal and Shelf Science 35, 545–563.
| Turbidity and salinity in a tropical northen Australian estuary and their infuence on fish distribution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXis1Smsbg%3D&md5=816c2d3bea8ffc470160df0e0b797bafCAS |
Daufresne, M., and Boët, P. (2007). Climate change impacts on structure and diversity of fish communities in rivers. Global Change Biology 13, 2467–2478.
| Climate change impacts on structure and diversity of fish communities in rivers.Crossref | GoogleScholarGoogle Scholar |
Davis, M. B., and Shaw, R. G. (2001). Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679.
| Range shifts and adaptive responses to Quaternary climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjt1elsrY%3D&md5=805fe853663d03b9bb185740297ce377CAS |
Dawson, M. N. (2001). Phylogeography in coastal marine animals: a solution from California? Journal of Biogeography 28, 723–736.
| Phylogeography in coastal marine animals: a solution from California?Crossref | GoogleScholarGoogle Scholar |
Dodds, W. K., Gido, K., Whiles, M. R., Fritz, K. M., and Matthews, W. J. (2004). Life on the edge: the ecology of Great Plains prairie streams. Bioscience 54, 205–216.
| Life on the edge: the ecology of Great Plains prairie streams.Crossref | GoogleScholarGoogle Scholar |
Douglas, M. R., Brunner, P. C., and Douglas, M. E. (2003). Drought in an evolutionary context: molecular variability in flannelmouth sucker (Catostomus latipinnis) from the Colorado River basin of western North America. Freshwater Biology 48, 1254–1273.
| Drought in an evolutionary context: molecular variability in flannelmouth sucker (Catostomus latipinnis) from the Colorado River basin of western North America.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt12iu7g%3D&md5=47bef95e75c5539df3de13cb4d302a2eCAS |
Edgar, G. J., and Barrett, N. S. (2000). Effects of catchment activities on macrofaunal assemblages in Tasmanian estuaries. Estuarine, Coastal and Shelf Science 50, 639–654.
| Effects of catchment activities on macrofaunal assemblages in Tasmanian estuaries.Crossref | GoogleScholarGoogle Scholar |
Edgar, G. J., Barrett, N. S., Graddon, D. J., and Last, P. R. (2000). The conservation significance of estuaries: a classification of Tasmanian estuaries using ecological, physical and demographic attributes as a case study. Biological Conservation 92, 383–397.
| The conservation significance of estuaries: a classification of Tasmanian estuaries using ecological, physical and demographic attributes as a case study.Crossref | GoogleScholarGoogle Scholar |
Edgar, G. J., Barrett, N. S., and Stuart-Smith, R. D. (2009). Exploited reefs protected from fishing transform over decades into conservation features otherwise absent from seascapes. Ecological Applications 19, 1967–1974.
| Exploited reefs protected from fishing transform over decades into conservation features otherwise absent from seascapes.Crossref | GoogleScholarGoogle Scholar |
Elith, J., Kearney, M., and Phillips, S. (2010). The art of modelling range-shifting species. Methods in Ecology and Evolution 1, 330–342.
| The art of modelling range-shifting species.Crossref | GoogleScholarGoogle Scholar |
Elliott, J. M., Hurley, M. A., and Elliott, J. A. (1997). Variable effects of droughts on the density of a sea-trout Salmo trutta population over 30 years. Journal of Applied Ecology 34, 1229–1238.
| Variable effects of droughts on the density of a sea-trout Salmo trutta population over 30 years.Crossref | GoogleScholarGoogle Scholar |
Ellison, J. C. (2005). Holocene palynology and sea-level change in two estuaries in southern Irian Jaya. Palaeogeography, Palaeoclimatology, Palaeoecology 220, 291–309.
| Holocene palynology and sea-level change in two estuaries in southern Irian Jaya.Crossref | GoogleScholarGoogle Scholar |
Elton, C. S. (1924). Periodic fluctuations in numbers of animals: their causes and effects. British Journal of Experimental Biology 2, 119–163.
