Climate change and Australian marine and freshwater environments, fishes and fisheries: synthesis and options for adaptation
John D. Koehn A E , Alistair J. Hobday B , Morgan S. Pratchett C and Bronwyn M. Gillanders DA Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, 123 Brown Street, Heidelberg, Vic. 3084, Australia.
B Climate Adaptation Flagship, CSIRO Marine and Atmospheric Research, Hobart, Tas. 7001, Australia.
C ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, Qld 4811, Australia.
D Southern Seas Ecology Laboratories, School of Earth and Environmental Sciences, University of Adelaide, SA 5005, Australia.
E Corresponding author. Email: john.koehn@dse.vic.gov.au
Marine and Freshwater Research 62(9) 1148-1164 https://doi.org/10.1071/MF11139
Submitted: 16 June 2011 Accepted: 10 August 2011 Published: 21 September 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Anthropogenic climate change is already apparent and will have significant, ongoing impacts on Australian fishes and their habitats. Even with immediate actions to reduce greenhouse gases, there will be sustained environmental changes. Therefore, it is necessary to consider appropriate adaptations to minimise detrimental impacts for both fishes and the human populations that utilise them. Climate change will have a range of direct effects on the physiology, fitness, and survivorship of Australia’s marine, estuarine and freshwater fishes, but also indirect effects via habitat degradation and changes to ecosystems. Effects will differ across populations, species and ecosystems, with some impacts being complex and causing unexpected outcomes. The range of adaptation options and necessary levels of intervention to maintain populations and ecosystem function will largely depend on the vulnerability of species and habitats. Climate change will also have an impact on people who depend on fishes for food or livelihoods; adapting to a new climate regime will mean trade-offs between biological assets and socioeconomic drivers. Models can be used to help predict trends and set priorities; however, they must be based on the best available science and data, and include fisheries, environmental, socioeconomic and political layers to support management actions for adaptation.
Additional keywords: adaptation, climate, estuaries, impacts, Indo Pacific, management.
Introduction
The rate at which the Earth’s climate has changed over the past century, and is projected to change over the rest of the current century, is unprecedented in the past 800 000 years (Steffen 2009). It is now unequivocal that human activities (e.g. greenhouse gas emissions) have caused or contributed to recent global climate change (IPCC 2007). The role of greenhouse gases is relatively well understood; expressed simplistically, climate change involves more energy trapped in the climate system as a result of increased greenhouse gases and the ‘greenhouse effect’, leading to increases in the Earth’s temperature and changes to global hydrological cycles (Fig. 1). The widespread increases in temperatures and associated environmental changes such as melting of glaciers and icecaps, sea level rises, increases in weather extremes (hot and cold days, storms, floods, droughts) will have an impact on aquatic ecosystems (marine, lake and riverine), their fishes and fisheries. Although these changes are often portrayed as complex and uncertain, most major potential impacts, at least conceptually, are reasonably well documented (Fig. 1) (IPCC 1995, 2001, 2007). Moreover, global temperatures will continue to increase over the next 30–40 years, regardless of further anthropogenic forcing, because of inertia in the climate system (IPCC 2007). As such, there is a critical need to act now, not just to address root causes of global climate change (mitigation) but to instigate management actions to assist populations, species and communities to cope with the expected changes (adaptation).

|
For biological systems, impacts of global climate change are compounded by the existing human alteration of natural systems, which have greatly undermined the resilience of populations, species and communities within marine and freshwater ecosystems. The cumulative effects of prior and ongoing stressors also make species, communities and ecosystems much more vulnerable to climate change (e.g. Schindler 2001; Valiela et al. 2001; Hughes et al. 2003; Dudgeon et al. 2006; Wooldridge 2009). This is particularly so for freshwater and estuarine ecosystems, which are affected by increasing development as well as land and water use within their catchments.
The impacts of climate change on fish and fisheries raise many concerns. Recreational and commercial fisheries not only contribute substantially to the Australian economy (e.g. Henry and Lyle 2003; Norman-Lopez et al. in press), but are also socially and culturally important (Rowland 2005). Recreational angling (marine, freshwater and estuarine) is an important leisure activity in Australia, with an annual participation rate of ~19.5%, a rate higher than for the rest of the world (Henry and Lyle 2003; Cooke and Cowx 2004). Australia’s freshwater fishes and their habitats, in particular, have suffered considerable declines in many regions (see Morrongiello et al. 2011a; Pratchett et al. 2011), with many species now being of conservation concern (Lintermans 2010) and populations in need of rehabilitation (Murray–Darling Basin Commission 2004). Changes to water flows on land can also affect estuarine and inshore marine fisheries (Gillanders et al. 2011) and cause fishers to change their fishing behaviour (e.g. use different methods, target different species).
Compared with the rest of the world, Australia has a small population and extensive access to marine resources (Hobday et al. 2008). These resources are increasingly being managed to ensure long-term sustainable use. However, climate change may significantly reduce sustainability and viability of aquatic populations, thereby undermining existing management structures. Climate change may also have an impact on the economic wealth, food security and political stability of neighbouring countries, which is of concern to Australia. In tropical island nations, fish and fisheries contribute substantially to subsistence and market-based economies. For several of the smaller Pacific island countries and territories (PICTs), fish is their most important renewable resource, with tuna stocks a particularly valuable asset (Bell et al. 2009). Fish consumption in many countries is remarkably high; national consumption of fish in six PICTs in Micronesia and Polynesia was at least twice the level needed to supply ~50% of the recommended protein requirements. In another five countries, national fish consumption was close to, or well in excess of, the 34–37 kg per year needed for good nutrition (Bell et al. 2009). This reliance on fish is largely due to limited options for agriculture or alternative sources of protein in these small island nations. Thus, maintaining the local supply of seafood in these countries is important for economic and social health (Bell et al. 2011).
The biological impacts of climate change will undoubtedly vary among populations, species and ecosystems, but there is not yet sufficient information (nor the harnessing of existing information) to establish clear hierarchies in the vulnerabilities of species to global climate change. The absence of this information is a major impediment to prioritising species conservation actions and for maximising sustainability and resilience of fisheries sectors faced with current and future climate change (Pratchett et al. 2011). The objectives of the present paper are five-fold. First, we provide a brief overview of the effects of climate change on aquatic systems, especially in Australia; second, we document the major environmental changes that have been observed and are projected to occur; third, we consider the impacts to Australian freshwater, estuarine and marine fishes and fisheries, considering their vulnerability to both direct and indirect effects; fourth, we consider the socioeconomic dimensions of fish and people; and finally, we explore adaptations to climate change, including major research needs and required management actions. This review is based on the plethora of recent studies that have considered potential effects of climate change in aquatic ecosystems as well as the work presented in this Special Issue and other presentations at the Climate change and the aquatic environment: the future for fish and fisheries symposium held in Melbourne by the Australian Society for Fish Biology in 2010 (Koehn 2011).
Effects of climate change on aquatic ecosystems
Freshwater and estuarine ecosystems
Freshwater and estuarine ecosystems are among the most affected by climate change (Pittock et al. 2008) because changes in both the quality and quantity of water affects habitat structure, nutrient loading and transport of materials and organisms. Variation in river flows over time greatly influences habitat patches (Thorp et al. 2006) and one of the impacts of climate change will be the rise in extreme events (e.g. floods, storms, severe droughts), causing shifts in the timing, frequency, duration and magnitude of hydrological events (Bates et al. 2008). Changes in flow dynamics resulting from climate change will therefore profoundly affect riverine habitats and the fishes that depend on them (Walker et al. 1995; Meyer et al. 1999; Aldous et al. 2011).
Australia is a large, flat (–15 m to 2229 m asl) continent of 7.6 million km2, covering a latitudinal range of 10°41′S to 43°38′S, and spanning climatic zones from tropical to temperate, with an immense arid interior and a wide range of habitat types. Rainfall over much of the continent is sporadic and river flows are the most variable in the world (Walker 1986), having a strong influence on freshwater and estuarine systems. Despite a relatively low diversity of freshwater fishes, Australia has high levels of endemism (Allen et al. 2002; Pusey et al. 2004), with many species needing careful conservation consideration (Lintermans 2010). Many Australian rivers are already affected by a range of other threats that have reduced and fragmented many fish populations (Morrongiello et al. 2011b; Pratchett et al. 2011). Increasing degradation of Australian freshwater systems will therefore lead to species extinctions, not just localised extirpations, and climate change may exacerbate existing impacts, including reducing the suitability of remnant or refuge habitats and the connectivity between them (Morrongiello et al. 2011b). Changes to species’ distributions are predicted, although a paucity of high mountains in Australia limits any possible altitudinal shifts for upland freshwater fish species as waters warm; the disconnected nature of catchments coupled with in-stream barriers further limit such range changes (Bond et al. 2011).
Changes to flow regimes may also have an impact on ecological processes such as ecosystem productivity and connectivity, and ultimately, on fish survival, growth and reproduction. Xenopoulos et al. (2005) suggested that 75% of freshwater fish throughout the world will become extinct through part of their range by 2070 because of reduced river discharges. Declines in precipitation and inflows, combined with increasing evaporation, will also reduce lake levels (Carpenter et al. 1992; Hughes 2003). The possible effects of climate change on lake ecosystems (see Jackson 2011; Naithani et al. 2011) has received little attention in Australia and this needs to be addressed as the impacts may be significant (see Morrongiello et al. 2011a). Freshwater flows through to estuarine and marine environments are also critically important for several species and fisheries (Gillanders and Kingsford 2002; Gillson 2011). Changes to river flows, however, cannot be viewed in isolation from other threats; in particular, such impacts must be linked to the rates of water extraction for agricultural irrigation, a major existing stressor to many river systems (Murray–Darling Basin Commission 2004; Morrongiello et al. 2011b; Pratchett et al. 2011) and one that will continue to be exacerbated by climate change.
Although estuaries are critical transition zones linking freshwater and marine habitats, they are some of the most degraded habitats on Earth (e.g. Jackson et al. 2001; Beck et al. 2011). Estuaries are differentially influenced by river, tide and wave dynamics, thereby affecting not only the form of the estuary but also water flow, nutrient cycling and ecosystem processes (Turner et al. 2004; Gillanders et al. 2011). Estuaries are, therefore, likely to be affected by the variables that influence both freshwater and marine systems. Potential impacts within estuaries may be harder to predict than those for marine systems, given the diversity of estuarine types, complex interactions and the need to downscale to finer-scale regional models. For example, changes to carbon cycling in estuaries may be expected through global changes, as well as changes to freshwater input, particularly in river-dominated systems (Jiang et al. 2008).
Trends in estuaries may also differ from those in marine systems. For example, salinity may increase in estuaries as freshwater flow decreases and evaporation increases through increased temperatures, although patterns may also depend on the impact of marine processes such as storm surges. Estuaries are highly productive systems (Beck et al. 2001), supporting not only resident fish but also species that spend time in estuaries for periods of their development or migrate through them to fresh- or saltwater (Elliott et al. 2007). Reduced river discharges may mean that fewer freshwater fish use estuaries. There may also be increased use of estuaries by marine stragglers and migrants (Elliott et al. 2007), although this will depend on how marine processes influence estuarine mouth morphology and whether there are continued connections between estuarine and marine waters. Estuarine species are typically able to tolerate a wide range of salinities; impacts on such species may depend on how high salinity levels reach.
