A test of large-scale reproductive migration in females of the amphidromous shrimp Macrobrachium acanthurus (Caridea : Palaemonidae) from south-eastern Brazil
Giovana Bertini A F , J. Antonio Baeza B C D and Eduardo Perez C EA LABCRUST (Laboratório de Biologia e Cultivo de Crustáceos), Universidade Estadual Paulista – UNESP - Campus de Registro – Rua Nelson Brihi Badur, 430 Registro – 11900-000, Brazil; NEBECC (Crustacean Biology, Ecology and Culture Study Group).
B Smithsonian Marine Station at Fort Pierce, 701 Seaway Drive, Fort Pierce, FL 34949, USA.
C Departamento de Biología Marina, Facultad de Ciencias del Mar, Universidad Católica del Norte, Larrondo 1281, Coquimbo, Chile.
D 132 Long Hall, Department of Biological Sciences, Clemson University, Clemson, SC 29634, USA.
E Centro de Estudios Avanzados en Zonas Aridas (CEAZA), Coquimbo, Chile.
F Corresponding author. Email: gibertini@registro.unesp.br
Marine and Freshwater Research 65(1) 81-93 https://doi.org/10.1071/MF13028
Submitted: 30 January 2013 Accepted: 20 June 2013 Published: 20 September 2013
Abstract
Macrobrachium acanthurus inhabits estuaries and rivers in the western Atlantic. It is not clear whether females migrate towards estuaries to hatch larvae, as reported for other congeneric shrimps. We tested whether females of M. acanthurus exhibit reproductive migrations. The population dynamics of this shrimp was studied in the Ribeira de Iguape River, Brazil. Four sites that differ in position with respect to the coast were sampled monthly during 2007. In M. acanthurus, reproduction was seasonal and reproductive intensity did not vary among study sites. Females brooding early and late embryos were found at all study sites during the reproductive season. No disappearance of reproductive females was observed at the study site located furthest away (~150 km) from the coast. Thus, reproductive females of M. acanthurus do not exhibit reproductive migrations towards estuaries to hatch larvae. Maturity in females was reached at smaller body sizes during the austral summer and spring compared with winter and autumn. Growth rate and body size was sex-specific; males grew slower but attained larger average and final body sizes than females. This information needs to be considered in assessing stocks and establishing sustainable management plans for M. acanthurus in Brazil.
Additional keywords: freshwater prawn, growth, maturity, reproduction, sex ratio
Introduction
Shrimps from the infraorder Caridea use a diverse range of habitats and exhibit considerable diversity in terms of life cycles (Bauer 2004; De Grave et al. 2009). Many caridean shrimps are symbiotic with sessile marine invertebrates, others inhabit deep-sea chemoautotrophic environments, a few have conquered semiterrestrial mangrove forests, and a relatively large number of species have colonised freshwater environments (Bauer 2004; De Grave et al. 2009). Most freshwater shrimps pertain to three different monophyletic groups (i.e. the families Atyidae and Xiphocarididae, and the genus Macrobrachium in the family Palaemonidae: see Bauer 2004, 2011) and the life cycle of these shrimps differs widely. In some species, the transition from the marine ancestral environment to the freshwater realm is complete; the entire life cycle takes place in freshwater and parental females brood a few large embryos that hatch either as a crawling juvenile or at an advanced larval stage (e.g. M. ferreirai: Magalhães and Walker 1988; Dugastella valentina: Cuesta et al. 2006). In other species, parental females brood numerous and small embryos that hatch at an early larval (Zoea) stage, the larval period is extended, and larvae do require marine or estuarine conditions to complete development. In some of these species with indirect development, each individual in the population exhibits amphidromous behaviour: larvae released in rivers drift downstream to marine or estuarine habitats and then, after settlement and metamorphosis, postlarvae migrate back to the freshwater environment (Bauer and Delahoussaye 2008; Bauer 2011). This amphidromous life cycle is also shared by a considerable number of freshwater fishes and mollusks (McDowall 2007).
The genus Macrobrachium, with 243 described species, has a worldwide tropical and subtropical distribution (De Grave and Fransen 2011). Some shrimps in this genus are rather small, attaining a total body length of no more than a few centimetres (e.g. M. jelskii) while other species attain large body sizes (>30 cm total length) (Holthuis 1980; Jayachandran 2001). Some species exhibit abbreviated larval development (M. ferreirae) while others have extended larval periods and exhibit an amphidromous life cycle (M. ohione) (Magalhães and Walker 1988; Bauer and Delahoussaye 2008).
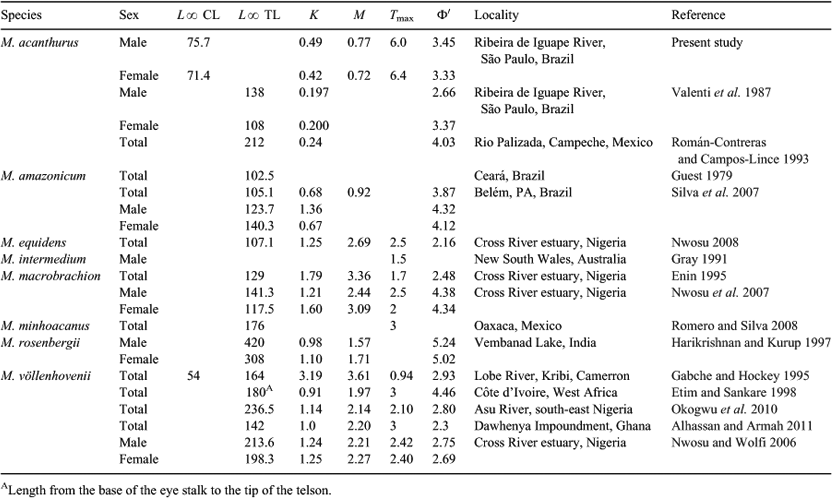
In this study, we were interested in examining the reproductive biology of the shrimp M. acanthurus, a common inhabitant of the western Atlantic (Holthuis 1980; Bond-Buckup and Buckup 1989; Melo 2003). M. acanthurus can be found in estuaries and in rivers up to ~300 km inland and individuals inhabit rocks, underneath death trees, and among aquatic vegetation (Coelho 1963). M. acanthurus exhibits indirect development and females hatch carnivorous planktonic larvae that take 30–40 days to metamorphose (Valenti 1985). In south-eastern Brazil, M. acanthurus reproduces year-round, but with the greatest intensity during the austral summer (Valenti et al. 1986). Males and females attain a maximum body length of 138 mm and 108 mm, respectively (Valenti et al. 1987). Given its large body size, M. acanthurus is exploited by artisanal fisheries in Central and South America, including Brazil (New et al. 2000).
Several details concerning the life history of M. acanthurus are not known. For instance, it is not clear whether females migrate towards estuaries to hatch larvae, as is reported to occur in other congeners (Mohamed and Rao 1971; Lee and Fielder 1979; Read 1985; Bauer and Delahoussaye 2008; John 2009; Rome et al. 2009; Olivier and Bauer 2011), or whether females hatch larvae anywhere along the river. Choudhury (1971) demonstrated that larvae of this species have the ability to live in freshwater for ~1 week after hatching. This suggests the absence of reproductive migrations in M. acanthurus. In contrast, other authors have suggested that females do migrate towards estuaries during each reproductive season (Hughes and Richard 1973; Chávez-Alarcón and Chávez 1976).