Fagan, W. F. (2002). Connectivity, fragmentation, and extinction risk in dendritic metapopulations. Ecology 83, 3243–3249.
| Connectivity, fragmentation, and extinction risk in dendritic metapopulations.Crossref | GoogleScholarGoogle Scholar |
Fagan, W. F., Unmack, P. J., Burgess, C., and Minckley, W. L. (2002). Rarity, fragmentation, and extinction risk in desert fishes. Ecology 83, 3250–3256.
| Rarity, fragmentation, and extinction risk in desert fishes.Crossref | GoogleScholarGoogle Scholar |
Faulks, L., Gilligan, D., and Beheregaray, L. (2010a). Evolution and maintenance of divergent lineages in an endangered freshwater fish, Macquaria australasica. Conservation Genetics 11, 921–934.
| Evolution and maintenance of divergent lineages in an endangered freshwater fish, Macquaria australasica.Crossref | GoogleScholarGoogle Scholar |
Faulks, L. K., Gilligan, D. M., and Beheregaray, L. B. (2010b). Clarifying an ambiguous evolutionary history: range-wide phylogeography of an Australian freshwater fish, the golden perch (Macquaria ambigua). Journal of Biogeography 37, 1329–1340.
| Clarifying an ambiguous evolutionary history: range-wide phylogeography of an Australian freshwater fish, the golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar |
Feely, R. A., Alin, S. R., Newton, J., Sabine, C. L., Warner, M., Devol, A., Krembs, C., and Maloy, C. (2010). The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuarine, Coastal and Shelf Science 88, 442–449.
| The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVKmu7w%3D&md5=bdff2f5f0d4fccba15ea3f97eb2fed6aCAS |
Figueira, W. F., and Booth, D. J. (2010). Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters. Global Change Biology 16, 506–516.
| Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters.Crossref | GoogleScholarGoogle Scholar |
Figueira, W. F., Biro, P., Booth, D., and Valenzuela, V. (2009). Performance of tropical fish recruiting to temperate habitats: role of ambient temperature and implications of climate change. Marine Ecology Progress Series 384, 231–239.
| Performance of tropical fish recruiting to temperate habitats: role of ambient temperature and implications of climate change.Crossref | GoogleScholarGoogle Scholar |
Fitzpatrick, R., Thomas, B., and Merry, R. (2009). Acid sulfate soils. In ‘Natural History of the Riverland and Murrayland’. (Ed. J. Jennings.) pp. 65–111. (Royal Society of South Australia (Inc.): Adelaide.)
Fletcher, W. J., and Head, F. (2006). ‘State of the Fisheries report 2005/06.’ (Department of Fisheries, Western Australia: Perth.)
Fodrie, F. J., Heck, K. L., Powers, S. P., Graham, W. M., and Robinson, K. L. (2010). Climate-related, decadal-scale assemblage changes of seagrass-associated fishes in the northern Gulf of Mexico. Global Change Biology 16, 48–59.
| Climate-related, decadal-scale assemblage changes of seagrass-associated fishes in the northern Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |
Gibson, C. A., Meyer, J. L., Poff, N. L., Hay, L. E., and Georgakakos, A. (2005). Flow regime alterations under changing climate in two river basins: implications for freshwater ecosystems. River Research and Applications 21, 849–864.
| Flow regime alterations under changing climate in two river basins: implications for freshwater ecosystems.Crossref | GoogleScholarGoogle Scholar |
Gill, H. S., Wise, B. S., Potter, I. C., and Chaplin, J. A. (1996). Biannual spawning periods and resultant divergent patterns of growth in the estuarine goby Pseudogobius olorum: temperature induced? Marine Biology 125, 453–466.
Gillanders, B. M., and Kingsford, M. J. (1998). Influence of habitat on abundance and size structure of a large temperate-reef fish, Achoerodus viridis (Pisces: Labridae). Marine Biology 132, 503–514.
| Influence of habitat on abundance and size structure of a large temperate-reef fish, Achoerodus viridis (Pisces: Labridae).Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M., and Kingsford, M. J. (2002). Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanography and Marine Biology 40, 233–309.
Gillanders, B. M., Able, K. W., Brown, J. A., Eggleston, D. B., and Sheridan, P. F. (2003). Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Marine Ecology Progress Series 247, 281–295.
| Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries.Crossref | GoogleScholarGoogle Scholar |
Gillson, J., Scandol, J., and Suthers, I. (2009). Estuarine gillnet fishery catch rates decline during drought in eastern Australia. Fisheries Research 99, 26–37.
| Estuarine gillnet fishery catch rates decline during drought in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Gomon, M. F., Glover, C. J. M., and Kuiter, R. H. (1994). ‘The Fishes of Australia’s South Coast.’ (State Print: Adelaide.)