Marine ecosystems
Marine ecosystems play a pivotal role in regulating the global climate, but are themselves sensitive to global climate change (Hoegh-Guldberg and Bruno 2010). The most striking effects of recent climate change recorded globally for marine ecosystems include (1) rapid declines in the spatial extent of Arctic sea ice (Wang and Overland 2009), (2) increasing loss of habitat-forming species (e.g. corals, giant kelp, seagrasses and mangroves) across many important coastal habitats (e.g. Short and Neckles 1999; Hoegh-Guldberg et al. 2007), (3) declines in ocean productivity (Behrenfeld et al. 2006; Polovina et al. 2008) and (4) shifts in the geographic distributions and latitudinal ranges of marine organisms (e.g. Perry et al. 2005; Last et al. 2011). These changes are caused or exacerbated by ubiquitous increases in surface temperatures across the world’s oceans, increases in the amount of CO2 dissolved in the ocean, and associated changes in the frequency and intensity of physical disturbances, sea-level rise, and changes in the position and strength of ocean current systems (Hoegh-Guldberg and Bruno 2010).
Ocean warming has been most pronounced in polar oceans, where temperatures have increased at more than twice the global average. As a consequence, the spatial extent of Arctic sea ice has declined by 42 000 km2 per year since 1979, and has been projected to disappear completely by 2037 (Wang and Overland 2009). Absolute temperature changes have been lower in tropical waters (typically <2°C increase), but have still had significant impacts because of the extreme temperature sensitivities of some key habitat-forming and foundation species (Hoegh-Guldberg et al. 2007). Scleractinian corals, for example, function exceedingly close to their upper thermal limit, whereby bleaching and death may occur when sea temperatures exceed normal local limits by as little as 1.0°C (Jokiel and Coles 1990). Consequently, coral reefs, together with polar ice caps, are regarded as the ecosystems most vulnerable to the sustained and ongoing impacts of global climate change (Walther et al. 2002). Corals, especially reef-building scleractinian corals, are fundamental to the functioning of coral-reef ecosystems, contributing to primary production, nutrient recycling and reef growth (Hoegh-Guldberg 2004). Removal or destruction of corals profoundly alters the structure and dynamics of coral-reef habitats, leading to catastrophic declines in the abundance of many reef-associated fishes, with further consequences for productivity and ecosystem function (e.g. Wilson et al. 2006; Munday et al. 2008; Pratchett et al. 2008).
Further effects of climate change on ocean-wide productivity are expected to result from large-scale changes in climatic conditions and ocean circulation. Previous climatic events, such as El Niño, have had major effects on the distribution or productivity of exploited fish populations (Lehodey et al. 1997; Worm et al. 2005; Ottersen et al. 2006), leading to widespread concern about future effects of global climate change (Brander 2007). Although there is considerable uncertainty about the effects of climate change on global fisheries production (Brander 2007), there is likely to be a major redistribution of fisheries productivity associated with shifts in climate envelopes and resultant geographical redistribution of key fisheries species (Cheung et al. 2010; Hobday 2010). Along with evidence of climate-related shifts in the distribution of marine fishes (e.g. Perry et al. 2005; Booth et al. 2011; Last et al. 2011), fisheries production in the tropics has been projected to decline by 40%, yet increase by a corresponding amount in high latitudes, as a result of large-scale redistribution of species (Cheung et al. 2010).
Climate change: documented changes and future projections
Global climate change is already having an impact on Australia’s aquatic environments, with evidence for global and regional warming both on land and in coastal waters (Lough and Hobday 2011). The climate system appears to be changing faster than thought earlier (Steffen 2009). Observations indicate that there has been an increase of ~1°C in the north and >2°C in southern Australia. Australian sea levels between 1990 and 2009 rose 1–3 cm decade–1 in the south and east and 7–10 cm decade–1 in the north and west (Lough and Hobday 2011). Rainfall has decreased in many areas although there is large spatial variation. El Niño/La Niña–Southern Oscillation events lead to large variability in coastal water temperatures and river discharges, with eastern Australian river discharge being most sensitive to these events. However, the high variability of Australian river flows (Walker 1986), together with a lack of collated data, complicate projections in freshwater systems (Lough and Hobday 2011). Climate-change strategies need to be undertaken at the basin-scale, and few river basins have adequate models to guide this management (Aldous et al. 2011).
Understanding past changes, as well as the degree of certainty surrounding projected changes in key environmental parameters (e.g. temperature, rainfall, wind and current speeds), relies on the availability and quality of appropriate climate data. Australia has many high-quality, consistent and ongoing records of land-based weather elements, enabling detection and, in some cases, attribution of recent trends in air temperatures and rainfall (Lough and Hobday 2011). Equivalent data are largely lacking for marine and, especially, estuarine and freshwater ecosystems. Much of the data relating changing oceanographic patterns in marine environments come from just four coastal monitoring sites that have been maintained for over 60 years (Thompson et al. 2009). This provides significant insights into both physical and chemical changes in the local marine environment (Thompson et al. 2009), but leaves considerable uncertainty regarding the generality of these results across different regions (Lough and Hobday 2011). For Australia’s freshwater ecosystems, there is considerable data on physical, chemical and biological parameters extending back to the early 20th century (e.g. river flows); however, there has not been any uniform, coordinated approach to data collection that might enable reliable spatial and temporal contrasts (Bond et al. 2008; Lough and Hobday 2011). By combining current datasets and historical perspectives (including palaeoclimatic reconstructions), it is clear that we are already in an era of rapidly changing climate. Although data for inland waters are less definitive, there is strong evidence for changes in rainfall and river flows (Lough and Hobday 2011).
Climate models can be used to help develop scenarios to direct potential adaptation options for management responses to climate change. There has been a range of models used to produce updated projections on emissions and climate-change scenarios (e.g. IPCC 1995, 2001, 2007; Moss et al. 2010). Because climate change is non-stationary and we are in the realm of new data ranges compared with historical patterns, some uncertainty in projections is to be expected. There is, of course, also variation in model projections (even for the same scenario) so model validation and refinement becomes an essential step (Hobday and Lough 2011).
Climate models project that sea-surface temperatures (SST) will rise by as much as 2.5°C by 2050 in south-eastern Australian coastal waters, with winter warming slightly stronger than summer warming. In other coastal regions of Australia, warming is projected to be 1–1.5°C by 2050, with the exception of the Bonney Upwelling region (Robe, South Australia to Portland, Victoria), where the rate of warming is projected to lag behind the global average. On land, continuing trends of reduced freshwater flow during El Niño years and enhanced flows during La Niña years are projected (Ward et al. 2010). Rainfall projections for 2030 show differences across the continent, with up to 10% less annual rainfall in south-eastern Australia, up to 20% less annual rainfall in south-western Australia, up to 10% more summer rainfall on the eastern coast, up to 10% more autumn rainfall inland, heavier rainfall where average rainfall increases or decreases slightly, and an increase in the intensity of tropical cyclones (Hobday and Lough 2011).
Different types of ecological models can be used in combination with climate models to project changes to fish and fisheries (Fulton 2010; Bond et al. 2011; Plagányi et al. 2011). These ecological models can focus on individual species to assess carrying capacity, reproductive potential, larval settlement and spatial distribution, or focus on populations to predict productivity and spatial distribution. Multi-species and ecosystem models can be used to investigate species replacement, dependent predator species and shifts in community composition. Models that include fishers (and thus economic, social and cultural considerations) can be used to project the income and employment effect of these ecological changes to the community, whereas end-to-end models incorporating these previous elements and management systems can be used to assess the adaptability of overlying governance structures (Fulton 2010; Plagányi et al. 2011).
Climate-change impacts on fishes
We classified the range of direct and indirect effects that climate change will have on freshwater, estuarine and marine fishes, fish populations or fisheries (Roessig et al. 2004; Munday et al. 2008; Pratchett et al. 2009) into primary, secondary and tertiary categories. Primary impacts are climate-related changes that directly affect the behaviour, physiology, fitness and survivorship of fishes via a ‘one-step’ process. Temperature, for example, exerts a major influence on metabolic processes for virtually all organisms, and increasing temperatures may directly change growth, reproduction and development of fishes (Pankhurst and Munday 2011). Secondary impacts mostly relate to changes in the quality or quantity of habitats, which indirectly affect fishes (via two steps). Such impacts include climate-induced degradation of coastal habitats (e.g. Pratchett et al. 2008, 2011), declines in nutrient delivery and availability (Behrenfeld et al. 2006), and reduced amounts of water in freshwater and estuarine habitats, which affect the local abundance and biomass of fishes. We define tertiary impacts as those that may occur as a result of several factors (see Balcombe et al. 2011) or involve multiple (two or more) steps, often with complicated feedbacks, causing unanticipated outcomes (sensu ‘ecological surprises’; e.g. Williams and Jackson 2007). Tertiary impacts may also involve combinations or permutations of many factors, which may be difficult to identify or resolve, and may take an extended period to become apparent. Direct and indirect impacts may affect different species and life stages differently (e.g. primary impacts on larval development, tertiary and secondary impacts on adult habitats). We acknowledge that there are links among primary, secondary and tertiary impacts, and that alternative considerations to classify diverse direct and indirect effects of climate change exist; our goal in using these categories is to recognise the different types and complexities of these impacts and to ensure that they are not neglected but are considered to guide development of research supporting robust management options.
Primary impacts
Temperature is one of the most fundamental variables affecting the lives of fishes. Being ectotherms, fish are subject to temperature effects on their physiological condition, development, growth rates, reproduction and behaviour (Pörtner and Farrell 2008). Increasing temperature will affect species differently, depending on whether they are at the extremes of their distribution and temperature tolerance. Fish occupy thermal preference windows that are much narrower than their thermal tolerance range (Pankhurst and Munday 2011). Climate change will lead to rising water temperatures, changing seasonal temperature profiles and higher maxima and minima, and there are many inhibitory effects on fish that may occur as a result of changed temperatures. Additional environmental stress can activate a physiological stress response leading to an internal coping strategy such as reproductive inhibition, which may divert energy from growth and reproduction to maintenance. Although this applies to fish in the wild, fish raised in aquaculture may also be exposed to altered thermal regimes, resulting in changes to growth and production (Pankhurst and Munday 2011).
Aside from affecting population size and dynamics, changes in temperature may also change phenology (Walther et al. 2002). Photoperiod and temperature are commonly viewed as the primary environmental determinants of reproductive development (Bromage et al. 2001), with acute temperature changes stimulating reproductive development in tropical and temperate species (Hilder and Pankhurst 2003; Pankhurst and King 2010). Thermal preference windows vary among species and environments and among life stages, with early life-history stages and reproducing adults being especially sensitive. Consequences include alterations to timing of spawning, reduced spawning and reproductive output, reduced egg size, size at hatching, developmental and feeding rates, number and quality of offspring, and swimming performances (especially for larvae), hence governing population dynamics and sustainability (Gaines et al. 2007; Pörtner and Farrell 2008; Donelson et al. 2010; Pankhurst and Munday 2011). Higher temperatures can cause accelerated development, with faster larval development and shorter larval duration, resulting in either higher survivorship or greater susceptibility to starvation because of higher metabolic rates that require more food and almost constant feeding. This can result in more variable recruitment (Munday et al. 2009; Donelson et al. 2010).