In this study, we tested whether or not parental females of M. acanthurus exhibit reproductive migrations – from fully freshwater environments to estuaries for larval hatching. For this purpose, we described the population dynamics of this species at a single basin (i.e. Ribeira de Iguape) but at different study sites located far away or close to the coast. If females do not exhibit reproductive migrations, we expected females brooding early- and late-stage embryos to be consistently present at different study sites along the river both far away (e.g. 150 km from the estuary) and close to the coast during the same reproductive season. Also, we determined site-specific population parameters such as sex ratio, size at first maturity, sex-specific growth rate, and maximum body size, and we compared the parameters above among localities to reveal further details about the life history of this economically valuable shrimp.
Materials and methods
Study site
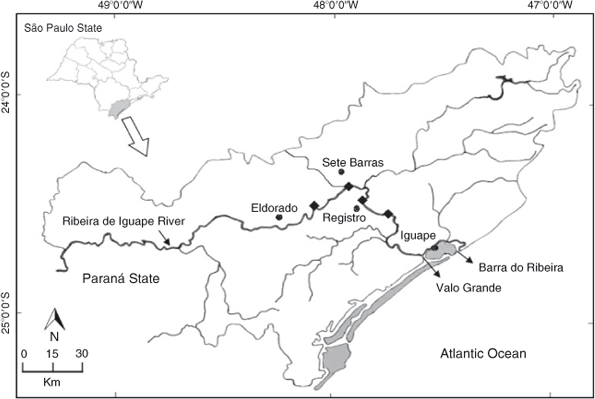
The Ribeira de Iguape basin (23°45′S, 46°45′W to 25°30′S, 50°W), covering an area of ~25 000 km2, is one of the largest basins in south-eastern Brazil (Fig. 1). Following Köppen’s climate classification, most of the Ribeira de Iguape basin has a climate type Af, characterised by rainy tropical weather, the absence of a dry season during the year, and an average precipitation of >60 mm during the driest months of the year (Rolim et al. 2007). In the upper portion of the Ribeira de Iguape River, currents are comparatively strong while in the lower portion of the river, near the estuary, water runs slowly, the river meanders, and swamps, marshes and other areas that surround the river potentially flood during the rainy season (Camargo et al. 1972).
|
Collection of M. acanthurus
Individuals of M. acanthurus were collected once per month, from January to December 2007, at four different sites in the Ribeira de Iguape River. Sampling sites were selected to characterise the reproductive biology of this shrimp along the length of the river. Iguape was located the closest to the mouth of the river (72 km from the mouth), and Registro (114 km), Sete Barras (138 km) and Eldorado (155 km) were progressively located more inland (Fig. 1).
At each study site, shrimps were sampled using a combination of minnow traps and kick nets. A total of 12 minnow traps (1 m length, 30 cm diameter, and 8 mm mesh pore) were baited with small pieces of banana and/or cow bones, placed either among the vegetation near the margin of the river (n = 6 traps) or at the bottom of the river (n = 6 traps), and retrieved after 24 h. In addition, two people with a single kick-net (0.5 m2, 5 mm mesh pore) sampled the margin of the river (against the current) during a total of 20 min specifically focusing on semisubmerged vegetation known to harbour M. acanthurus. Thus, we consistently used a total of 12 replicates (sampling units) per month and per study site throughout the entire sampling period. The rationale for using different sampling methodologies and for sampling different microhabitats at each study site was to capture all ontogenetic stages (recruits, juveniles, and adults), sexes, and body sizes of M. acanthurus in their natural proportions. Thus, we produced a representative picture of the shrimp population structure and sex ratio at each study site throughout the year.
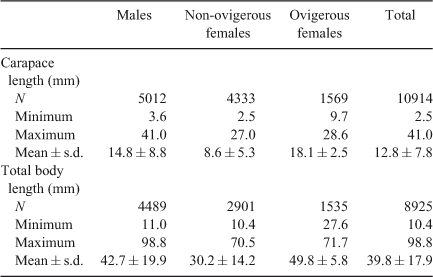
Immediately after collection, shrimps were placed in plastic bags within an ice chest and transported alive to the Laboratory of Crustacean Biology and Cultivation (LABCRUST), UNESP-Registro, Registro, where shrimps were frozen until they were measured and sexed (see below). In total, we collected 20 159 shrimps (n = 11 987, 2878, 2746, and 2548 specimens in Iguape, Registro, Eldorado, and Sete Barras, respectively) during the entire study period. Logistic and time constraints did not permit measuring and sexing each collected individual in such a large sample. Thus, we followed the criterion of Wenner et al. (1991) (also employed in Baeza et al. 2010) to obtain a representative sample from each study site and sampling date. We measured and sexed all individuals in samples comprising 80 or fewer shrimps. In samples containing 80–160 shrimps, we measured 80 haphazardly selected specimens. Lastly, we measured and sexed 50% or 25% of all shrimps in samples comprising 160–320 individuals or more than 321 individuals, respectively. In total, 10 914 shrimps (54.14% of the total capture) were sexed and measured during this study.
The carapace length (CL) of each selected shrimp >10.0 mm CL was measured with a caliper (to the nearest 0.1 mm) as the distance between the orbital angle and the posterior margin of the carapace. Specimens <10.0 mm CL were measured to the nearest 0.025 mm under the stereomicroscope using a graduated ocular micrometer. Shrimps were classified as males or females according to the presence or absence, respectively, of appendix masculina on the second pleopods and gonopores on the coxae of the fifth pereopods. Lastly, each female was classified according to the presence or absence (brooding or non-brooding, respectively) of embryos and the developmental stage of the embryos (early, intermediate, and late) beneath the abdomen: early-stage embryos are those that have been spawned recently and have no eyes and a uniformly distributed yolk; intermediate-stage embryos have visible but not well developed eyes; late-stage embryos have little yolk, well developed eyes, chromatophores, and appendages.
Population dynamics of M. acanthurus
Previous studies on the population dynamics of other shrimp species have shown differences in growth parameters between the sexes (e.g. Andrade and Pérez 2004, 2007). Therefore, during this study, we calculated growth parameters separately for males and females of M. acanthurus. Also, we aimed to study age and growth schedules of M. acanthurus separately for each study site (see Results). Lastly, we also conducted an analysis that included shrimps collected from all study sites to provide a general picture of the life-history schedule of M. acanthurus across the entire basin.
For each study site and for all study sites grouped together, the age and growth of M. acanthurus were determined using length–frequency distributions (LFDs) (Andrade and Pérez 2004, 2007; Baeza et al. 2010). We reconstructed monthly LFDs using 3-mm-CL size intervals, as this scale proved useful for the detection of modes during preliminary data analyses. Next, growth parameters were fitted to our data using the von Bertalanffy growth curve without seasonality (Sparre and Venema 1997):
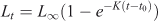
where Lt is the average length estimated at age t, L∞ is the asymptotic maximum length, K is a curvature parameter defining how quickly L∞ is achieved and t0 is the theoretical age at which shrimps would have zero length. This von Bertalanffy model is a useful expression of growth in shrimps (Silliman 1969; Parrack 1979; García and Le Reste 1987) although crustaceans exhibit a discontinuous growth pattern. We estimated the different growth parameters using the software LFDA5 (Kirkwood et al. 2001). This software uses Rn as a measure of goodness of fit for estimated growth parameters. Rn ranges between 0 and 1 and values closer to unity indicate better goodness of fit.
For each sex, expected longevity was estimated using the values obtained above for the different growth parameters. We assumed a minimal number of years necessary for individuals of each sex to reach L∞ (Pauly 1991),
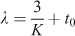
A proxy of the instantaneous rate of natural mortality M for each sex was estimated using Alagaraja’s equation described by Sparre and Venema (1995) as:
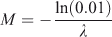
where λ corresponds to longevity of M. acanthurus calculated as the time needed by a cohort to reach maximum average length (estimated by the von Bertalanffy growth equation). Natural mortality was estimated by determining when 99% of a cohort disappears from the population.