Growns, I. (2008). The influence of changes to river hydrology on freshwater fish in regulated rivers of the Murray–Darling basin. Hydrobiologia 596, 203–211.
| The influence of changes to river hydrology on freshwater fish in regulated rivers of the Murray–Darling basin.Crossref | GoogleScholarGoogle Scholar |
Harris, J. (1986). Reproduction of the Australian bass, Macquaria novemaculeata (Perciformes: Percichthyidae) in the Sydney basin. Marine and Freshwater Research 37, 209–235.
| Reproduction of the Australian bass, Macquaria novemaculeata (Perciformes: Percichthyidae) in the Sydney basin.Crossref | GoogleScholarGoogle Scholar |
Harrison, T. D., and Whitfield, A. K. (2006). Temperature and salinity as primary determinants influencing the biogeography of fishes in South African estuaries. Estuarine, Coastal and Shelf Science 66, 335–345.
| Temperature and salinity as primary determinants influencing the biogeography of fishes in South African estuaries.Crossref | GoogleScholarGoogle Scholar |
Haslett, S. K., Strawbridge, F., Martin, N. A., and Davies, C. F. C. (2001). Vertical saltmarsh accretion and its relationship to sea-level in the Severn Estuary, UK: an investigation using foraminifera as tidal indicators. Estuarine, Coastal and Shelf Science 52, 143–153.
| Vertical saltmarsh accretion and its relationship to sea-level in the Severn Estuary, UK: an investigation using foraminifera as tidal indicators.Crossref | GoogleScholarGoogle Scholar |
Hicks, A., Barbee, N. C., Swearer, S. E., and Downes, B. J. (2010). Estuarine geomorphology and low salinity requirement for fertilisation influence spawning site location in the diadromous fish, Galaxias maculatus. Marine and Freshwater Research 61, 1252–1258.
| Estuarine geomorphology and low salinity requirement for fertilisation influence spawning site location in the diadromous fish, Galaxias maculatus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVagt7vN&md5=f19a2e19168886bbc9351ed26ededed1CAS |
Hindell, J. S. (2007). Determining patterns of use by black bream Acanthopagrus butcheri (Munro, 1949) of re-established habitat in a south-eastern Australian estuary. Journal of Fish Biology 71, 1331–1346.
| Determining patterns of use by black bream Acanthopagrus butcheri (Munro, 1949) of re-established habitat in a south-eastern Australian estuary.Crossref | GoogleScholarGoogle Scholar |
Hindell, J. S., Jenkins, G. P., and Womersley, B. (2008). Habitat utilisation and movement of black bream Acanthopagrus butcheri (Sparidae) in an Australian estuary. Marine Ecology Progress Series 366, 219–229.
| Habitat utilisation and movement of black bream Acanthopagrus butcheri (Sparidae) in an Australian estuary.Crossref | GoogleScholarGoogle Scholar |
Hobday, X., and Lough, X. (2011). Projected climate changes in Australian marine and freshwater environments. Marine and Freshwater Research 62, 1000–1014.
| Projected climate changes in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Hoffmann, A., and Willi, Y. (2008). Detecting genetic responses to environmental change. Nature Reviews. Genetics 9, 421–432.
| Detecting genetic responses to environmental change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvFKrtLw%3D&md5=ee65e950ed4b196afdecc5caa77ad3b5CAS |
Hofmann, G. E. (2005). Patterns of Hsp gene expression in ectothermic marine organisms on small to large biogeographic scales. Integrative and Comparative Biology 45, 247–255.
| Patterns of Hsp gene expression in ectothermic marine organisms on small to large biogeographic scales.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlt12rur0%3D&md5=18725ba1c5c81c87d7a7ea1176376616CAS |
Holbrook, S. J., Kingsford, M. J., Schmitt, R. J., and Stephens, J. S. (1994). Spatial and temporal patterns in assemblages of temperate reef fish. American Zoologist 34, 463–475.