Changes in seawater chemistry (acidification) associated with increasing CO2 concentrations (Doney et al. 2007) pose a major problem for calcifying organisms (Orr et al. 2005), although high pCO2 in water can also affect acid–base balance (acidosis) for non-calcifying organisms (Connell and Russell 2010). Fish are generally good at regulating their acid–base balance, although early life-history stages may be highly susceptible to acidosis because they have a large surface area to volume ratio. Unexpectedly, elevated CO2 concentrations have also been shown to affect larval sensory ability and behaviour, leaving larvae unable to discriminate between ecologically important chemical cues and potentially resulting in higher predation rates (Munday et al. 2009). This highlights the potential for discovery of more ecological surprises with further research into the effects of climate change in aquatic ecosystems.
Few studies have investigated the effects of elevated pCO2 on estuarine organisms, especially for non-calcifying organisms. Estuarine environments may be more susceptible to reduced pH because they are shallower, less saline and have lower alkalinity than marine waters (Miller et al. 2009). In addition, there may also be other sources of CO2 compared with marine waters (Miller et al. 2009). Estuarine fish species live in complex and dynamic environments that are influenced by varying salinities that result from the mixing of marine and freshwater. Salinity affects the metabolism of fish through influences on osmoregulation and oxygen consumption, with extremes of salinity and rapid changes causing stress. The spatial distribution of fish along estuaries can indicate the tolerable salinity range of species. The Coorong estuary in South Australia provides a clear indication of how salinity may affect fish distribution because it ranges in salinity from fresh–brackish water in Lake Alexandrina through to extreme hypersalinity at the southern end (Gillanders et al. 2011). Only one species of fish (small-mouth hardyhead, Atherinosoma microstoma) is found in the most hypersaline waters, whereas a combination of estuarine and marine species is found near the Murray mouth (Noell et al. 2009).
A variety of other primary impacts has affected or is projected to affect marine fishes, including changes to currents (Poloczanska et al. 2007) and timing of seasonal upwelling (Nieblas et al. 2009). As for the previously discussed impacts, these can affect several life stages, from larvae to adults, in their feeding, migration, adult reproduction and larval dispersal. Similar impacts from changing flows are expected in freshwater and estuarine environments, particularly for diadromous and other migratory species. For example, the distances that larval riverine fish can drift depend on the flow at the time, and hence, this distance may be greatly reduced with lower flows (Koehn et al. 2004).
Research on primary impacts is needed to improve the performance of models that predict the future distribution or abundance of fish species (e.g. Robinson et al. 2011). Experimental approaches may be appropriate in many cases for determining tolerances for drivers such as temperature or salinity; however; they limit the number of species that can be considered. The performance of different life stages is also an important consideration, because bottlenecks may occur at fertilisation or during larval development. Managers might need projections of future primary variables to evaluate adaptation options; however, some variables (e.g. temperature and rainfall) are more readily available than others (e.g. runoff and pH) (Hobday and Lough 2011).
Secondary impacts
The most immediate effects of climate change for many fishes will be caused by changes in availability, structure and connections of critical aquatic habitats (Pratchett et al. 2011). Climate-induced changes to habitat structure have already been recorded in many aquatic ecosystems, where they have had significant effects on both abundance and diversity of fishes (Swales et al. 1999; Wilson et al. 2006; Pratchett et al. 2008). Changes to habitats will result from increasing temperature (Hoegh-Guldberg et al. 2007), increased severity of tropical cyclones (Madin and Connolly 2006), changes in the amount, seasonality, or patterns of rainfall and subsequent streamflow (Bates et al. 2008; CSIRO 2008), sea-level rise (Short and Neckles 1999), and/or changes to ocean circulation and current patterns (Munday et al. 2009). Each of these factors is likely to have specific effects in different ecosystems and their relative importance will vary by habitat (Tables 1, 2).

|

|
In coastal habitats, key habitat-forming species (e.g. seagrasses, coral and kelp) are sensitive to a wide range of environmental changes resulting from climate change, including changes in temperature, salinity, water clarity and nutrient loads, elevated CO2 concentrations and ocean acidification, as well as the frequency and/or severity of disturbance from cyclones and storm events (e.g. Hughes et al. 2003; Harley et al. 2006; Waycott et al. 2007; Russell et al. 2009). Seagrass beds and kelp forests are facing extensive degradation and loss throughout the world, mostly as a result of eutrophication and sedimentation (Connell et al. 2008; Gorman et al. 2009; Waycott et al. 2009), or increases in the local abundance of destructive herbivores (Steneck et al. 2002). Sensitivity of these habitat-forming plant species to chronic disturbances is also exacerbated by physiological stress associated with increasing temperatures (Steneck et al. 2002; Waycott et al. 2009). The most devastating effects of climate change on coral reefs have been large-scale and severe episodes of coral bleaching, resulting from high sea surface temperatures (Goreau et al. 2000). Effects of increasing temperature on coral-dominated habitats will also be further exacerbated by ocean acidification (Hoegh-Guldberg et al. 2007), which reduces coral growth and increases susceptibility to coral bleaching.
In freshwater systems, changes to flow will have major effects on habitat structure (Walker et al. 1995). In south-eastern Australia, for example, reductions in freshwater flows as a result of climate change (Van Dijk et al. 2006; CSIRO 2008) will exacerbate the existing declines in the distribution and abundance of native fish species attributable to water retention, diversion and extraction (Gehrke and Harris 2000), as exemplified during the recent ‘millenium’ drought (2003–2010) (Bond et al. 2008, 2011; Murphy and Timbal 2008; Timbal and Jones 2008). Coupled with increasing water demands by human societies, climate change will put increasing pressure on environmental flows critical for sustaining freshwater habitats. Other potential effects include decreases in the frequency and magnitude of floodplain inundation, leading to increased salinity, altered sediment and nutrient delivery, anoxic episodes, and increased incidence of algal blooms (Van Dijk et al. 2006) and high concentrations of other chemicals such as polyphenols (Morrongiello et al. 2011c). Many river systems that already suffer from high salinities will be further affected by low flows and reduced flushing events. Reduced flows will have an impact on connectivity between the main river and floodplain habitats and this may then have impacts on fish species that use both riverine and floodplain and/or wetland habitats (e.g. spangled perch, Leiopotherapon unicolor; Pusey et al. 2004).
The greatest effect of climate change on intertidal habitats (including mangroves and salt marshes) is expected to arise as a result of increasing sea levels. Sea-level rise will lead to a redistribution (where possible) of intertidal and shallow coastal habitats, as well as increased seawater intrusion into estuaries and rivers (Short and Neckles 1999). However, development and modification of coastal habitats has introduced physical barriers to landward migration of wetlands along much of Australia’s coastline (Lovelock and Ellison 2007), leading to contractions in habitat area (Doody 2004). The loss of tidal wetlands has significant ecological and geophysical impacts, including destabilisation of coastal shorelines and sediments, increased contamination and eutrophication of coastal ecosystems, leading to lower productivity. Moreover, these habitats often sustain unique assemblages of fishes as well as many other important species such as migratory shorebirds (Valiela et al. 2009). Many commercially important fish species may also use these habitats for their juvenile development (Beck et al. 2001). Secondary impacts of climate change on estuarine fishes may occur through changes in connectivity between either estuarine and freshwaters or estuarine and marine waters. For example, lowered freshwater input may reduce flushing of estuarine mouths such that they remain closed for longer. Closure of estuarine mouths may prevent fish movement between estuaries and marine waters and potentially affect species that need to move to marine waters to breed or those that use estuaries for juvenile development (Gillanders et al. 2011).
Tertiary impacts
Tertiary impacts of climate change are less easily defined than are primary (direct) and secondary (indirect) effects; however, they arise from multiple changes, species or habitat interactions or impacts caused by consequent socio-economic changes. Tertiary impacts are complicated by the many attributes, combinations or permutations of factors that may be affected (see Balcombe et al. 2011; Bond et al. 2011; Pratchett et al. 2011), are less predictable and are the impacts that we are least aware of or prepared for. Stressors other than climate change may also be important, such as fishing, agriculture, coastal development, habitat degradation and water storage and extraction. The range of impacts, their variable time frames, complexity of interactions and the uncertainty of outcomes mean that tertiary impacts will be difficult to predict and manage. These impacts, however, are likely to be more prevalent in marine and estuarine systems where the interactions with population and land-use pressures are the most evident (Tables 1, 2).
As yet, there are few quantified examples of tertiary climate-change effects on fishes. Therefore, we mention only potential impacts, in part to challenge the research and management communities to think beyond the direct primary impacts and secondary habitat impacts. One example is the climate-mediated arrival of a new sea urchin species to Tasmania that has led to disruption of ecosystem structure and a decline in abundance of other species, including fish (Ling et al. 2009). Range expansions of other species, either alien or native to other regions of Australia, may be able to occupy new habitats (see Bond et al. 2011; Booth et al. 2011), leading to unanticipated ecosystem impacts (e.g. Sorte et al. 2010). In terrestrial systems, increased fire frequency as a result of drier and hotter conditions may lead to changes in sedimentation inputs to freshwater systems, and subsequent habitat and species impacts (Morrongiello et al. 2011b). There are considerable interactions between water temperature, salinity, flows and dissolved oxygen and nutrients that can affect marine and freshwater habitats and species, with increases in anoxia recently recognised as climate-related phenomena (e.g. Brewer and Peltzer 2009). This is most evident when fish kills occur (e.g. Koehn 2005) but non-critical effects may go less noticed.
Understanding and predicting tertiary impacts is likely to be facilitated through modelling approaches rather than through experimental manipulations, because of the spatial scale, time lags before the impact is realised, and synergistic effects (Plagányi et al. 2011). Discussion of potential impacts when developing model scenarios should be wide-ranging, because ecological surprises may come from outside the traditional sphere (Fulton 2010). Long-term monitoring as part of understanding tertiary impacts will also be important for both historical insight and model validation (Brander 2010), although attribution may be difficult. Different ecosystems and their components have different values that will be affected by different climate-change drivers, with different resultant impacts. These impacts need to be gauged in light of other existing and future potential stressors (Table 2), so that appropriate management decisions can be made.
Climate change, fish and people
Climate change affects people, livelihoods, social and physical wellbeing, cultures, and traditional and subsistence fisheries, causing considerable social impacts (Allison et al. 2009; Bell et al. 2009). Social changes can lead to additional changes beyond fishes, their habitats and fisheries. Understanding human decision-making entails more than just understanding economics, and links the impacts on land and its people to those of the fishery. Indeed, many of the poorest people may be those most at risk (Allison et al. 2009; Plagányi et al. 2011). Although biology-based modelling approaches and predictions are included in many papers in this Special Issue, Plagányi et al. (2011) advocate an holistic approach to modelling ecosystems and their dependant Australian and Pacific communities.
In Australia, dependence on fish and fish-derived income is low compared with the regional neighbours. The implications of climate change for fisheries and aquaculture in the Pacific Community (Melanesia, Micronesia and Polynesia) must be overlaid on the regional changes that are already occurring and the role of fisheries and aquaculture in the lives of the people (Bell et al. 2011). Key drivers of change in the Pacific Community are as follows: population growth and urbanisation, governance and political stability; global economic conditions; status of fisheries in other oceans; markets and trade; fuel costs; technology and innovation; foreign aid; and climate change. Government roles in these areas include management of fisheries and aquaculture, maintaining food security and livelihoods, and management of government revenue and economic growth. It is important to determine the vulnerability of these roles to climate change and other major drivers and adapt them to maintain the benefits of fisheries and aquaculture. For example, determining how much fish will be needed for future food security (per capita fish consumption), how many livelihoods fish resources and aquaculture can sustain (% coastal households selling fish), and how tuna can best contribute to government revenue and economic growth (government revenue and GDP) are all key factors (Bell et al. 2011) that must be integrated into predictive climate-impact models (Plagányi et al. 2011).