Lastly, we were interested in measuring variability in size at first maturity and sex ratio in M. acanthurus throughout the sampling period at the different study sites. Sex ratio was calculated as the number of males divided by the total number of individuals [males plus females] in the population. For each sampling date and site, the observed proportion of males to females was tested for deviations from a 1 : 1 sex ratio using a binomial test (Wilson and Hardy 2002). In females, size at first maturity (L50) was estimated as the CL at which the probability of brooding embryos was 0.5 using logistic regression (Wilson and Hardy 2002). The analysis of size at first maturity was conducted on a per season rather than a monthly basis given the low frequency of females brooding embryos during most months (see Results).
Results
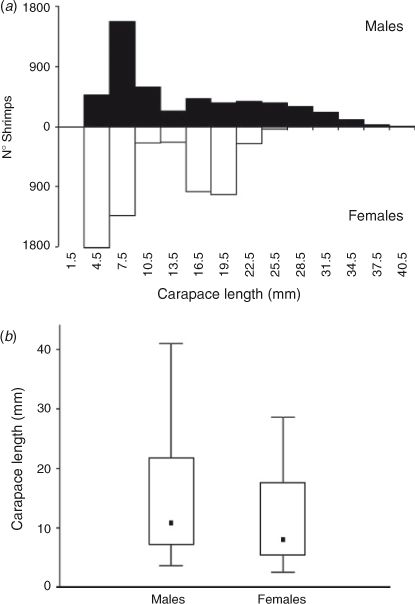
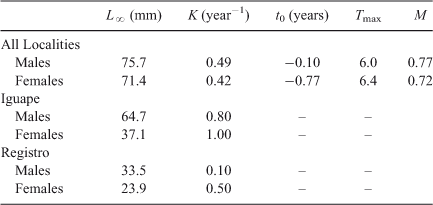
Considering all study sites and months, shrimps varied in body size between 2.5 and 41 mm CL with an average (±s.d.) of 12.8 (±7.8) mm CL (Table 1). A Kolmogorov–Smirnov test demonstrated significant differences in size–frequency distribution between the two sexes (P < 0.05). The median size (50-percentile) was 11 mm CL in males and ~8 mm CL in females. All percentiles were higher for males when compared with those for female shrimps (Fig. 2).
|
|
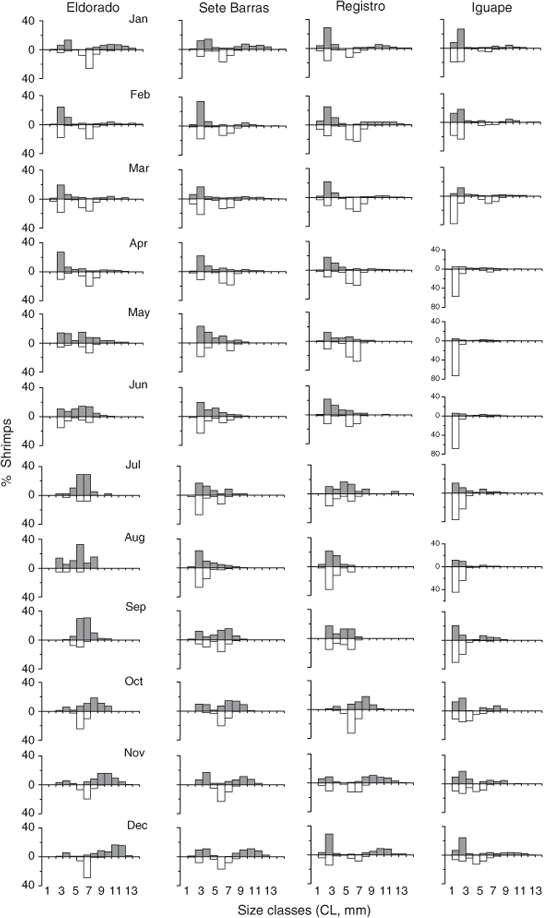
Males and females, both large and small, were found during most of the year at all study sites (Fig. 3).
|
The population-wise von Bertalanffy equations estimated for males and females were
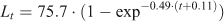
and
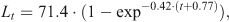
respectively. Natural mortality was slightly greater in males (0.77 year–1) than in females (0.72 year–1). In turn, longevity was slightly shorter in males (6 years) than in females (6.4 years) (Table 2).
|
Small shrimps (<9.0 mm CL) were intermittently found at all study sites (Fig. 3). However, they were more frequently observed at Iguape from April to August compared with the remaining study sites and months. Thus, recruitment in M. acanthurus occurs throughout the river continuum, but is more intense in Iguape during the autumn and winter period.
The greatest differences in population dynamics of M. acanthurus occurred between Eldorado and Iguape, located the furthest from, and closest to, the coast, respectively (Table 2). In Iguape, the population was dominated by small male and female shrimps during the entire year (Fig. 3). The relative abundance of shrimps with intermediate and large body sizes increased only during October and December. By contrast, Eldorado was characterised by the presence of intermediate and large shrimps during most of the year. However, during the spring and summer months (September–December), small recruits were not found at this site (Fig. 3). The largest shrimps in our samples were collected at Eldorado. At Sete Barras and Registro, size–frequency distributions were more similar to those observed at Eldorado and Iguape, respectively. In Sete Barras, shrimps from all body size classes were present during most of the year but small shrimps were absent during October, November, and May. In Registro, the population was dominated by small male and female shrimps during the entire year but adults were common from October to April.
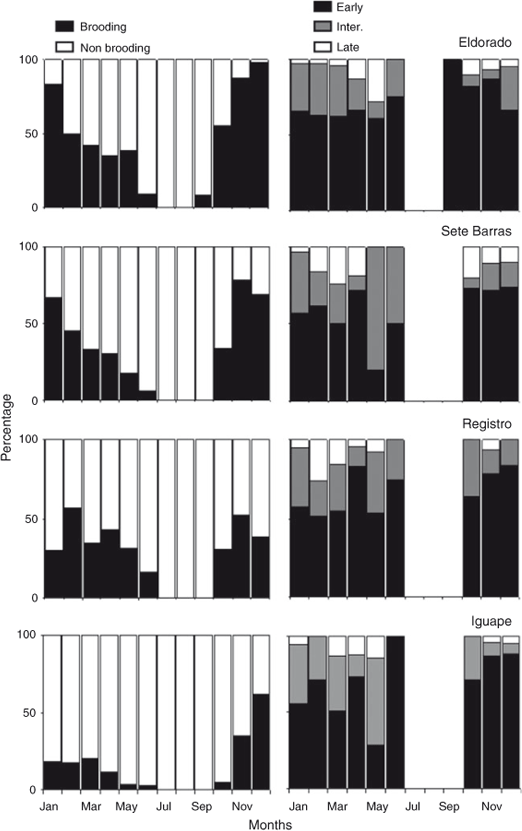
The percentage of brooding females varied considerably during the study period at all study sites (Fig. 4). Brooding females dominated the population (>50%) from January to March but their relative abundance decreased substantially from April to June at all study sites. No brooding females were found during July and August at all study sites. Starting in September, the percentage of brooding females increased to dominate the population during October and December. Interestingly, from January to May, the percentage of brooding females at Iguape and Registro was low compared with that observed at Sete Barras and Eldorado during the same period. Nonetheless, this relatively low percentage of brooding females is explained by the relatively high abundance of small juvenile females at these two sites during the same months. Thus, M. acanthurus reproduces seasonally throughout the studied river portion and there is no indication of females moving towards the mouth of the river during the most intense reproductive period.