Ishimatsu, A., Hayashi, M., and Kikkawa, T. (2008). Fishes in high-CO2, acidified oceans. Marine Ecology Progress Series 373, 295–302.
| Fishes in high-CO2, acidified oceans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVCrsLo%3D&md5=4929319c5a0d6651489b1368b768a496CAS |
Jenkins, G. P. (2005). Influence of climate on the fishery recruitment of a temperate, seagrass-associated fish, the King George whiting Sillaginodes punctata. Marine Ecology Progress Series 288, 263–271.
| Influence of climate on the fishery recruitment of a temperate, seagrass-associated fish, the King George whiting Sillaginodes punctata.Crossref | GoogleScholarGoogle Scholar |
Jenkins, G. P., Black, K. P., Wheatley, M. J., and Hatton, D. N. (1997). Temporal and spatial variability in recruitment of a temperate, seagrass-associated fish is largely determined by physical processes in the pre- and post-settlement phases. Marine Ecology Progress Series 148, 23–35.
| Temporal and spatial variability in recruitment of a temperate, seagrass-associated fish is largely determined by physical processes in the pre- and post-settlement phases.Crossref | GoogleScholarGoogle Scholar |
Kennish, M. J. (2001). Coastal salt marsh systems in the US: a review of anthropogenic impacts. Journal of Coastal Research 17, 731–748.
Kennish, M. J. (2002). Environmental threats and environmental future of estuaries. Environmental Conservation 29, 78–107.
| Environmental threats and environmental future of estuaries.Crossref | GoogleScholarGoogle Scholar |
Kingsford, M. J., and Carlson, I. J. (2010). Patterns of distribution and movement of fishes, Ophthalmolepis lineolatus and Hypoplectrodes maccullochi, on temperate rocky reefs of south eastern Australia. Environmental Biology of Fishes 88, 105–118.
| Patterns of distribution and movement of fishes, Ophthalmolepis lineolatus and Hypoplectrodes maccullochi, on temperate rocky reefs of south eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Kritzer, J. P., and Sale, P. F. (2004). Metapopulation ecology in the sea: from Levins’ model to marine ecology and fisheries science. Fish and Fisheries 5, 131–140.
| Metapopulation ecology in the sea: from Levins’ model to marine ecology and fisheries science.Crossref | GoogleScholarGoogle Scholar |
Labbe, T. R., and Fausch, K. D. (2000). Dynamics of intermittent stream habitat regulate persistence of a threatened fish at multiple scales. Ecological Applications 10, 1774–1791.
| Dynamics of intermittent stream habitat regulate persistence of a threatened fish at multiple scales.Crossref | GoogleScholarGoogle Scholar |
Lamontagne, S., Hicks, W., Fitzpatrick, R., and Rogers, S. (2004). Survey and description of sulfidic materials in wetlands of the Lower River Murray floodplains: implications for floodplain salinity management. CSIRO Land and Water technical report no. 28/04, Bentley, WA.
Last, P. R., White, W. T., Gledhill, D. C., Hobday, A. J., Brown, R., Edgar, G. J., and Pecl, G. (2011). Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Global Ecology and Biogeography 20, 58–72.
| Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices.Crossref | GoogleScholarGoogle Scholar |
Leis, J. M. (2006). Are larvae of demersal fishes plankton or nekton? In ‘Advances in Marine Biology’. (Eds J. S. Alan and W. S. David.) pp. 57–141. (Academic Press: London.)
Ling, S. D., and Johnson, C. R. (2009). Population dynamics of an ecologically important range-extender: kelp beds versus sea urchin barrens. Marine Ecology Progress Series 374, 113–125.
| Population dynamics of an ecologically important range-extender: kelp beds versus sea urchin barrens.Crossref | GoogleScholarGoogle Scholar |
Ling, S. D., Johnson, C. R., Ridgway, K., Hobday, A. J., and Haddon, M. (2009). Climate-driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics. Global Change Biology 15, 719–731.
| Climate-driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics.Crossref | GoogleScholarGoogle Scholar |
Llewellyn, L. (1973). Spawning, development, and temperature tolerance of the spangled perch, Madigania unicolor (Gunther), from inland waters in Australia. Australian Journal of Marine and Freshwater Research 24, 73–94.