One of the key stressors to freshwater and estuarine ecosystems (although these can also affect inshore marine ecosystems) is the extraction or diversion of river flows for domestic or agricultural use. This is particularly prevalent in south-eastern Australia such as in the Murray–Darling Basin (Murray–Darling Basin Commission 2004; Pratchett et al. 2011). A drier climate will place greater need for this scarce resource, although it may also cause changes to crop selection, irrigation practices, environmental water allocations and rural population demographics. Little consideration has been given to the impact of climate change on recreational activities (e.g. Hadwen and Bunn 2004; Ligare et al. 2011) and such assessment is needed for recreational angling in Australia. Recreational anglers may change fishing locations or the species targeted (e.g. from cold-water salmonids to warm-water native species), resulting in changes to population take rates.
Finally, many of the non-climate drivers that have negative impacts on fish are a result of human population growth. Thus, there may be a need to consider spatial and temporal variation in human populations and their likely responses to their world caused by changes in climate. Although this research area may be beyond the traditional remit of ‘fish’ scientists, partnerships with social scientists will enrich our understanding beyond biological impacts and will be critical in the design of effective adaptation options.
Adapting to climate change
Mitigation of climate change (e.g. reducing atmospheric levels of greenhouse gas and cutting overall emissions) is a significant global challenge, whereas adaptation (adjustment in natural or human systems) will be essential to minimise effects of climate change at more local scale. Adaptation in natural (e.g. changes to fish and the ecosystem) and human (e.g. changes to fishers, processors, divers, communities) systems can be autonomous (= passive) or directed (= active). Both types of adaptation will be important for the species and the humans that manage, exploit and rely on them. For example, plasticity in biological response underpins autonomous adaptability in many species, and autonomous changes to human communities and socioeconomic systems will also occur, given the historical flexibility of human populations.
Autonomous adaptation may not be sufficient to cope given the magnitude of the projected change, the unprecedented rate of that change, and the additional stressors that natural and human systems encounter. Thus, there is a critical need to undertake directed adaptation actions that may reduce or delay climate impacts, or provide opportunity for more autonomous adaptation, and it is directed adaptation that we consider in detail in this section. Directed adaptation strategies should aim to increase the flexibility in management of vulnerable species and ecosystems (Hulme 2005) and build adaptive capacity in human systems (e.g. Marshall 2010). The range of potential directed adaptation strategies differ in scale of intervention for both human (e.g. CSIRO 2011, Chapter 7) and natural systems (e.g. Dawson et al. 2011). In natural systems, for example, the least dramatic adaptation options include improving existing management, whereas the most dramatic options include assisted migration and ex situ conservation (Dawson et al. 2011).
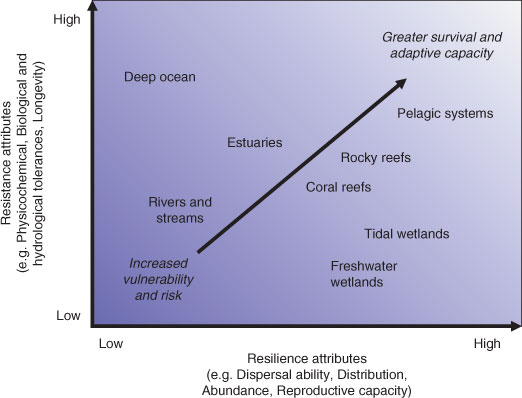
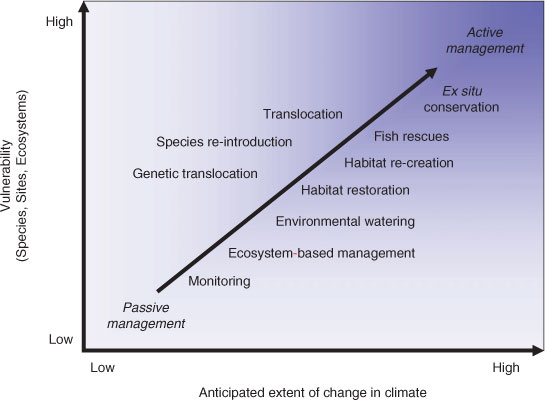
Although multi-species or ecosystem management may be preferred, there is often an imperative to manage individual species (e.g. species of conservation concern). One method for setting species priorities is to assess their vulnerability to climate-change impacts by considering the biological attributes of species relating to resistance (ability to withstand) and resilience (ability to recover). Such considerations have been necessitated for freshwater fishes in south-eastern Australia as a consequence of the recent drought, with species such as the barred galaxias, Galaxias fuscus, deemed highly vulnerable and at the greatest risk, whereas others such as golden perch, Macquaria ambigua, being considered less so (see Crook et al. 2010). Species, habitats or ecosystem components could be assessed and prioritised in this way (Fig. 2), remembering that their vulnerability may also be affected by other existing or predicted stressors. Such traits, together with the exposure to climate-change impacts need to be examined before appropriate (possibly directed) adaptation options are explored. In cases where the anticipated climate change is low and vulnerability is low, existing, passive management approaches may be appropriate (Fig. 3). When the anticipated change is high and the species is more vulnerable, more urgent, directed, active management efforts may be needed to ensure suitable outcomes. Such management is more likely to be applied to individual species, populations or sites and, in some cases, suboptimal decisions made on a range of social and economic grounds may be required (e.g. prohibitive costs to maintain some species in the wild).
|
|
Existing management of natural systems can be improved by consideration of future biological change. For example, projection of species’ future range and abundance changes would enable strategic directed adaptation and planning of management responses in fisheries. If the primary relationship is known, management can also track changes in the physical variables and manage against this change. Although single-species (e.g. Robinson et al. 2011) and multi-species models are important in generating these projections, ecosystem and end-to-end models that include economic, social and human-learning components that provide procedure frameworks (with their feedback loops) are considered the most useful tools to develop and test adaptation options in response to changes in fish stocks (Fulton 2010; Plagányi et al. 2011). Unfortunately, these models often need data on a broader suite of species, which is not always available, although input and participation by fishers can help to offset this shortcoming (Plagányi et al. 2011). It is important to remember that the future will be different from the past; hence, projections may be difficult to make and have varying degrees of uncertainty. Any adaptation option should be robust to this uncertainty (Hobday et al. 2008).
Intermediate directed adaptation options to improve the coping ability of natural systems include habitat restoration, particularly for freshwater (e.g. Murray–Darling Basin Commission 2004; Nicol et al. 2004) and coastal (e.g. Levin et al. 1996) systems, and habitat enhancement, such as artificial habitat creation for marine systems (e.g. Davis 1995; Abelson 2006) (Fig. 3). Enhancement of native populations, via releases of captive-bred individuals, is also another intermediate strategy (e.g. Lorenzen et al. 2010), although these release options are not without their own constraints (e.g. Meffe 1992; Lorenzen et al. 2010). Importantly, there is a significant cost associated with restoration of aquatic environments, such that established intervention strategies developed for restoration following small-scale disturbances (e.g. ship groundings; Jaap 2000) will be prohibitively expensive to scale-up and effectively address regional and global effects of climate change.
Existing management approaches suffice for some species whose natural dispersal mechanisms are adequate for them to adapt and move with environmental changes, whereas for others, the rates of change to habitats caused by climate change may be too rapid or extreme. One of the more controversial management actions suggested for the survival of threatened species is that of ‘assisted migration’ or ‘translocation’ (e.g. Hoegh-Guldberg et al. 2008; Dawson et al. 2011). Although potentially necessary to ensure survival of individual species, extensive movement of threatened species may jeopardise the local diversity and ecosystem function of recipient regions (Davidson and Simkanin 2008; Minteer and Collins 2010). Concerns have been raised as to why, when, where, what and how such actions may be undertaken (Richardson et al. 2009; Minteer and Collins 2010). Such movements, if they were to occur, should be undertaken within robust, transparent protocols that can determine whether such actions are feasible, will meet defined objectives and are economically efficient, ecologically safe and socially acceptable (e.g. Richardson et al. 2009; Olden et al. 2011).
One area where assisted migration may be a legitimate option is for native freshwater fishes that are in vulnerable, isolated habitats, with little chance of successful migration to other habitats (Olden et al. 2011). One such example may be the critically endangered barred galaxias that survives in isolated populations in small upland tributaries of the Goulburn River catchment in south-eastern Australia. The species requires cool water temperatures and is at great risk from predation by introduced trout (Salmo trutta and Oncorhynchus mykiss) in the lower river reaches. The catchments of many of the remaining populations have been badly affected by recent bushfires, with severe impacts on habitat quality. Fish from some affected populations have already been taken from the wild and housed in aquaria while stream habitats have recovered (i.e. fish rescue, Fig. 3) (Arthur Rylah Institute for Environmental Research, T. Raadik, unpubl. data). The establishment of additional populations at other sites, however, would reduce future risk for this species. Such movements of fish already occur in freshwater systems, with stocking and translocations of native species (Olden et al. 2011).
There is particular concern for the impacts of climate change on threatened species. A search of completed national recovery plans for fish listed under the Environment Protection and Biodiversity Conservation Act 1999 (http://www.environment.gov.au/biodiversity/threatened/recovery-list-common.html, accessed 20 July 2011), revealed that scant attention has been paid to this threat to these listed species thus far. Of the 26 species included in recovery plans (19 freshwater and 7 marine fishes), climate change was mentioned only for six of the freshwater species. Only in one case (barred galaxias) could it be argued that this issue was adequately addressed in recovery actions, despite several other species likely to be under considerable threat from climate-change impacts. No mention of climate change was made for the marine species (sharks and handfishes), however these are unlikely to be under immediate risk as a result of climate change. This omission of climate change possibly reflects the widespread, difficult nature of this ‘big picture’ threat, as there is little that can be done in an overall sense by actions that the plans may realistically address. With consideration of the species-specific impacts, however, there are adaptive actions that could be considered and included (e.g. environmental watering, species or genetic translocation, Fig. 3). In light of the many potential impacts of climate change, this threat should be specifically considered when recovery plans are being written and reviewed, and addressed where appropriate.
Overall, planned adaptation through changes to biological or human systems should focus on ‘win–win’, or ‘no-regrets’ options. In the Pacific, for example, fisheries management-adaptation options have focussed on those that can restore and sustain coastal and freshwater fisheries (Bell et al. 2011). Some key projections suggest that abundance of skipjack tuna, Katsuwonus pelamis, will increase in the eastern Pacific (Lehodey et al. 1997; Bell et al. 2011). Increased access to tuna for subsistence fishers, along with low-cost, in-shore fish-aggregating devices, and improvements to storing and distributing tuna will help local fishers. Abundances of freshwater fish may also increase with increases in rainfall in equatorial regions, and so protection of forest cover in catchments to maintain water quality and developing pond aquaculture facilities are also options.