|
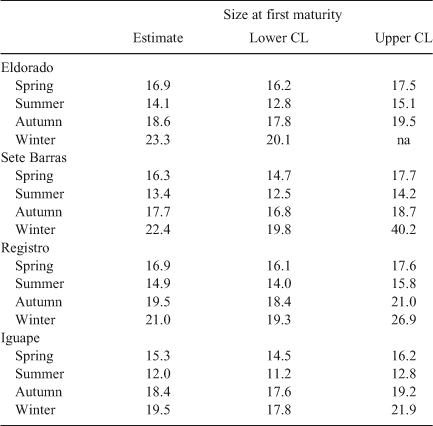
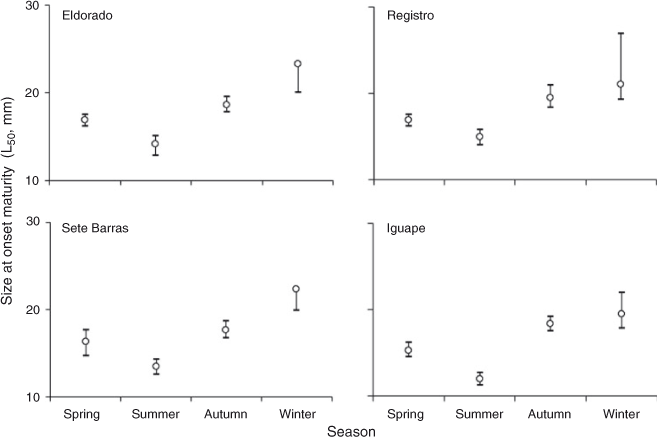
Size at first maturity varied between 12.0 mm CL (95% confidence intervals [CI] = 11.2–12.7 mm CL) at Iguape and 23.3 mm CL (CI = 20.1–n.a. mm CL) at Eldorado (Table 3). Size at first maturity was invariably attained at smaller body sizes during the summer and spring seasons than during the winter and autumn seasons at all study sites (Fig. 5).
|
|
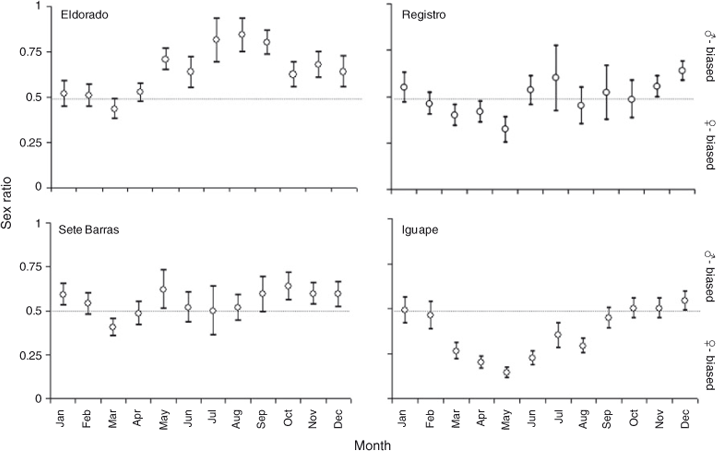
Sex ratios varied between 0.14 (May, at Iguape) and 0.85 (August, at Eldorado) during the study period. Equal sex ratios were observed during most of the year at Sete Barras and Registro. At Eldorado, located the farthest away from the coast, sex ratio was skewed towards males during most of the year (Fig. 6). In turn, at Iguape, located the closest to the coast, sex ratio was skewed towards females during most of the year (Fig. 6).
|
Discussion
In the shrimp M. acanthurus, reproduction at the Ribeira de Iguape River is seasonal, peaking during the warmer months of the year, decreasing during spring and autumn, and almost completely ceasing during the coldest months of the year (July–September). Furthermore, seasonal reproductive activity did not vary much among study sites. Importantly, females carrying embryos in all stages of development were found at all study sites. Also, females of all body sizes were found year around at all study sites. Although, the sex ratio was biased in favour of females from March to September at Iguape, no evident disappearance of large reproductive females (brooding early and late embryos) was observed at this and other study sites located 10–100s of kilometres away from the coast that could have indicated a massive large-scale female reproductive migration. Altogether, the information above indicates that reproductive females of the amphidromous shrimp M. acanthurus do not migrate considerable distances (i.e. hundreds of kilometres) down river to hatch larvae during the reproductive season.
That females of M. acanthurus hatch larvae kilometres away from the coast might imply, at first glance, considerable osmoregulatory stress and decreased survivorship for the larvae as they are expected to spend relatively long periods in fresh water before they reach the estuary. Nonetheless, laboratory experiments have shown that the early larval stages of M. acanthurus can and do survive in fresh water for up to 5–8 days and that the first zoea larva is lecitothropic (Choudhury 1971). Recent studies have also demonstrated that other diadromous species of Macrobrachium are transient hyper–hypo-osmoregulators (with isosmotic points at salinities ~17–21) and that the early larval stages are those with the greatest osmoregulatory ability (Charmantier and Anger 2011). The above suggests that early larvae of M. acanthurus (and other congeneric species) are ‘well equiped’ from a physiological perspective to spend relatively long periods experiencing fully freshwater conditions during transportation to estuaries with proper conditions (more stable and higher salt concentrations) to complete development. Importantly, in the laboratory, larval development of M. acanthurus is successfully accomplished in salinities of 15–20 ppt (Choudhury 1971). In turn, in vitro larvae experiencing salinities above 33 and below 5 ppt, respectively, usually die within 10 days’ exposure (Choudhury 1971). The above suggests that estuarine conditions represent an obligatory requisite for successful development in M. acanthurus.
Importantly, the greatest reproductive activity of M. acanthurus (November–March) coincides with the wet season at the studied latitude, characterised by strong river runoff and currents. Indeed, average water currents during the wet season at the Ribeira de Iguape River vary between 3.5 km h–1 and 4.2 km h–1 (measurements taken during spring and summer of the years 2004, 2005, 2011, and 2012 at Eldorado, Sete Barras, and Registro: information available at Departamento de Águas e Energia Elétrica do estado de São Paulo [www.daee.sp.gov.br]). Taking into account the average water currents above and the distance between Eldorado and the coast, larvae hatched at this study site located the farthest away from the coast (~150 km) need ~37 h for passive transportation to the estuary. Certainly, this period of passive larval transportation is much shorter than the longest period reported for larvae to survive when exposed to fully freshwater conditions (up to 5–8 days: Choudhury 1971). Overall, river conditions during the main reproductive season do facilitate larval transport in M. acanthurus and larvae are fully equipped to resist riverine conditions during passive transportation towards the estuary at the Ribeira de Iguape River.
The reproductive behaviour herein reported for M. acanthurus differs from that observed in several other congeneric species. Parental females migrate towards river mouths and/or estuaries to hatch larvae in M. idella (Mohamed and Rao 1971), M. australiense (Lee and Fielder 1979), M. petersi (Read 1985), M. rosenbergii (John 2009) and M. ohione (Bauer and Delahoussaye 2008; Rome et al. 2009; Olivier and Bauer 2011). On the other hand, the reproductive behaviour herein reported for M. acanthurus is similar to that observed in several other congeneric species. Females exhibit no obvious migrations and newly hatched zoea I larvae drift down river aided by water currents in M. malcolmsonii (Rao 1986; Prasad and Kanaujia 2006), M. gangeticum (Rao 1986; Prasad and Kanaujia 2006), M. nobilii (Balasundaram and Pandian 1982), M. carcinus (March et al. 1998), M. crenulatum (March et al. 1998), M. faustinum (March et al. 1998), M. heterochirus (March et al. 1998), and M. lanchesteri (Phone et al. 2005). This life-history variability suggests that Macrobrachium shrimps can be used as a model system to understand those conditions driving amphidromy in organisms that have colonised freshwater environments.