| Spawning, development, and temperature tolerance of the spangled perch, Madigania unicolor (Gunther), from inland waters in Australia.Crossref | GoogleScholarGoogle Scholar |
Lough, J. M. (2008). Shifting climate zones for Australia’s tropical marine 604 ecosystems. Geophysical Research Letters 35, L14708.
| Shifting climate zones for Australia’s tropical marine 604 ecosystems.Crossref | GoogleScholarGoogle Scholar |
Lough, J. M., and Hobday, J. A. (2011). Observed climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 984–999.
| Observed climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Lyons, J., Stewart, J. S., and Mitro, M. (2010). Predicted effects of climate warming on the distribution of 50 stream fishes in Wisconsin, USA. Journal of Fish Biology 77, 1867–1898.
| Predicted effects of climate warming on the distribution of 50 stream fishes in Wisconsin, USA.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cbnt1SmtQ%3D%3D&md5=b9c400978e0c89ba95a6798b9e16eeb1CAS |
Mackenzie, B. R., Gislason, H., Mollmann, C., and Koster, F. W. (2007). Impact of 21st century climate change on the Baltic Sea fish community and fisheries. Global Change Biology 13, 1348–1367.
| Impact of 21st century climate change on the Baltic Sea fish community and fisheries.Crossref | GoogleScholarGoogle Scholar |
Malcolm, H., Jordan, A., and Smith, S. (2010). Biogeographical and cross-shelf patterns of reef fish assemblages in a transition zone. Marine Biodiversity 40, 181–193.
| Biogeographical and cross-shelf patterns of reef fish assemblages in a transition zone.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M. (1992). Habitat changes and declines of freshwater fish in Australia: what is the evidence and do we need more. In ‘Sustainable Fisheries through Sustaining Fish Habitat. Australian Society for Fish Biology Workshop’. (Ed. D. A. Hancock.) pp. 118–123. (Bureau of Resource Sciences Proceedings: Canberra.)
Mazumder, D., Saintilan, N., and Williams, R. J. (2006). Trophic relationships between itinerant fish and crab larvae in a temperate Australian saltmarsh. Marine and Freshwater Research 57, 193–199.
| Trophic relationships between itinerant fish and crab larvae in a temperate Australian saltmarsh.Crossref | GoogleScholarGoogle Scholar |
Meynecke, J. O., Lee, S. Y., Duke, N., and Warnken, J. (2006). Effect of rainfall as a component of climate change on estuarine fish production in Queensland. Estuarine, Coastal and Shelf Science 69, 491–504.
| Effect of rainfall as a component of climate change on estuarine fish production in Queensland.Crossref | GoogleScholarGoogle Scholar |
Meynecke, J. O., Lee, S. Y., and Duke, N. C. (2008). Linking spatial metrics and fish catch reveals the importance of coastal wetland connectivity to inshore fisheries in Queensland, Australia. Biological Conservation 141, 981–996.
| Linking spatial metrics and fish catch reveals the importance of coastal wetland connectivity to inshore fisheries in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Minns, C., and Moore, J. (1995). Factors limiting the distributions of Ontario’s freshwater fishes: the role of climate and other variables, and the potential impacts of climate change. Canadian Special Publication of Fisheries and Aquatic Sciences 121, 137–160.
Morrongiello, J., Crook, D., King, A., Ramsey, D., and Brown, P. (2011). Impacts of drought and predicted effects of climate change on fish growth in temperate Australian lakes. Global Change Biology 17, 745–755.
| Impacts of drought and predicted effects of climate change on fish growth in temperate Australian lakes.Crossref | GoogleScholarGoogle Scholar |
Munday, P. L., Jones, G. P., Sheaves, M., Williams, A., and Goby, G. (2007). Vulnerability of fishes of the Great Barrier Reef to climate change. In ‘Climate Change and the Great Barrier Reef: a Vulnerability Assessment.’ (Eds J. E. Johnson and P. A. Marshall.) pp. 357–391. (Great Barrier Reef Marine Park Authority and Australian Greenhouse Office: Townsville, Qld.)