Adaptation options for climate change cannot be considered in isolation. Some of the more radical adaptation options may not be permitted, given legislative obligations to threatened species, existing stressors may limit the range of options (e.g. coastal rock walls that may prevent landward retreat of marine or estuarine habitats), or interactions with options being pursued in other sectors can lead to unwanted outcomes (e.g. maladaptation). For example, some climate-change adaptation options (e.g. tree planting in catchments) may further exacerbate problems in adjacent fish habitats (e.g. reducing stream flows and nullify habitat restoration efforts). In considering future options, management and societal values might also need to change, and so stakeholder engagement and consultative processes will be crucial.
Conclusions
Climate change is already affecting many aspects (conservation, recreational, commercial and subsistence) of marine and freshwater ecosystems, their fishes, and their human uses (Bell et al. 2009), both in market and non-market senses (such as biodiversity and ecosystem services). Climate change is likely to have an increasingly important influence on the structure and dynamics of Australia’s aquatic ecosystems. It is often difficult, however, to distinguish specific effects of climate change against the back-drop of other disturbances affecting fishes and their habitats. Climate change cannot become an excuse for failure to address such threats, but must be managed in the context of existing and future pressures (e.g. exploitation rates). Indeed, improved management of other anthropogenic disturbances will help maximise adaption options available to minimise the impacts of climate change.
The biological impacts of climate change will differ across populations, species and ecosystems and there is a need to consider potential thresholds and tipping points, risks and consequences, time frames and limiting factors. Importantly, there will be ‘winners and losers’ for species, locations and habitats, and some impacts will be complex and cause unexpected outcomes. Models can be used to help provide predictions and trends and to set priorities; however, they must be based on the best available science, supported by adequate data to reduce model uncertainty. To provide a basis for adaptive management, such models need to overlay fisheries and environmental data with socio-economic and political layers (Plagányi et al. 2011). Priorities must be set for management actions and the benefits quantified so the benefits of adaptive actions can be balanced against the costs of increased severity of actions and impacts into the future. The future of Australia’s unique aquatic systems depends on getting this balance right.
Acknowledgements
We thank all authors (54 in total) for their contributions to the twelve publications submitted for this Special Issue. Thanks go to the Australian Society for Fish Biology Conference Organising Committee led by Martin Gomon, the symposium steering committee (John Koehn, Philip Munday, Alistair Hobday, Ned Pankhurst, Dave Crook and Jeff Leis) and participants at the Climate change and the aquatic environment: the future for fish and fisheries symposium held at the Melbourne Museum, Carlton, Melbourne, 12–14 July 2010. Key sponsors of this event were the Museum of Victoria, Fisheries Research and Development Corporation and Department of Sustainability and Environment, Victoria. We thank Nick Bond and the two anonymous referees for constructive comments on this paper and Andrew Boulton and Leanne Hamilton for assistance with production of this Special Issue.
References
Abelson, A. (2006). Artificial reefs vs coral transplantation as restoration tools for mitigating coral reef deterioration: benefits, concerns and proposed guidelines. Bulletin of Marine Science 78, 151–159.Aldous, A., Fitzsimons, J., Richter, B., and Bach, L. (2011). Droughts, floods and freshwater ecosystems: evaluating climate change impacts and developing adaptation strategies. Marine and Freshwater Research 62, 223–231.
| Droughts, floods and freshwater ecosystems: evaluating climate change impacts and developing adaptation strategies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsVKktrw%3D&md5=6a1ffe0cf925eda9340ec29be4eee8f8CAS |
Allen, G. R., Midgely, S. H., and Allen, M. (2002). ‘Field Guide to the Freshwater Fishes of Australia.’ (Western Australia Museum: Perth.)
Allison, E. H., Perry, A. L., Badjeck, M.-C., Adger, W. N., Brown, K., Halls, A. S., Pilling, G. M., Reynolds, J. D., Andrew, N. L., and Dulvy, N. K. (2009). Vulnerability of national economies to the impacts of climate change on fisheries. Fish and Fisheries 10, 173–196.
| Vulnerability of national economies to the impacts of climate change on fisheries.Crossref | GoogleScholarGoogle Scholar |
Balcombe, S. R., Sheldon, F., Capon, S. J., Bond, N. R., Hadwen, W. L., Marsh, N., and Bernays, S. J. (2011). Climate-change threats to native fish in degraded rivers and floodplains of the Murray–Darling Basin, Australia. Marine and Freshwater Research 62, 1099–1114.
| Climate-change threats to native fish in degraded rivers and floodplains of the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Bates, B. C., Kundzewicz, Z. W., Wu, S., and Palutikof, J. P. (Eds) (2008). ‘Climate Change and Water. Paper of the Intergovernmental Panel on Climate Change.’ (IPCC Secretariat: Geneva.)
Beck, M. W., Heck, K. L., Able, K. W., Childers, D. L., Eggleston, D. B., Gillanders, B. M., Halpern, B., Hays, C. G., Hoshino, K., Minello, T. J., Orth, R. J., Sheridan, P. F., and Weinstein, M. P. (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51, 633–641.
| The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates.Crossref | GoogleScholarGoogle Scholar |
Beck, M. W., Brumbaugh, R. D., Airoldi, L., Carranza, A., Coen, L. D., Crawford, C., Defeo, O., Edgar, G. J., Hancock, B., Kay, M. C., Lenihan, H. S., Luckenbach, M. W., Toropova, C. L., Zhang, G., and Guo, X. (2011). Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61, 107–116.
| Oyster reefs at risk and recommendations for conservation, restoration, and management.Crossref | GoogleScholarGoogle Scholar |
Behrenfeld, M. J., O’Malley, R. T., Siegel, D. A., McClain, C. R., Sarmiento, J. L., Feldman, G. C., Milligan, A. J., Falkowski, P. G., Letelier, R. M., and Boss, E. S. (2006). Climate-driven trends in contemporary ocean productivity. Nature 444, 752–755.
| Climate-driven trends in contemporary ocean productivity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Ontb3M&md5=27c4d34b7d6d360938ad6fa0b17b3d8eCAS |
Bell, J. D., Kronen, M., Vunisea, A., Nash, W. J., Keeble, G., Demmke, A., Pontifex, S., and Andréfouet, S. (2009). Planning the use of fish for food security in the Pacific. Marine Policy 33, 64–76.
| Planning the use of fish for food security in the Pacific.Crossref | GoogleScholarGoogle Scholar |
Bell, J., Johnson, J., and Hobday, A. (Eds) (2011). ‘Vulnerability of Fisheries and Aquaculture in the Pacific to Climate Change.’ (Secretariat of the Pacific Community: Noumea Cedex.)
Bond, N. R., Lake, P. S., and Arthington, A. H. (2008). The impacts of drought on freshwater ecosystems: an Australian perspective. Hydrobiologia 600, 3–16.
| The impacts of drought on freshwater ecosystems: an Australian perspective.Crossref | GoogleScholarGoogle Scholar |
Bond, N., Thomson, J., Reich, P., and Stein, J. (2011). Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Marine and Freshwater Research 62, 1043–1061.
| Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Booth, D. J., Bond, N., and Macreadie, P. (2011). Detecting range shifts among Australian fishes in response to climate change. Marine and Freshwater Research 62, 1027–1042.
| Detecting range shifts among Australian fishes in response to climate change.Crossref | GoogleScholarGoogle Scholar |
Brander, K. M. (2007). Climate change and food security special feature: global fish production and climate change. Proceedings of the National Academy of Sciences, USA 104, 19 709–19 714.
| Climate change and food security special feature: global fish production and climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitFSltQ%3D%3D&md5=9880833ae7c80313735f426cecd5f1e2CAS |
Brander, K. (2010). Impacts of climate change on fisheries. Journal of Marine Systems 79, 389–402.
| Impacts of climate change on fisheries.Crossref | GoogleScholarGoogle Scholar |
Brewer, P. G., and Peltzer, E. T. (2009). Limits to marine life. Science 324, 347–348.
| Limits to marine life.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFant7g%3D&md5=1896181d98cb614a66153e780b29d4ccCAS |
Bromage, N., Porter, M., and Randall, C. (2001). The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197, 63–98.
| The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjsFalsb0%3D&md5=89a575d6b5bc291ba0d8f7a77373c3baCAS |
Carpenter, S. R., Fisher, S. G., Grimm, N. B., and Kitchell, J. F. (1992). Global change and freshwater ecosystems. Annual Review of Ecology and Systematics 23, 119–139.
| Global change and freshwater ecosystems.Crossref | GoogleScholarGoogle Scholar |
Cheung, W. W. L., Lam, V. W. Y., Sarmiento, J. L., Kearney, K., Watson, R., Zeller, D., and Pauly, D. (2010). Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Global Change Biology 16, 24–35.
| Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change.Crossref | GoogleScholarGoogle Scholar |
Connell, S. D., and Russell, B. D. (2010). The direct effects of increasing CO2 and temperature on non-calcifying organisms: increasing the potential for phase shifts in kelp forests. Proceedings of the Royal Society B, Biological Sciences 277, 1409–1415.
| The direct effects of increasing CO2 and temperature on non-calcifying organisms: increasing the potential for phase shifts in kelp forests.Crossref | GoogleScholarGoogle Scholar |
Connell, S. D., Russell, B. D., Turner, D. J., Shepherd, S. A., Kildea, T., Miller, D., Airoldi, L., and Cheshire, A. (2008). Recovering a lost baseline: missing kelp forests from a metropolitan coast. Marine Ecology Progress Series 360, 63–72.
| Recovering a lost baseline: missing kelp forests from a metropolitan coast.Crossref | GoogleScholarGoogle Scholar |
Cooke, S. J., and Cowx, I. G. (2004). The role of recreational fishing in the global fish crisis. Bioscience 54, 857–859.
| The role of recreational fishing in the global fish crisis.Crossref | GoogleScholarGoogle Scholar |
Crook, D. A., Reich, P., Bond, N. R., McMaster, D., Koehn, J. D., and Lake, P. S. (2010). Using biological information to support proactive strategies for managing freshwater fish during drought. Marine and Freshwater Research 61, 379–387.
| Using biological information to support proactive strategies for managing freshwater fish during drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjvFSjs7g%3D&md5=80d269acaf21f989f18f33c88f071d64CAS |
CSIRO (2008). Water availability in the Murray–Darling Basin. A report to the Australian Government from the CSIRO Murray–Darling Basin Sustainable Yields Project, CSIRO, Canberra.
CSIRO (2011). Climate change: science and solutions for Australia. CSIRO, Canberra. Available at www.csiro.au/resources/Climate-Change-Book.html [accessed 9 June 2011].