This study suggests that M. acanthurus grows quickly. Females attain 23 and 37 mm CL two and 12 months after larval settlement, respectively. Also, males attain a smaller body size than females: 12 and 32 mm CL, two and 12 months after larval settlement, respectively. Previous studies have reported rapid growth rates in other species of Macrobrachium (Kanaujia et al. 1997; Romero and Silva 2008; Alhassan and Armah 2011) (see review in Table 4). Also, sex-specific growth rates, as shown here for M. acanthurus, have been reported before in other crustaceans (e.g. penaeid shrimps: Andrade and Pérez 2004; caridean shrimps: Sánchez-Palacios et al. 2008). Our results agree, but only partially, with those of Choudhury (1970) who reported an initial body size of 6.5 mm total body length (TL) and 27 mm TL for juveniles 40 days and two months after metamorphosis, respectively (data obtained from laboratory-reared specimens after larval development). Using previously published equations that relate CL to TL (CL = 0.533 × TL and CL = 0.5043 × TL in males and females, respectively: Albertoni et al. 2002), our results fit well with those previously reported by Choudhury (1970) for males but not for females. An overestimation of the t0 parameter, herein reported to be 0.77 in females versus 0.11 in males, might explain differences in initial body size between our study and that of Choudhury (1970). Such initial body size is expected to be similar in males and females. Studies examining differences in initial body size (i.e. at metamorphosis) between the two sexes are needed to reveal whether or not differences in growth rate between the two sexes are natural or due to parameter overestimation.
In M. acanthurus, males grow more slowly than females but attain larger average and final body sizes. This pattern of sexual dimorphism (males > females) agrees with previous observations on the same species (see Table 4 and references therein). Nonetheless, the maximum body sizes herein reported for males and females are larger than those reported before for the same species. For instance, Valenti et al. (1987) reported maximum body sizes of 138 mm TL (or 69.5 mm CL calculated using the equation that relates CL to TL: Albertoni et al. 2002) and 108 mm TL (or 57.6 mm CL) in males and females, respectively. The reasons for dissimilarities between our results and those of Valenti et al. (1987) remain to be addressed. Sexual dimorphism is also known in several other species of Macrobrachium (e.g. M. rosenbergii: Sampaio and Valenti 1996; M. olfersi: Mossolin and Bueno 2003; M. iheringi: Fransozo et al. 2004). Our results in M. acanthurus support the notion that classical sexual dimorphism (males > females) is more common than reverse sexual dimorphism (e.g. females > males in M. ohione: Bauer and Caskey 2006) within the genus. In M. acanthurus, sexual dimorphism might be explained by a ‘large male advantage’. Large body size might increase male mating opportunities if monopolisation of receptive females occurs via overt aggression, as reported before in other congeneric shrimps (e.g. M. rosenbergii: Sampaio and Valenti 1996). Experiments examining male mating tactics in the presence and absence of receptive females might help reveal the conditions that explain sexual dimorphism in M. acanthurus.
Based on logistic regression, size at first sexual maturity (CL50) in females of M. acanthurus varied between 12.0 and 23.3 mm CL among sites throughout the study period. Importantly, visual examination of the data indicated no major differences in size at first maturity among study sites. However, size at first maturity was invariably attained at smaller body sizes during summer and spring than during winter and autumn at all study sites. Studies on seasonal variation in size at first maturity are rare among crustaceans, including freshwater shrimps. However, shifts in size at first maturity in fish are commonly reported (Beacham 1983; Gerritsen et al. 2003; Armstrong et al. 2004). Among shrimps, Oh et al. (1999) compared size at first maturity in Crangon crangon over four years and observed no major differences in this parameter through time. On the other hand, in agreement with our results in M. acanthurus, size at first maturity is reported to decrease during warm months and when reproduction is intense in other shrimps (e.g. Parhippolyte misticia: Onaga et al. 2012). Laboratory experiments have demonstrated that temperature and photoperiod affect the timing of size at (female) maturity in protandric simultaneous hermaphroditic shrimps (Bauer 2002). In M. acanthurus, a decrease in size at first maturity during the main breeding season (when environmental conditions are optimal for reproduction) might be adaptive if early-maturing females produce more broods than late-maturing females. Additional experimental studies are needed to understand abiotic and biotic conditions driving the timing of size at first sexual maturity in M. acanthurus.
We have reviewed previous studies reporting size at first sexual maturity in M. acanthurus along the Brazilian coast and found that our estimates (12.0–23.3 mm CL) are considerably larger to those reported by Anger and Moreira (1998) (6.9 mm CL) for M. acanthurus inhabiting São Sebastião, northern coast of São Paulo State. A posteriori, we believe that our values are overestimated due to the relatively large number of adult females not brooding embryos observed at each study site throughout the year. Indeed, the body size of the smallest female carrying eggs observed during this study was 9.7 mm CL. This value is similar to that reported by Anger and Moreira (1998). Overall, uncertainty in size at maturity might have important implications for fisheries stock assessment (J. Anderson and J. A. Baeza, unpubl. data). We argue in favour of studies constructing an age-structured model to evaluate the effect of uncertainty in size at maturity on population assessments of M. acanthurus.
In M. acanthurus, sex ratio varied considerably (between 0.14 and 0.85) among study sites throughout the study period. Equal sex ratios were observed during most of the year in Sete Barras and Registro. At Eldorado, located the farthest away from the coast, the population was dominated by males during most of the year. In turn, at Iguape, located the closest to the coast, sex ratios were skewed towards females during most of the year. The differences in sex ratio among localities are puzzling. For gonochoric (separate sexes) species such as M. acanthurus, sex allocation theory predicts equal sex ratios as parents should produce an equal number of male and female offspring because of frequency-dependent selection against the more common sex in the population (Fisher 1930). However, female or male skewed population-wise sex ratios have been reported before in other shrimps (Alon and Stancyk 1982; Bauer and Abdalla 2001; Fransozo et al. 2004; Mejía-Ortíz and Alvarez 2010) and major causes for imbalances in sex ratio among marine invertebrates include sex-specific growth and/or mortality rates (Wenner 1972), sex-dependant migration (Wenner 1972; Costa et al. 2010), sex-dependant mating behaviours (Willson and Pianka 1963; Castilho et al. 2008), and/or sampling error (Bolaños et al. 2012). Sex-dependant migration does not appear to occur in the studied river. Also, we have taken precautions during this study to avoid any sampling error that might result in sex ratio biases. However, we cannot discard sex-specific growth rates, mortality rates, and/or mating behaviours driving biased sex ratios in some but not in other localities.
Here we have shown that reproduction is seasonal in the amphidromous shrimp M. acanthurus and that females do not migrate down river towards the estuary to hatch larvae. Indeed, we have shown that parental females of M. acanthurus liberate offspring throughout the river, even when inhabiting sites located hundreds of kilometres from the coast. Also, population-level reproductive parameters, including size at first maturity and sex ratio, vary considerably at moderate spatial scales throughout the year. The information generated in this study needs to be considered in assessing stocks and establishing sustainable management plans for M. acanthurus in south-eastern Brazil.