Munday, P. L., Leis, J. M., Lough, J. M., Paris, C. B., Kingsford, M. J., Berumen, M. L., and Lambrechts, J. (2009). Climate change and coral reef connectivity. Coral Reefs 28, 379–395.
| Climate change and coral reef connectivity.Crossref | GoogleScholarGoogle Scholar |
Munday P. L.Dixson D. L.McCormick M. I.Meekan M.Ferrari M. C. O.Chivers D. P. (2010 ). Replenishment of fish populations is threatened by ocean acidification. Proceedings of the National Academy of Sciences, USA 107 , 12 930–12 934
Murray, A. M., and Wilson, M. V. H. (2009). A new late Cretaceous macrosemiid fish (Neopterygii, Halecostomi) from Morocco, with temporal and geographical range extensions for the family. Palaeontology 52, 429–440.
| A new late Cretaceous macrosemiid fish (Neopterygii, Halecostomi) from Morocco, with temporal and geographical range extensions for the family.Crossref | GoogleScholarGoogle Scholar |
Newbrey, M. G., Murray, A. M., Wilson, M. V. H., Brinkman, D. B., and Neuman, A. G. (2009). Seventy-five-million-year-old tropical tetra-like fish from Canada tracks Cretaceous global warming. Proceedings. Biological Sciences 276, 3829–3833.
| Seventy-five-million-year-old tropical tetra-like fish from Canada tracks Cretaceous global warming.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1Mnlt1Omsw%3D%3D&md5=28d9d8433000cb7ee7fbfbb4823e348cCAS |
Newton, G. M. (1996). Estuarine ichthyoplankton ecology in relation to hydrology and zooplankton dynamics in a salt-wedge estuary. Marine and Freshwater Research 47, 99–111.
| Estuarine ichthyoplankton ecology in relation to hydrology and zooplankton dynamics in a salt-wedge estuary.Crossref | GoogleScholarGoogle Scholar |
Nicholson, G., Jenkins, G. P., Sherwood, J., and Longmore, A. (2008). Physical environmental conditions, spawning and early-life stages of an estuarine fish: climate change implications for recruitment in intermittently open estuaries. Marine and Freshwater Research 59, 735–749.
| Physical environmental conditions, spawning and early-life stages of an estuarine fish: climate change implications for recruitment in intermittently open estuaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVaisrvK&md5=cb0a33c233eaae3d2506d58b67a49195CAS |
Nilsson, G. E., Crawley, N., Lunde, G., and Munday, P. L. (2009). Elevated temperature reduces the respiratory scope of coral reef fishes. Global Change Biology 15, 1405–1412.
| Elevated temperature reduces the respiratory scope of coral reef fishes.Crossref | GoogleScholarGoogle Scholar |
Nye, J. A., Link, J. S., Hare, J. A., and Overholtz, W. J. (2009). Changing spatial distribution of fish stocks in relation to climate and population size on the northeast United States continental shelf. Marine Ecology Progress Series 393, 111–129.
| Changing spatial distribution of fish stocks in relation to climate and population size on the northeast United States continental shelf.Crossref | GoogleScholarGoogle Scholar |
O’Connor, M. I., Bruno, J. F., Gaines, S. D., Halpern, B. S., Lester, S. E., Kinlan, B. P., and Weiss, J. M. (2007). Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proceedings of the National Academy of Sciences, USA 104, 1266–1271.
| Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht12jtL4%3D&md5=6c1c390793bbdd6527a6927e7658fc2eCAS |
Palmer, M. A., Reidy, C., Nilsson, C., Florke, M., Alcamo, J., Lake, P. S., and Bond, N. (2008). Climate change and the world’s river basins: anticipating response options. Frontiers in Ecology and the Environment 6, 81–89.
| Climate change and the world’s river basins: anticipating response options.Crossref | GoogleScholarGoogle Scholar |
Pankhurst, N. W., and Munday, P. L. (2011). Effects of climate change on fish reproduction and early life history changes. Marine and Freshwater Research 62, 1062–1081.
| Effects of climate change on fish reproduction and early life history changes.Crossref | GoogleScholarGoogle Scholar |
Parmesan, C., Gaines, S., Gonzalez, L., Kaufman, D. M., Kingsolver, J., Peterson, A. T., and Sagarin, R. (2005). Empirical perspectives on species borders: from traditional biogeography to global change. Oikos 108, 58–75.
| Empirical perspectives on species borders: from traditional biogeography to global change.Crossref | GoogleScholarGoogle Scholar |
Pearce, A. F., and Hutchins, J. B. (2009). Oceanic processes and the recruitment of tropical fish at Rottnest Island (Western Australia). Journal of the Royal Society of Western Australia 92, 179–195.