Davidson, I., and Simkanin, C. (2008). Skeptical of assisted colonization. Science 322, 1048–1049.
| Skeptical of assisted colonization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVegsL3F&md5=a7c99f3d40c44e3edacdba2985f7b149CAS |
Davis, G. E. (1995). Recruitment of juvenile abalone (Haliotis spp.) measured in artificial habitats. Marine and Freshwater Research 46, 549–554.
| Recruitment of juvenile abalone (Haliotis spp.) measured in artificial habitats.Crossref | GoogleScholarGoogle Scholar |
Dawson, T. P., Jackson, S. T., House, J. I., Prentice, I. C., and Mace, G. M. (2011). Beyond predictions: biodiversity conservation in a changing climate. Science 332, 53–58.
| Beyond predictions: biodiversity conservation in a changing climate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvVyisbk%3D&md5=d2ff014b49e420753bbcae68d31c18edCAS |
Donelson, J. M., Munday, P. L., McCormick, M. I., Pankhurst, N. W., and Pankhurst, P. M. (2010). Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Marine Ecology Progress Series 401, 233–243.
| Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish.Crossref | GoogleScholarGoogle Scholar |
Doney, S. C., Mahowald, N., Lima, I., Feely, R. A., Mackenzie, F. T., Lamarque, J.-F., and Rasch, P. J. (2007). The impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system. Proceedings of the National Academy of Sciences, USA 104, 14 580–14 585.
| The impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVOlt7bI&md5=97274467032576adf438233df9485c3fCAS |
Doody, J. P. (2004). ‘Coastal squeeze’ – an historical perspective. Journal of Coastal Conservation 10, 129–138.
| ‘Coastal squeeze’ – an historical perspective.Crossref | GoogleScholarGoogle Scholar |
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z., Knowler, D. J., Lévêque, C., Naiman, R. J., Prieur-Richard, A.-H., Soto, D., Stiassny, M. L. J., and Sullivan, C. A. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews of the Cambridge Philosophical Society 81, 163–182.
| Freshwater biodiversity: importance, threats, status and conservation challenges.Crossref | GoogleScholarGoogle Scholar |
Elliott, M., Whitfield, A. K., Potter, I. C., Blaber, S. J. M., Cyrus, D. P., Nordlie, F. G., and Harrison, T. D. (2007). The guild approach to categorizing estuarine fish assemblages: a global review. Fish and Fisheries 8, 241–268.
| The guild approach to categorizing estuarine fish assemblages: a global review.Crossref | GoogleScholarGoogle Scholar |
Fulton, E. A. (2010). Approaches to end-to-end models. Journal of Marine Systems 81, 171–183.
| Approaches to end-to-end models.Crossref | GoogleScholarGoogle Scholar |
Gaines, S. D., Gaylord, B., Gerber, L. R., Hastings, A., and Kinlan, B. P. (2007). Connecting places. The ecological consequences of dispersal in the sea. Oceanography 20, 90–99.
Gehrke, P. C., and Harris, J. H. (2000). Large-scale patterns in species richness and composition of temperate riverine fish communities. Marine and Freshwater Research 51, 165–182.
| Large-scale patterns in species richness and composition of temperate riverine fish communities.Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M., and Kingsford, M. J. (2002). Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanography and Marine Biology: an Annual Review 40, 233–309.
Gillanders, B. M., Elsdon, T. S., Halliday, I. A., Jenkins, G. P., Robins, J. B., and Valesini, F. J. (2011). Potential effects of climate change on Australian estuaries and fish-utilising estuaries: a review. Marine and Freshwater Research 62, 1115–1131.
| Potential effects of climate change on Australian estuaries and fish-utilising estuaries: a review.Crossref | GoogleScholarGoogle Scholar |
Gillson, J. (2011). Freshwater flow and fisheries production in estuarine and coastal systems: where a drop of rain is not lost. Reviews in Fisheries Science 19, 168–186.
| Freshwater flow and fisheries production in estuarine and coastal systems: where a drop of rain is not lost.Crossref | GoogleScholarGoogle Scholar |
Goreau, T. F., McClanahan, T., Hayes, R. L., and Strong, A. (2000). Conservation of coral reefs after the 1998 global bleaching event. Conservation Biology 14, 5–15.
| Conservation of coral reefs after the 1998 global bleaching event.Crossref | GoogleScholarGoogle Scholar |
Gorman, D., Russell, B. D., and Connell, S. D. (2009). Land-to-sea connectivity: linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts. Ecological Applications 19, 1114–1126.
| Land-to-sea connectivity: linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts.Crossref | GoogleScholarGoogle Scholar |
Hadwen, W. L., and Bunn, S. E. (2004). Tourists increase the contribution of autochthonous carbon to littoral zone food webs in oligotrophic dune lakes. Marine and Freshwater Research 55, 701–708.
| Tourists increase the contribution of autochthonous carbon to littoral zone food webs in oligotrophic dune lakes.Crossref | GoogleScholarGoogle Scholar |
Harley, C. D. G., Hughes, A. R., Hultgren, K. M., Miner, B. G., Sorte, C. J. B., Thornber, C. S., Rodriguez, L. F., Tomanek, L., and Williams, S. L. (2006). The impacts of climate change in coastal marine systems. Ecology Letters 9, 228–241.
| The impacts of climate change in coastal marine systems.Crossref | GoogleScholarGoogle Scholar |
Henry, G. W., and Lyle, J. M. (2003). ‘The National Recreational and Indigenous Fishing Survey. FRDC Project No. 99/158.’ (Australian Government Department of Agriculture, Fisheries and Forestry: Canberra.)
Hilder, M. L., and Pankhurst, N. W. (2003). Evidence that temperature change cues reproductive development in the spiny damselfish, Acanthochromis polyacanthus. Environmental Biology of Fishes 66, 187–196.
| Evidence that temperature change cues reproductive development in the spiny damselfish, Acanthochromis polyacanthus.Crossref | GoogleScholarGoogle Scholar |
Hobday, A. J. (2010). Ensemble analysis of the future distribution of large pelagic fishes in Australia. Progress in Oceanography 86, 291–301.
| Ensemble analysis of the future distribution of large pelagic fishes in Australia.Crossref | GoogleScholarGoogle Scholar |
Hobday, A. J., and Lough, J. M. (2011). Projected climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 1000–1014.
| Projected climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Hobday, A. J., Poloczanska, E. S., and Matear, R. (2008). Implications of climate change for Australian fisheries and aquaculture: a preliminary assessment. Report to the Department of Climate Change, Canberra, Australia. Available at http.//www.cmar.csiro.au/climateimpacts/reports.htm [accessed 8 June 2011].
Hoegh-Guldberg, O. (2004). Coral reefs in a century of rapid environmental change. Symbiosis 37, 1–31.
Hoegh-Guldberg, O., and Bruno, J. F. (2010). The impact of climate change on the world’s marine ecosystems. Science 328, 1523–1528.
| The impact of climate change on the world’s marine ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsVWnt7Y%3D&md5=a2e5b8b5397c5584684eafb902fb3e95CAS |
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., Harvel, C. D., Sale, P. F., Edwards, A. J., Caldeira, K., Knowlton, N., Eakin, C. M., Iglesias-Prieto, R., Muthiga, N., Bradbury, R. H., Dubi, A., and Hatziolos, M. E. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742.
| Coral reefs under rapid climate change and ocean acidification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVWhu7fN&md5=b074c829a513c1a5f7a4dd1596f39ea3CAS |
Hoegh-Guldberg, O., Hughes, L., McIntyre, S., Lindenmayer, D. B., Parmesan, C., Possingham, H. P., and Thomas, C. D. (2008). Assisted colonisation and rapid climate change. Science 321, 345–346.
| Assisted colonisation and rapid climate change.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cvlvFWqsg%3D%3D&md5=2fd0e496e29dfd4ac47d0598881a15e6CAS |
Hughes, L. (2003). Climate change and Australia: trends, projections and impacts. Austral Ecology 28, 423–443.
| Climate change and Australia: trends, projections and impacts.Crossref | GoogleScholarGoogle Scholar |
Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., Grosberg, R., Hoegh-Guldberg, O., Jackson, J. B. C., Kleypas, J., Lough, J. M., Marshall, P., Nyström, M., Palumbi, S. R., Pandolfi, J. M., Rosen, B., and Roughgarden, J. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933.
| Climate change, human impacts, and the resilience of coral reefs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt1elsb4%3D&md5=51509a1cdf062823fc9b4614ec8c4f8fCAS |
Hulme, P. E. (2005). Adapting to climate change: is there scope for ecological management in the face of a global threat? Journal of Applied Ecology 42, 784–794.
| Adapting to climate change: is there scope for ecological management in the face of a global threat?Crossref | GoogleScholarGoogle Scholar |
IPCC (1995) IPCC second assessment. Climate change 1995. A report of the Intergovernmental Panel on Climate Change. Available at http://www.ipcc.ch/pdf/climate-changes-1995/ipcc-2nd-assessment/2nd-assessment-en.pdf [accessed 8 June 2011].
IPCC (2001). IPCC third assessment report. Climatic change 2001. Available at www.ipcc.ch/publications_and_data/publications_and_data_reports.shtml [accessed 8 June 2011].
IPCC (2007). ‘Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.’ (Cambridge University Press: Cambridge, UK.)
Jaap, W. C. (2000). Coral reef restoration. Ecological Engineering 15, 345–364.
| Coral reef restoration.Crossref | GoogleScholarGoogle Scholar |
Jackson, J. B. C., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., Bradbury, R. H., Cooke, R., Erlandson, J., Estes, J. A., Hughes, T. P., Kidwell, S., Lange, C. B., Lenihan, H. S., Pandolfi, J. M., Peterson, C. H., Steneck, R. S., Tegner, M. J., and Warner, R. R. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637.
| Historical overfishing and the recent collapse of coastal ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXls1Khu7o%3D&md5=f44c167fa2cc9b6564255af8f98a5d44CAS |
Jackson, L. J. (2011). Conservation of shallow lakes given an uncertain, changing climate: challenges and opportunities. Aquatic Conservation: Marine and Freshwater Ecosystems 21, 219–223.
Jiang, L. Q., Cai, W. J., and Wang, Y. C. (2008). A comparative study of carbon dioxide degassing in river- and marine-dominated estuaries. Limnology and Oceanography 53, 2603–2615.
| A comparative study of carbon dioxide degassing in river- and marine-dominated estuaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVOgu7nE&md5=e07852ffca329ce74109ac8977ac0658CAS |
Jokiel, P. L., and Coles, S. L. (1990). Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 8, 155–162.
| Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. (2005). The loss of valuable Murray cod in fish kills: a science and management perspective. In ‘Management of Murray Cod in the Murray–Darling Basin: Statement, Recommendations and Supporting Papers. Proceedings of a Workshop Held in Canberra, ACT, 3–4 June 2004’. (Eds M. Lintermans and B. Phillips.) pp. 73–82. (Murray–Darling Basin Commission and Cooperative Research Centre for Freshwater Ecology: Canberra.) Available at www2.mdbc.gov.au/NFS/nfs_publications.html [accessed 8 June 2011].
Koehn, J. D. (2011). Climate change and Australian marine and freshwater environments, fishes and fisheries: introduction. Marine and Freshwater Research 62, 981–983.
| Climate change and Australian marine and freshwater environments, fishes and fisheries: introduction.Crossref | GoogleScholarGoogle Scholar |
Koehn, J., Stuart, I., and Crook, D. (2004). Linking the ecological importance of downstream fish movements to management of Murray–Darling Basin fish populations. In ‘Management of Murray Cod in the Murray–Darling Basin: Statement, Recommendations and Supporting Papers. Proceedings of a Workshop Held in Canberra, ACT, 3–4 June 2004’. (Eds M. Lintermans and B. Phillips.) pp. 67–78. (Murray–Darling Basin Commission and Cooperative Research Centre for Freshwater Ecology: Canberra.) Available at www2.mdbc.gov.au/NFS/nfs_publications.html [accessed 8 June 2011].