Acknowledgements
GB thanks the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for providing financial support (05/56131-0). We are also thankful to students and technicians for their help during field work. Sampling was conducted according to the São Paulo state and Brazilian federal laws. JAB is most grateful to Maria Lucia Negreiros-Fransozo, Paula Araujo, Adilson Fransozo, Alexandre Oliveira de Almeida, Ricardo Cunha Lima and the Sociedade Brasileira de Carcinologia that make possible his visit to Brazil during 2010 and this collaboration. This is contribution number 924 of the Smithsonian Marine Station at Fort Pierce.
References
Albertoni, E., Palma-Silva, C., and Esteves, F. (2002). Distribution and growth in adults of Macrobrachium acanthurus (Decapoda, Palaemonidae) in a tropical coastal lagoon, Brazil. Revista Brasileira de Zoologia 19, 61–70.| Distribution and growth in adults of Macrobrachium acanthurus (Decapoda, Palaemonidae) in a tropical coastal lagoon, Brazil.Crossref | GoogleScholarGoogle Scholar |
Alhassan, E., and Armah, A. (2011). Population dynamics of the African river prawn, Macrobrachium vollenhovenii, in Dawhenya impoundment. Turkish Journal of Fisheries and Aquatic Sciences 11, 115–121.
| Population dynamics of the African river prawn, Macrobrachium vollenhovenii, in Dawhenya impoundment.Crossref | GoogleScholarGoogle Scholar |
Alon, N. C., and Stancyk, S. E. (1982). Variation in life-history patterns of the grass shrimp Palaemonetes pugio in two South Carolina estuarine systems. Marine Biology 68, 265–276.
| Variation in life-history patterns of the grass shrimp Palaemonetes pugio in two South Carolina estuarine systems.Crossref | GoogleScholarGoogle Scholar |
Andrade, G., and Pérez, E. P. (2004). Age and growth of the white shrimp Litopenaeus schmitti in western Venezuela. Interciencia 29, 212–218.
Andrade, G., and Pérez, E. P. (2007). Comparación de métodos de estimación de crecimiento del camarón blanco Litopenaeus schmitti en el Occidente de Venezuela. Interciencia 32, 41–47.
Anger, K., and Moreira, G. S. (1998). Morphometric and reproductive traits of tropical caridean shrimps. Journal of Crustacean Biology 18, 823–838.
| Morphometric and reproductive traits of tropical caridean shrimps.Crossref | GoogleScholarGoogle Scholar |
Armstrong, M. J., Gerritsen, H. D., Allen, M., McCurdy, W. J., and Peel, J. A. D. (2004). Variability in maturity and growth in a heavily exploited stock: cod (Gadus morhua L.) in the Irish Sea. ICES Journal of Marine Science 61, 98–112.
| Variability in maturity and growth in a heavily exploited stock: cod (Gadus morhua L.) in the Irish Sea.Crossref | GoogleScholarGoogle Scholar |
Baeza, J. A., Braga, A. A., López-Greco, L. S., Perez, E., Negreiros-Fransozo, M. L., and Fransozo, A. (2010). Population dynamics, sex ratio and size at sex change in a protandric-simultaneous hermaphrodite, the spiny shrimp Exhippolysmata oplophoroides. Marine Biology 157, 2643–2653.
| Population dynamics, sex ratio and size at sex change in a protandric-simultaneous hermaphrodite, the spiny shrimp Exhippolysmata oplophoroides.Crossref | GoogleScholarGoogle Scholar |
Balasundaram, C., and Pandian, T. J. (1982). Yolk energy utilization in Macrobrachium nobilii (Henderson & Mathai). Journal of Experimental Marine Biology and Ecology 61, 125–131.
| Yolk energy utilization in Macrobrachium nobilii (Henderson & Mathai).Crossref | GoogleScholarGoogle Scholar |
Bauer, R. T. (2002). Test of hypotheses on the adaptive value of an extended male phase in the hermaphroditic shrimp Lysmata wurdemanni (Caridea: Hippolytidae). The Biological Bulletin 203, 347–357.
| Test of hypotheses on the adaptive value of an extended male phase in the hermaphroditic shrimp Lysmata wurdemanni (Caridea: Hippolytidae).Crossref | GoogleScholarGoogle Scholar | 12480725PubMed |
Bauer, R. T. (2004). ‘Remarkable Shrimps: Natural History and Adaptations of the Carideans.’ (University of Okalahoma Press: Norman, OK.)
Bauer, R. T. (2011). Amphidromy and migrations of freshwater shrimps. I. Costs, benefits, evolutionary origins, and an unusual case of amphidromy. In ‘New Frontiers in Crustacean Biology. Proceedings of the TCS Summer Meeting, Tokyo, 20–24 September 2009’. (Ed. A. Asakura.) pp. 145–156. (Brill: Leiden, The Netherlands.)
Bauer, R. T., and Abdalla, J. A. (2001). Male mating tactics in the shrimp Palaemonetes pugio (Decapoda, Caridea): precopulatory mate guarding vs. pure searching. Ethology 107, 185–199.
| Male mating tactics in the shrimp Palaemonetes pugio (Decapoda, Caridea): precopulatory mate guarding vs. pure searching.Crossref | GoogleScholarGoogle Scholar |
Bauer, R. T., and Caskey, J. L. (2006). Flagellar setae of the second antennae in decapod shrimps: sexual dimorphism and possible role in detection of contact sex pheromones. Invertebrate Reproduction & Development 49, 51–60.
| Flagellar setae of the second antennae in decapod shrimps: sexual dimorphism and possible role in detection of contact sex pheromones.Crossref | GoogleScholarGoogle Scholar |
Bauer, R. T., and Delahoussaye, J. (2008). Life history migrations of the amphidromous river shrimp Macrobrachium ohione from a continental large river system. Journal of Crustacean Biology 28, 622–632.
| Life history migrations of the amphidromous river shrimp Macrobrachium ohione from a continental large river system.Crossref | GoogleScholarGoogle Scholar |
Beacham, T. D. (1983). Variability in median size and age at sexual maturity of Atlantic cod (Gadus morhua) on the Scotian shelf in the northwest Atlantic Ocean. Fishery Bulletin 81, 303–312.
Bolaños, J. A., Baeza, J. A., Hernandez, J. E., Lira, C., and López, R. (2012). Population dynamics and reproductive output of the nonindigenous crab Charybdis hellerii in the southeastern Caribbean Sea. Journal of the Marine Biological Association of the United Kingdom 92, 469–474.
| Population dynamics and reproductive output of the nonindigenous crab Charybdis hellerii in the southeastern Caribbean Sea.Crossref | GoogleScholarGoogle Scholar |
Bond-Buckup, G., and Buckup, L. (1989). Os Palaemonidae de águas continentais de Brasil Meridional (Crustacea, Decapoda). Revista Brasileira de Biologia 49, 883–896.
Camargo, J. C., Pinto, S. A. F., and Troppmair, H. (1972). Estudo fitogeográfico ecológico da Bacia Hidrográfica Paulista do Rio da Ribeira. São Paulo, Instituto de Geografia da USP, Séria Biogeografia No. 5.
Castilho, A. L., Costa, R. C., Fransozo, A., and Negreiros-Fransozo, M. L. (2008). Reproduction and recruitment of the South American red shrimp Pleoticus muelleri (Crustacea, Solenoceridae), from the southeastern coast of Brazil. Marine Biology Research 4, 361–368.
| Reproduction and recruitment of the South American red shrimp Pleoticus muelleri (Crustacea, Solenoceridae), from the southeastern coast of Brazil.Crossref | GoogleScholarGoogle Scholar |
Charmantier, G., and Anger, K. (2011). Ontogeny of osmoregulatory patterns in the South American shrimp Macrobrachium amazonicum: loss of hypo-regulation in a land-locked population indicates phylogenetic separation from estuarine ancestors. Journal of Experimental Marine Biology and Ecology 396, 89–98.
| Ontogeny of osmoregulatory patterns in the South American shrimp Macrobrachium amazonicum: loss of hypo-regulation in a land-locked population indicates phylogenetic separation from estuarine ancestors.Crossref | GoogleScholarGoogle Scholar |
Chávez-Alarcón, Z., and Chávez, E. A. (1976). Introducción al conocimiento de la biologia del langostino Macrobrachium carcinus (L.) en el Estado de Veracruz. In ‘Memorias del Simposium de la Biología y Dinámica Poblacional de Camarones, Guaymas, Sonora, 8–13 Agosto 1976’. pp 13–23. (México).