Perry, G., and Bond, N. R. (2009). Spatially-explicit modelling of habitat dynamics and fish population persistence in an intermittent lowland stream. Ecological Applications 19, 731–746.
| Spatially-explicit modelling of habitat dynamics and fish population persistence in an intermittent lowland stream.Crossref | GoogleScholarGoogle Scholar |
Perry, A. L., Low, P. J., Ellis, J. R., and Reynolds, J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915.
| Climate change and distribution shifts in marine fishes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlsVWmtbg%3D&md5=b99cf337ddaccb32659fd2b7ab3c4576CAS |
Poff, L. N., and Allan, D. J. (1995). Functional organization of stream fish assemblages in relation to hydrological variability. Ecology 76, 606–627.
| Functional organization of stream fish assemblages in relation to hydrological variability.Crossref | GoogleScholarGoogle Scholar |
Power, M., and Attrill, M. J. (2002). Factors affecting long-term trends in the estuarine abundance of pogge (Agonus cataphractus). Estuarine, Coastal and Shelf Science 54, 941–949.
| Factors affecting long-term trends in the estuarine abundance of pogge (Agonus cataphractus).Crossref | GoogleScholarGoogle Scholar |
Power, M., and Attrill, M. J. (2003). Long-term trends in the estuarine abundance of Nilsson’s pipefish (Syngnathus rostellatus Nilsson). Estuarine, Coastal and Shelf Science 57, 325–333.
| Long-term trends in the estuarine abundance of Nilsson’s pipefish (Syngnathus rostellatus Nilsson).Crossref | GoogleScholarGoogle Scholar |
Power, M., and Attrill, M. J. (2007). Temperature-dependent temporal variation in the size and growth of Thames estuary smelt Osmerus eperlanus. Marine Ecology Progress Series 330, 213–222.
| Temperature-dependent temporal variation in the size and growth of Thames estuary smelt Osmerus eperlanus.Crossref | GoogleScholarGoogle Scholar |
Pratchett, M. S., Munday, M. S., Wilson, S. K., Graham, N. A. J., Cinner, J. E., Bellwood, D. R., Jones, G. P., Polunin, N. V. C., and Mcclanahan, T. R. (2008). Effects of climate-induced coral bleaching on coral-reef fishes: ecological and economic consequences. Oceanography and Marine Biology: An Annual Review 46, 251–296.
| Effects of climate-induced coral bleaching on coral-reef fishes: ecological and economic consequences.Crossref | GoogleScholarGoogle Scholar |
Pratchett, M. S., Bay, L. K., Gehrke, P. C., Koehn, J. D., Osborne, K., Pressey, R. L., Sweatman, H. P. A., and Wachenfeld, D. (2011). Contribution of climate change to degradation and loss of critical fish habitats in Australian marine and freshwater environments. Marine and Freshwater Research 62, 1062–1081.
| Contribution of climate change to degradation and loss of critical fish habitats in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Read, C. I., Bellwood, D. R., and Van Herwerden, L. (2006). Ancient origins of Indo-Pacific coral reef fish biodiversity: a case study of the leopard wrasses (Labridae: Macropharyngodon). Molecular Phylogenetics and Evolution 38, 808–819.
| Ancient origins of Indo-Pacific coral reef fish biodiversity: a case study of the leopard wrasses (Labridae: Macropharyngodon).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvVWnsbs%3D&md5=fa29abee457c29e898f739f97db22fabCAS |
Ridgway, K. R. (2007). Long-term trend and decadal variability of the southward penetration of the East Australian Current. Geophysical Research Letters 34, 22 921–22 936.
| Long-term trend and decadal variability of the southward penetration of the East Australian Current.Crossref | GoogleScholarGoogle Scholar |
Roy, P. S., Williams, R. J., Jones, A. R., Yassini, I., Gibbs, P. J., Coates, B., West, R. J., Scanes, P. R., Hudson, J. P., and Nichol, S. (2001). Structure and function of south-east Australian estuaries. Estuarine, Coastal and Shelf Science 53, 351–384.