Last, P. R., White, W. T., Gledhill, D. C., Hobday, A. J., Brown, R., Edgar, G. J., and Peci, G. (2011). Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Global Ecology and Biogeography 20, 58–72.
| Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices.Crossref | GoogleScholarGoogle Scholar |
Lehodey, P., Bertignac, M., Hampton, J., Lewis, A., and Picaut, J. (1997). El Niño Southern Oscillation and tuna in the western Pacific. Nature 389, 715–718.
| El Niño Southern Oscillation and tuna in the western Pacific.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmvVSht70%3D&md5=d9af2ad15fefac46942c59c7ee71572aCAS |
Levin, L. A., Talley, D., and Thayer, G. (1996). Succession of macrobenthos in a created saltmarsh. Marine Ecology Progress Series 141, 67–82.
| Succession of macrobenthos in a created saltmarsh.Crossref | GoogleScholarGoogle Scholar |
Ligare, S. T., Viers, J. H., Null, S. E., Rheinheimer, D. E., and Mount, J. F. (2011). Non-uniform changes to whitewater recreation in California’s Sierra Nevada from regional climate warming. River Research and Applications , .
| Non-uniform changes to whitewater recreation in California’s Sierra Nevada from regional climate warming.Crossref | GoogleScholarGoogle Scholar |
Ling, S. D., Johnson, C. R., Ridgway, K., Hobday, A. J., and Haddon, M. (2009). Climate driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics. Global Change Biology 15, 719–731.
| Climate driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2010). Conservation status of Australian fishes – 2010. Australian Society for Fish Biology Newsletter 40, 79–82.
Lorenzen, T., Leber, K. M., and Blankenship, H. L. (2010). Responsible approach to marine stock enhancement: an update. Reviews in Fisheries Science 18, 189–210.
| Responsible approach to marine stock enhancement: an update.Crossref | GoogleScholarGoogle Scholar |
Lough, J. M., and Hobday, A. J. (2011). Observed climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 984–999.
| Observed climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Lovelock, C. E., and Ellison, J. C. (2007). Vulnerability of mangroves and tidal wetlands of the Great Barrier Reef to climate change. In ‘Climate change and the Great Barrier Reef: A vulnerability assessment.’ (Eds J. E. Johnson, and P. A. Marshall) pp. 237–269. (Great Barrier Reef Marine Park Authority and Australian Greenhouse Office: Townsville)
Madin, J. S., and Connolly, S. R. (2006). Ecological consequences of major hydrological disturbances on coral reefs. Nature 444, 477–480.
| Ecological consequences of major hydrological disturbances on coral reefs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1aku7zP&md5=04736d1b2272d19b660781f637a700f4CAS |
Marshall, N. A. (2010). Understanding social resilience to climate variability in primary enterprises and industries. Global Environmental Change 20, 36–43.
| Understanding social resilience to climate variability in primary enterprises and industries.Crossref | GoogleScholarGoogle Scholar |
Meffe, G. K. (1992). Techno-arrogance and halfway technologies: salmon hatcheries on the Pacific Coast of North America. Conservation Biology 6, 350–354.
| Techno-arrogance and halfway technologies: salmon hatcheries on the Pacific Coast of North America.Crossref | GoogleScholarGoogle Scholar |
Meyer, J. L., Sale, M. J., Mulholland, P. J., and Poff, N. L. (1999). Impacts of climate change on aquatic ecosystem functioning and health. Journal of the American Water Resources Association 35, 1373–1386.
| Impacts of climate change on aquatic ecosystem functioning and health.Crossref | GoogleScholarGoogle Scholar |
Miller, A. W., Reynolds, A. C., Sobrino, C., and Riedel, G. F. (2009). Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries. PLoS ONE 4, e5661.
| Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries.Crossref | GoogleScholarGoogle Scholar |
Minteer, B. A., and Collins, J. P. (2010). Move it or lose it? The ecological ethics of relocating species under climate change. Ecological Applications 20, 1801–1804.
| Move it or lose it? The ecological ethics of relocating species under climate change.Crossref | GoogleScholarGoogle Scholar |
Morrongiello, J. R., Crook, D. A., King, A. J., Ramsey, D. S., and Brown, P. (2011a). Impacts of drought and predicted effects of climate change on fish growth in temperate Australian lakes. Global Change Biology 17, 745–755.
| Impacts of drought and predicted effects of climate change on fish growth in temperate Australian lakes.Crossref | GoogleScholarGoogle Scholar |
Morrongiello, J. R., Beatty, S. J., Bennett, J. C., Crook, D. A., Ikedife, D. N. E. N., Kennard, M. J., Kerezsy, A., Lintermans, M., McNeil, D. G., Pusey, B. J., and Rayner, T. (2011b). Climate change and its implications for Australia’s freshwater fish. Marine and Freshwater Research 62, 1082–1098.
| Climate change and its implications for Australia’s freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Morrongiello, J. R., Bond, N. R., Crook, D. A., and Wong, B. B. M. (2011c). Eucalyptus leachate inhibits reproduction in a freshwater fish. Freshwater Biology , .
| Eucalyptus leachate inhibits reproduction in a freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Moss, R. H., Edmonds, J. A., Hibbard, K. A., Manning, M. R., Rose, S. K., van Vuuren, D. F., Carter, T. R., Emori, S., Kainuma, M., Kram, T., Meehl, G. A., Mitchel, J. F. B., Nakicenovic, N., Riahi, K., Smith, S. J., Stouffer, R. J., Thomson, A. M., Weyant, J. P., and Wilbanks, T. J. (2010). The next generation of scenarios for climate change research and assessment. Nature 463, 747–756.
| The next generation of scenarios for climate change research and assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvVKqs7w%3D&md5=4695ffc4d8a122b482b24da99ae49afaCAS |
Munday, P. L., Jones, G. P., Pratchett, M. S., and Williams, A. (2008). Climate change and the future for coral reef fishes. Fish and Fisheries 9, 261–285.
| Climate change and the future for coral reef fishes.Crossref | GoogleScholarGoogle Scholar |
Munday, P. L., Leis, J. M., Lough, J. M., Paris, C. B., Kingsford, M. J., Berumen, M. L., and Lambrechts, J. (2009). Climate change and coral reef connectivity. Coral Reefs 28, 379–395.
| Climate change and coral reef connectivity.Crossref | GoogleScholarGoogle Scholar |
Murphy, B. F., and Timbal, B. (2008). A review of recent climate variability and climate change in southeastern Australia. International Journal of Climatology 28, 859–879.
| A review of recent climate variability and climate change in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Murray–Darling Basin Commission (2004). ‘Native Fish Strategy for the Murray–Darling Basin 2003–2013.’ (Murray–Darling Basin Commission: Canberra.) Available at www.mdbc.gov.au [accessed 8 June 2011].
Naithani, J., Plisnier, P.-D., and Deleersnijder, E. (2011). Possible effects of global climate change on the ecosystem of Lake Tanganyika. Hydrobiologia 671, 147–163.
| Possible effects of global climate change on the ecosystem of Lake Tanganyika.Crossref | GoogleScholarGoogle Scholar |
Nicol, S. J., Lieschke, J., Lyon, J., and Koehn, J. D. (2004). Observations on the distribution and abundance of carp and native fish, and their responses to a habitat restoration trial in the Murray River, Australia. New Zealand Journal of Marine and Freshwater Research 38, 541–551.
| Observations on the distribution and abundance of carp and native fish, and their responses to a habitat restoration trial in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
Nieblas, A. E., Sloyan, B. M., Hobday, A. J., Coleman, R., and Richardson, A. J. (2009). Variability of biological production in low wind-forced regional upwelling systems: a case study off southeastern Australia. Limnology and Oceanography 54, 1548–1558.
| Variability of biological production in low wind-forced regional upwelling systems: a case study off southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Noell, C. J., Ye, Q., Short, D. A., Bucater, L. B., and Wellman, N. R. (2009). Fish assemblages of the Murray Mouth and Coorong region, South Australia, during an extended drought period. CSIRO, Water for a Healthy Country National Research Flagship, Canberra.
Norman-Lopez, A., Pascoe, S., and Hobday, A. J. (In press). Economic impacts of ignoring climate change for Australian fisheries and associated sectors. Climate Change Economics. , .
Olden, J. D., Kennard, M. J., Lawler, J. J., and Poff, N. L. (2011). Challenged and opportunities in implementing managed relocation for conservation of freshwater species. Conservation Biology 25, 40–47.
| Challenged and opportunities in implementing managed relocation for conservation of freshwater species.Crossref | GoogleScholarGoogle Scholar |
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R. M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R. G., Plattner, G.-K., Rodgers, K. B., Sabine, C. L., Sarmiento, J. L., Schlitzer, R., Slater, R. D., Totterdell, I. J., Weirig, M.-F., Yamanaka, Y., and Yool, A. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686.
| Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVCjsL%2FE&md5=9390bc0fada8743ec20f80a7ffe47e99CAS |
Ottersen, G., Hjermann, D., and Stenseth, N. C. (2006). Changes in spawning stock structure strengthens the link between climate and recruitment in a heavily fished cod stock. Fisheries Oceanography 15, 230–243.
| Changes in spawning stock structure strengthens the link between climate and recruitment in a heavily fished cod stock.Crossref | GoogleScholarGoogle Scholar |
Pankhurst, N. W., and King, H. R. (2010). Temperature and salmonid reproduction: implications for aquaculture. Journal of Fish Biology 76, 69–85.
| Temperature and salmonid reproduction: implications for aquaculture.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cjnsV2qtQ%3D%3D&md5=d0d625b57b8a103bea2b8bc79dc91636CAS |
Pankhurst, N. W., and Munday, P. L. (2011). Effects of climate change on fish reproduction and early life history stages. Marine and Freshwater Research 62, 1015–1026.
| Effects of climate change on fish reproduction and early life history stages.Crossref | GoogleScholarGoogle Scholar |
Perry, A. L., Low, P. J., Ellis, J. R., and Reynolds, J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915.
| Climate change and distribution shifts in marine fishes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlsVWmtbg%3D&md5=b99cf337ddaccb32659fd2b7ab3c4576CAS |
Pittock, J., Hansen, L. J., and Abell, R. (2008). Running dry: freshwater biodiversity, protected areas and climate change. Biodiversity 9, 30–38.
Plagányi, E. E., Bell, J. D., Bustamante, R. H., Dambacher, J. M., Dennis, D. M., Dichmont, C. M., Dutra, L. X. C., Fulton, E. A., Hobday, A. J., van Putten, E. I., Smith, F., Smith, A. D. M., and Zhou, S. (2011). Modelling climate-change effects on Australian and Pacific aquatic ecosystems: a review of analytical tools and management implications. Marine and Freshwater Research 62, 1132–1147.
| Modelling climate-change effects on Australian and Pacific aquatic ecosystems: a review of analytical tools and management implications.Crossref | GoogleScholarGoogle Scholar |
Poloczanska, E. S., Babcock, R. C., Butler, A., Hobday, A. J., Hoegh-Guldberg, O., Kunz, T. J., Matear, R., Milton, D. A., Okey, T. A., and Richardson, A. J. (2007). Climate change and Australian marine life. Oceanography and Marine Biology: An Annual Review 45, 407–478.