Choudhury, P. C. (1970). Complete larval development of the palaemonid shrimp Macrobrachium acanthurus (Wiegmann, 1836), reared in the laboratory. Crustaceana 18, 113–132.
| Complete larval development of the palaemonid shrimp Macrobrachium acanthurus (Wiegmann, 1836), reared in the laboratory.Crossref | GoogleScholarGoogle Scholar |
Choudhury, P. C. (1971). Laboratory rearing of larvae of the palaemonid shrimp Macrobrachium acanthurus (Wiegmann, 1836). Crustaceana 21, 113–126.
| Laboratory rearing of larvae of the palaemonid shrimp Macrobrachium acanthurus (Wiegmann, 1836).Crossref | GoogleScholarGoogle Scholar |
Coelho, P. A. (1963). Observações preliminares sobre a biologia e a pesca de camarões do gênero Macrobrachium Bate, 1868 (Decapoda: Palaemonidae) no Estado de Pernambuco. Trabalhos do Instituto Oceanográfico da Universidade de Recife 3/4, 75–81.
Cuesta, J. A., Palacios-Theil, E., Drake, P., and Rodríguez, A. (2006). A new rare case of parental care in decapods. Crustaceana 79, 1401–1405.
| A new rare case of parental care in decapods.Crossref | GoogleScholarGoogle Scholar |
Costa, R. C., Branco, J. O., Machado, I. F., Campos, B. R., and Avila, M. G. (2010). Population biology of shrimp Artemesia longinaris Bate, 1888 (Crustacea, Decapoda, Penaeidae) from the south coast of Brazil. Journal of the Marine Biological Association of the United Kingdom 90, 663–669.
| Population biology of shrimp Artemesia longinaris Bate, 1888 (Crustacea, Decapoda, Penaeidae) from the south coast of Brazil.Crossref | GoogleScholarGoogle Scholar |
De Grave, S., and Fransen, C. H. J. M. (2011). Carideorum Catalogus: The Recent species of the Dendrobranchiate, Stenopodidean, Procarididean and Caridean shrimps (Crustacea: Decapoda). Zoologische Mededeelingen 85, 195–589.
De Grave, S., Pentcheff, N. D., Ahyong, S. T., Chan, T.-Y., Crandall, K. A., Dworschak, P. C., Felder, D. L., Feldmann, R. M., Fransen, C. H. J. M., Goulding, L. Y. D., Lemaitre, R., Low, M. E. Y., Martin, J. W., Ng, P. K. L., Schweitzer, C. E., Tan, S. H., Tshudy, D., and Wetzer, R. A. (2009). Classification of living and fossil genera of decapod crustaceans. The Raffles Bulletin of Zoology 21, 1–109.
Enin, U. I. (1995). First estimates of growth, mortality and recruitment parameters of Macrobrachium macrobrachion Herklots 1851, in the Cross River Estuary, Nigeria. Dana 11, 29–38.
Etim, L., and Sankare, Y. (1998). Growth and mortality, recruitment and yield of the freshwater shrimp Macrobrachium völlenhovenii, Herklots 1851 (Crustacea, Palaemonidae) in the Fahe reservoir, Côte d’Ivoire, West Africa. Fisheries Research 38, 211–223.
| Growth and mortality, recruitment and yield of the freshwater shrimp Macrobrachium völlenhovenii, Herklots 1851 (Crustacea, Palaemonidae) in the Fahe reservoir, Côte d’Ivoire, West Africa.Crossref | GoogleScholarGoogle Scholar |
Fisher, R. A. (1930). ‘The Genetical Theory of Natural Selection.’ (Oxford University Press: New York.)
Fransozo, A., Rodrigues, F. D., Freire, F. A. M., and Costa, R. C. (2004). Reproductive biology of the freshwater prawn Macrobrachium iheringi (Ortmann, 1897) (Decapoda, Caridea, Palaemonoidea) in the Botucatu region, São Paulo, Brazil. Nauplius 12, 119–126.
Gabche, C. E., and Hockey, H. U. P. (1995). Growth and mortality of the giant African river prawn Macrobrachium völlenhovenii (Herklots: Crustacea: Palaemonidae) in the Lobe River, Cameroon: a preliminary evaluation. Journal of Shellfish Research 14, 185–190.
García, S., and Le Reste, L. (1987). Ciclos vitales, dinámica, explotación y ordenación de las poblaciones de camarones peneidos costeros. Documento Técnico de Pesca No. 203, FAO. Rome, Italy.
Gerritsen, H. D., Armstrong, M. J., Allen, M., McCurdy, W. J., and Peel, J. A. D. (2003). Variability in maturity and growth in a heavily exploited stock: whiting (Merlangius merlangus L.) in the Irish Sea. Journal of Sea Research 49, 69–82.
| Variability in maturity and growth in a heavily exploited stock: whiting (Merlangius merlangus L.) in the Irish Sea.Crossref | GoogleScholarGoogle Scholar |
Gray, C. A. (1991). Temporal variability in the demography of the palaemonid prawn Macrobrachium intermedium in two seagrasses. Marine Ecology Progress Series 75, 227–237.
| Temporal variability in the demography of the palaemonid prawn Macrobrachium intermedium in two seagrasses.Crossref | GoogleScholarGoogle Scholar |
Guest, W. C. (1979). Laboratory life history of the palaemonid shrimp Macrobrachium amazonicum (Heller) (Decapoda, Palaemonidae). Crustaceana 37, 141–152.
| Laboratory life history of the palaemonid shrimp Macrobrachium amazonicum (Heller) (Decapoda, Palaemonidae).Crossref | GoogleScholarGoogle Scholar |
Harikrishnan, M., and Kurup, B. M. (1997). Growth, mortality and exploitation of male and female populations of Macrobrachium rosenbergii (de Man) in the Vembanad Lake, India. Indian Journal of Fisheries 44, 337–344.
Holthuis, L. B. (1980). FAO species catalogues. Shrimp and prawns of the world. An annotated catalogue of species on interest to fisheries. FAO Fisheries Synopsis, Rome.
Hughes, D. A., and Richard, J. D. (1973). Some current-directed movements of M. acanthurus (Wiegmann, 1836) (Decapoda, Palaemonidae) under laboratory conditions. Ecology 54, 927–929.
| Some current-directed movements of M. acanthurus (Wiegmann, 1836) (Decapoda, Palaemonidae) under laboratory conditions.Crossref | GoogleScholarGoogle Scholar |
Jayachandran, K. V. (2001). ‘Palaemonid Prawns – Biodiversity, Taxonomy, Biology and Management.’ (Science Publishers Inc.: Enfield, NH.)