| Structure and function of south-east Australian estuaries.Crossref | GoogleScholarGoogle Scholar |
Saintilan, N., and Williams, R. J. (1999). Mangrove transgression into saltmarsh environments in south-east Australia. Global Ecology and Biogeography 8, 117–124.
| Mangrove transgression into saltmarsh environments in south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Sanborn, S., and Bledsoe, B. (2006). Predicting streamflow regime metrics for ungauged streams in Colorado, Washington, and Oregon. Journal of Hydrology 325, 241–261.
| Predicting streamflow regime metrics for ungauged streams in Colorado, Washington, and Oregon.Crossref | GoogleScholarGoogle Scholar |
Sarre, G. A. (1999). Age compositions, growth rates, reproductive biology and diets of the black bream Acanthopagrus butcheri in four estuaries and a coastal saline lake in south-western Australia. Ph.D. Thesis, Murdoch University, Perth.
Shine, R. (2010). The ecological impact of invasive cane toads (Bufo marinus) in Australia. The Quarterly Review of Biology 85, 253–291.
| The ecological impact of invasive cane toads (Bufo marinus) in Australia.Crossref | GoogleScholarGoogle Scholar |
Sorte, C. J. B., Williams, S. L., and Carlton, J. T. (2010). Marine range shifts and species introductions: comparative spread rates and community impacts. Global Ecology and Biogeography 19, 303–316.
| Marine range shifts and species introductions: comparative spread rates and community impacts.Crossref | GoogleScholarGoogle Scholar |
Steffe, A. S., Murphy, J. J., Chapman, D. J., and Gray, C. C. (2005). An assessment of changes in the daytime recreational fishery of Lake Macquarie following the establishment of a ‘Recreational Fishing Haven’. Fisheries final report series, NSW Department of Primary Industries, Sydney.
Stein, J. L. (2006). A continental landscape framework for systematic conservation planning for Australian rivers and streams. PhD Thesis. Australian National University, Canberra.
Stuart-Smith, R. D., Barrett, N. S., Stevenson, D. G., and Edgar, G. J. (2010). Stability in temperate reef communities over a decadal time scale despite concurrent ocean warming. Global Change Biology 16, 122–134.
| Stability in temperate reef communities over a decadal time scale despite concurrent ocean warming.Crossref | GoogleScholarGoogle Scholar |
Tilman, D. (1987). On the meaning of competition and the mechanisms of competitive superiority. Functional Ecology 1, 304–315.
| On the meaning of competition and the mechanisms of competitive superiority.Crossref | GoogleScholarGoogle Scholar |
Unmack, P. J. (2000). The genus Hypseleotris of southeastern Australia: its identification and breeding biology. Fishes of Sahul 14, 647–657.
Unmack, P. J. (2001). Fish persistence and fluvial geomorphology in central Australia. Journal of Arid Environments 49, 653–669.
| Fish persistence and fluvial geomorphology in central Australia.Crossref | GoogleScholarGoogle Scholar |
Van Winkle, W., Rose, K., Shuter, B., Jager, H. I., and Holcomb, B. D. (1997). Effects of climatic temperature change on growth, survival, and reproduction of rainbow trout: predictions from a simulation model. Canadian Journal of Fisheries and Aquatic Sciences 54, 2526–2542.
| Effects of climatic temperature change on growth, survival, and reproduction of rainbow trout: predictions from a simulation model.Crossref | GoogleScholarGoogle Scholar |
Wernberg, T., Russell, B., Moore, P., Ling, S., Smale, D., Campbell, A., Coleman, M. A., Steinberg, P. D., Kendrick, G. A., and Connell, S. D. (2011). Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. Journal of Experimental Marine Biology and Ecology , .
| Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming.Crossref | GoogleScholarGoogle Scholar |
Willis, K. J., Bailey, R. M., Bhagwat, S. A., and Birks, H. J. B. (2010). Biodiversity baselines, thresholds and resilience: testing predictions and assumptions using palaeoecological data. Trends in Ecology & Evolution 25, 583–591.
| Biodiversity baselines, thresholds and resilience: testing predictions and assumptions using palaeoecological data.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cfjt1OrtA%3D%3D&md5=400bbe3882b067b728aff00c0d6b3259CAS |