Polovina, J. J., Howell, E. A., and Abecassis, M. (2008). Ocean’s least productive waters are expanding. Geophysical Research Letters 35, L03618.
| Ocean’s least productive waters are expanding.Crossref | GoogleScholarGoogle Scholar |
Pörtner, H. O., and Farrell, A. P. (2008). Physiology and climate change. Science 322, 690–692.
| Physiology and climate change.Crossref | GoogleScholarGoogle Scholar |
Pratchett, M. S., Munday, P. L., Wilson, S. K., Graham, N. A. J., Cinner, J. E., Bellwood, D. R., Jones, G. P., Polunin, N. V. C., and Mcclanahan, T. R. (2008). Effects of climate-induced coral bleaching on coral-reef fishes: ecological and economic consequences. Oceanography and Marine Biology: An Annual Review 46, 251–296.
| Effects of climate-induced coral bleaching on coral-reef fishes: ecological and economic consequences.Crossref | GoogleScholarGoogle Scholar |
Pratchett, M. S., Wilson, S. K., Graham, N. A. J., Munday, M. S., Jones, G. P., Polunin, N. V. (2009). Multi-scale temporal effects of climate-induced coral bleaching on motile reef organisms. In ‘Coral Bleaching: Patterns and Processes, Causes and Consequences’. (Eds M. van Oppen and J. Lough.) pp. 139–158. (Springer-Verlag: Heidelberg, Germany.)
Pratchett, M. S., Bay, L. K., Gehrke, P. C., Koehn, J. D., Osborne, K., Pressey, R. L., Sweatman, H. P. A., and Wachenfeld, D. (2011). Contribution of climate change to degradation and loss of critical fish habitats in Australian marine and freshwater environments. Marine and Freshwater Research 62, 1062–1081.
| Contribution of climate change to degradation and loss of critical fish habitats in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Pusey, B. J., Kennard M. J., and Arthington, A. H. (2004). ‘Freshwater Fishes of North-eastern Australia.’ (CSIRO Publishing: Melbourne.)
Richardson, D. M., Hellmann, J. J., McLachlan, J. S., Sax, D. F., Schwartz, M. W., Gonzalez, P., Brennan, E. J., Camacho, A., Root, T. L., Sala, O. E., Schneider, S. H., Ashe, D. M., Rappaport Clark, J., Early, R., Etterson, J. R., Fielder, E. D., Gill, J. L., Minteer, B. A., Polasky, S., Safford, H. D., Thompson, A. R., and Vellend, M. (2009). Multidimensional evaluation of managed relocation. Proceedings of the National Academy of Sciences, USA 106, 9721–9724.
| Multidimensional evaluation of managed relocation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotFGjsr8%3D&md5=f43b29491a927df51a27ef2656996925CAS |
Robinson, L. M., Elith, J., Hobday, A. J., Pearson, R. G., Kendall, B. E., Possingham, H. P., and Richardson, A. J. (2011). Pushing the limits in marine species distribution modelling: lessons from the land present challenges and opportunities. Global Ecology and Biogeography , .
| Pushing the limits in marine species distribution modelling: lessons from the land present challenges and opportunities.Crossref | GoogleScholarGoogle Scholar |
Roessig, J. M., Woodley, C. M., Cech, J. J., and Hansen, L. J. (2004). Effects of global climate change on marine and estuarine fishes and fisheries. Reviews in Fish Biology and Fisheries 14, 251–275.
| Effects of global climate change on marine and estuarine fishes and fisheries.Crossref | GoogleScholarGoogle Scholar |
Rowland, S. J. (2005). Overview of the history, fishery, biology and aquaculture of Murray cod (Maccullochella peelii peellii). In ‘Management of Murray Cod in the Murray–Darling Basin: Statement, Recommendations and Supporting Papers. Proceedings of a Workshop Held in Canberra, ACT, 3–4 June 2004’. (Eds M. Lintermans and B. Phillips.) pp. 38–61. (Murray–Darling Basin Commission and Cooperative Research Centre for Freshwater Ecology: Canberra.) Available at http://www2.mdbc.gov.au/NFS/nfs_publications.html [accessed 8 June 2011].
Russell, B. D., Thompson, J. I., Falkenberg, L. J., and Connell, S. D. (2009). Synergistic effects of climate change and local stressors: CO2 and nutrient-driven change in subtidal rocky habitats. Global Change Biology 15, 2153–2162.
| Synergistic effects of climate change and local stressors: CO2 and nutrient-driven change in subtidal rocky habitats.Crossref | GoogleScholarGoogle Scholar |
Schindler, D. W. (2001). The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Canadian Journal of Fisheries and Aquatic Sciences 58, 18–29.
| The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium.Crossref | GoogleScholarGoogle Scholar |
Short, F. T., and Neckles, H. A. (1999). The effects of global climate change on seagrasses. Aquatic Botany 63, 169–196.
| The effects of global climate change on seagrasses.Crossref | GoogleScholarGoogle Scholar |
Sorte, C. J. B., Williams, S. L., and Carlton, J. T. (2010). Marine range shifts and species introductions: comparative spread rates and community impacts. Global Ecology and Biogeography 19, 303–316.
| Marine range shifts and species introductions: comparative spread rates and community impacts.Crossref | GoogleScholarGoogle Scholar |
Steffen, W. L. (2009). ‘Climatic Change 2009: Faster Change and More Serious Risks.’ (Australian Government, Department of Climate Change: Canberra.)
Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., and Tegner, M. J. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation 29, 436–459.
| Kelp forest ecosystems: biodiversity, stability, resilience and future.Crossref | GoogleScholarGoogle Scholar |
Swales, S., Storey, A. W., Roderick, I. D., and Figa, B. S. (1999). Fishes of floodplain habitats of the Fly River system, Papua New Guinea, and changes associated with El Niño droughts and algal blooms. Environmental Biology of Fishes 54, 389–404.
| Fishes of floodplain habitats of the Fly River system, Papua New Guinea, and changes associated with El Niño droughts and algal blooms.Crossref | GoogleScholarGoogle Scholar |
Thorp, J. H., Thoms, M. C., and Delong, M. D. (2006). The riverine ecosystem synthesis: biocomplexity in river networks across space and time. River Research and Applications 22, 123–147.
Thompson, P. A., Baird, M. E., Ingelton, T., and Dublin, M. A. (2009). Long-term changes in temperate Australian coastal waters: implications for phytoplankton. Marine Ecology Progress Series 394, 1–19.
| Long-term changes in temperate Australian coastal waters: implications for phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVOmtA%3D%3D&md5=cb15666157e6dd1464014b0c9734ddd1CAS |
Timbal, B., and Jones, D. A. (2008). Future projections of winter rainfall in southeast Australia using a statistical downscaling technique. Climatic Change 86, 165–187.
| Future projections of winter rainfall in southeast Australia using a statistical downscaling technique.Crossref | GoogleScholarGoogle Scholar |
Turner, L., Tracey, D., Tilden, J., and Dennison, W. C. (2004). ‘Where River Meets Sea: Exploring Australia’s Estuaries.’ (CRC for Coastal Zone Estuary and Water Management: Brisbane.)
Valiela, I., Bowen, J. L., and York, J. K. (2001). Mangrove forests: one of the world’s threatened major tropical environments. Bioscience 51, 807–815.
| Mangrove forests: one of the world’s threatened major tropical environments.Crossref | GoogleScholarGoogle Scholar |
Valiela, I., Kinney, E., Bulbertson, J., Peacock, E., and Smith, S. (2009). Global losses of mangroves and salt marshes. In ‘Global Loss of Coastal Habitats; Rates, Causes and Consequences.’ (Ed C. M. Duarte) pp. 107–138 (Fundación BBVA: Bilbao.)
Van Dijk, A., Evans, R., Hairsine, P., Khan, S., Nathan, R., Paydar, Z., Viney, N., and Zhang, L. (2006). ‘Risks to the Shared Water Resources of the Murray–Darling Basin’. (Murray-Darling Basin Commission: Canberra.)
Walker, K. F. (1986). The Murray–Darling river system. In ‘The Ecology of River Systems’. (Eds B.R. Davies and K.F. Walker.) pp. 631–659. (Dr W Junk Publishers: Dordrecht, The Netherlands.)
Walker, K. F., Sheldon, F., and Puckridge, J. T. (1995). A perspective on dryland river ecosystems. Regulated Rivers: Research and Management 11, 85–104.
| A perspective on dryland river ecosystems.Crossref | GoogleScholarGoogle Scholar |
Walther, G-R., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., Fromentin, J.-M., Hoegh-Guldberg, O., and Bairlein, F. (2002). Ecological responses to recent climate change. Nature 416, 389–395.
| Ecological responses to recent climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XislantL8%3D&md5=585bf263e6cdaf7f555072db8bf3257dCAS |
Wang, M., and Overland, J. E. (2009). A sea ice free summer Arctic within 30 years? Geophysical Research Letters 36, L07502.
| A sea ice free summer Arctic within 30 years?Crossref | GoogleScholarGoogle Scholar |
Ward, P. J., Beets, W., Bouwer, L. M., Aerts, J. C. J. H., and Renssen, H. (2010). Sensitivity of river discharge to ENSO. Geophysical Research Letters 37, L12402.
| Sensitivity of river discharge to ENSO.Crossref | GoogleScholarGoogle Scholar |
Waycott, M., Collier, C., McMahon, K., Ralph, P., McKenzie, L., Udy, J., and Grech, A. (2007). Vulnerability of seagrasses in the Great Barrier Reef to climate change. In ‘Climate Change and the Great Barrier Reef: A Vulnerability Assessment’. (Eds J. E. Johnson and P. A. Marshall.) pp. 193–235. (Great Barrier Reef Marine Park Authority and Australian Greenhouse Office: Townsville.)
Waycott, M., Duarte, C. M., Carruthers, T. J. B., Orth, R. J., Dennison, W. C., Olyarnik, S., Calladine, A., Fourqurean, J. W., Heck, J. L., Hughes, R. A., Kendrick, G. A., Kenworthy, W. J., Short, F. T., and Williams, S. L. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences, USA 106, 12 377–12 381.
| Accelerating loss of seagrasses across the globe threatens coastal ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpslGjsbo%3D&md5=34dbad507adea00cecbd4b5134a0e888CAS |
Williams, J. W., and Jackson, S. T. (2007). Novel climates, no-analog communities, and ecological surprises. Frontiers in Ecology and the Environment 5, 475–482.
| Novel climates, no-analog communities, and ecological surprises.Crossref | GoogleScholarGoogle Scholar |
Wilson, S. K., Graham, N. A. J., Pratchett, M. S., Jones, G. P., and Polunin, N. V. C. (2006). Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Global Change Biology 12, 2220–2234.
| Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient?Crossref | GoogleScholarGoogle Scholar |
Wooldridge, S. A. (2009). Water quality and coral bleaching thresholds: formalising the linkage for the inshore reefs of the Great Barrier Reef, Australia. Marine Pollution Bulletin 58, 745–751.
| Water quality and coral bleaching thresholds: formalising the linkage for the inshore reefs of the Great Barrier Reef, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFWgtL4%3D&md5=354b158ca6baf31b16f1636cd4dc5f4bCAS |
Worm, B., Oschlies, A., Lotze, H. K., and Myers, R. A. (2005). Global patterns of predator diversity in the open oceans. Science 309, 1365–1369.
| 1:CAS:528:DC%2BD2MXovVOjtL8%3D&md5=e1988d9d7f5ccb6e71653ad5158fffdcCAS |
Xenopoulos, M. A., Lodge, D. M., Alcamo, J., Märker, M., Shulze, K., and Van Vuuren, D. P. (2005). Scenarios of freshwater fish extinctions from climate change and water withdrawl. Global Change Biology 11, 1557–1564.
| Scenarios of freshwater fish extinctions from climate change and water withdrawl.Crossref | GoogleScholarGoogle Scholar |