John, E. (2009). Physico-chemical studies of river Pumba and distribution of prawn, Macrobrachium rosenbergii. Journal of Environmental Biology 30, 709–712.
| 1:CAS:528:DC%2BD1MXht1WisrfK&md5=013e004c2e4efbe0993c4d8cf5b6462bCAS | 20136053PubMed |
Kanaujia, D., Mohanty, A., and Tripathi, S. (1997). Growth and production of Indian river prawn Macrobrachium malcolmsonii (H. Milne Edwards) under pond conditions. Aquaculture 154, 79–85.
| Growth and production of Indian river prawn Macrobrachium malcolmsonii (H. Milne Edwards) under pond conditions.Crossref | GoogleScholarGoogle Scholar |
Kirkwood, G. P., Aukland, R., and Zara, S. J. (2001). Length frequency distribution analysis (LFDA). Version 5.0. MRAG Ltd. London, UK.
Lee, C. L., and Fielder, D. R. (1979). A mass migration of the freshwater prawn, Macrobrachium australiense Holthuis, 1950 (Decapoda, Palaemonidae). Crustaceana 37, 219–222.
| A mass migration of the freshwater prawn, Macrobrachium australiense Holthuis, 1950 (Decapoda, Palaemonidae).Crossref | GoogleScholarGoogle Scholar |
Magalhães, C., and Walker, I. (1988). Larval development and ecological distribution of central amazonian palaemonid shrimps (Decapoda, Caridea). Crustaceana 55, 279–292.
| Larval development and ecological distribution of central amazonian palaemonid shrimps (Decapoda, Caridea).Crossref | GoogleScholarGoogle Scholar |
March, J., Benstead, G. J. P., Pringle, C. M., and Scatena, F. N. (1998). Migratory drift of larval freshwater shrimps in two tropical streams, Puerto Rico. Freshwater Biology 40, 261–273.
| Migratory drift of larval freshwater shrimps in two tropical streams, Puerto Rico.Crossref | GoogleScholarGoogle Scholar |
McDowall, R. M. (2007). On amphidromy, a distinct form of diadromy in aquatic organisms. Fish and Fisheries 8, 1–13.
| On amphidromy, a distinct form of diadromy in aquatic organisms.Crossref | GoogleScholarGoogle Scholar |
Mejía-Ortíz, L. M., and Alvarez, F. (2010). Seasonal patterns in the distribution of three species of freshwater shrimp, Macrobrachium spp., along an altitudinal river gradient. Crustaceana 83, 385–397.
| Seasonal patterns in the distribution of three species of freshwater shrimp, Macrobrachium spp., along an altitudinal river gradient.Crossref | GoogleScholarGoogle Scholar |
Melo, G. A. S. (2003).’ Manual de Identificação dos Crustacea Decapoda de Água Doce do Brasil.’ (Centro Universitário São Camilo, Museu de Zoologia, Universidade de São Paulo: Loyola.)
Mohamed, K. H., and Rao, V. (1971). Estuarine phase in the life-history of the commercial prawns of the west coast of India. Journal of the Marine Biological Association of India 13, 149–161.
Mossolin, E. C., and Bueno, S. L. S. (2003). Relative growth of the second pereiopod in Macrobrachium olfersi (Wiegmann, 1836) (Decapoda, Palaemonidae). Crustaceana 76, 363–376.
| Relative growth of the second pereiopod in Macrobrachium olfersi (Wiegmann, 1836) (Decapoda, Palaemonidae).Crossref | GoogleScholarGoogle Scholar |
New, M. B., D’abramo, L. R., Valenti, W. C., and Singholka, S. (2000). Sustainability of freshwater prawn culture. In ‘Freshwater Prawn Culture: The Farming of Macrobrachium rosenbergii’. (Eds M. B. New and W. C. Valenti.) pp. 429–434. (Blackwell Science: Oxford.)
Nwosu, F. M. (2008). Growth and mortality of the rough river prawn Macrobrachium equidens Dana, 1852 (Crustacea, Palaemonidae) in the Cross River Estuary, southeast Nigeria. Journal of Food Agriculture and Environment 6, 186–189.
Nwosu, F. M., and Wolfi, M. (2006). Population dynamics of the giant African river prawn Macrobrachium vollenhovenii Herklots 1857 (Crustacea, Palaemonidae) in the Cross River estuary, Nigeria, west Africa Journal of Applied Ecology 9, 1–18.
Nwosu, F. M., Holzlöhner, S., and Enin, U. I. (2007). The exploited population of the brackish river prawn (Macrobrachium macrobrachion Herklots, 1851) in the Cross River estuary, Nigeria. Scientia Marina 71, 115–121.
Oh, C. W., Hartnoll, R. G., and Nash, R. D. M. (1999). Population dynamics of the common shrimp, Crangon crangon (L.), in Port Erin Bay, Isle of Man, Irish Sea. ICES Journal of Marine Science 56, 718–733.
| Population dynamics of the common shrimp, Crangon crangon (L.), in Port Erin Bay, Isle of Man, Irish Sea.Crossref | GoogleScholarGoogle Scholar |
Okogwu, O. I., Ajuogu, J. C., and Nwani, C. D. (2010). Artisanal fishery of the exploited population of Macrobrachium vollenhovenii Herklot 1857 (Crustacea; Palaemonidae) in the Asu River, southeast Nigeria. Acta Zoologica Lituanica 20, 98–106.
| Artisanal fishery of the exploited population of Macrobrachium vollenhovenii Herklot 1857 (Crustacea; Palaemonidae) in the Asu River, southeast Nigeria.Crossref | GoogleScholarGoogle Scholar |
Olivier, T. J., and Bauer, R. T. (2011). Female downstream-hatching migration of the river shrimp Macrobrachium ohione in the lower Mississippi River and the Atchafalaya River. American Midland Naturalist 166, 379–393.
| Female downstream-hatching migration of the river shrimp Macrobrachium ohione in the lower Mississippi River and the Atchafalaya River.Crossref | GoogleScholarGoogle Scholar |
Onaga, T., Fiedler, C., and Baeza, J. A. (2012). Protandric simultaneous hermaphroditism in Parahippolyte misticia (Crustacea: Decapoda: Hippolytidae): implications for the evolution of mixed sexual systems in marine shrimps. Journal of Crustacean Biology 32, 383–394.
| Protandric simultaneous hermaphroditism in Parahippolyte misticia (Crustacea: Decapoda: Hippolytidae): implications for the evolution of mixed sexual systems in marine shrimps.Crossref | GoogleScholarGoogle Scholar |
Parrack, M. (1979). Aspects of brown shrimp, Penaeus aztecus, growth in the northern Gulf of Mexico. Fishery Bulletin 76, 827–836.
Pauly, D. (1991). Growth performance in fishes: rigorous description of patterns as a basis for understanding causal mechanisms. Aquabyte 4, 3–6.
Phone, H., Suzuki, H., and Ohtomi, J. (2005). Reproductive biology of the freshwater palaemonid prawn, Macrobrachium lanchesteri (De man, 1911) from Myanmar. Crustaceana 78, 201–213.
| Reproductive biology of the freshwater palaemonid prawn, Macrobrachium lanchesteri (De man, 1911) from Myanmar.Crossref | GoogleScholarGoogle Scholar |
Prasad, S., and Kanaujia, D. R. (2006). Availability and breeding behaviour of ganga river prawn Macrobrachium gangeticum (Bate) and Macrobrachium malcolmsonii (H.M. Edwards). Asian Fisheries Science 19, 377–388.
Rao, K. J. (1986). Studies on maturation, breeding, fecundity and sex ratio in Macrobrachium malcolmsonii (H. Milne Edwards) from Kolleru Lake. Journal of Aquatic Biology 4, 62–72.
Read, G. H. L. (1985). Factors affecting the distribution and abundance of Macrobrachium petersi (Hilgendorf) in the Keiskamma River and Estuary, South Africa. Estuarine, Coastal and Shelf Science 21, 313–324.
| Factors affecting the distribution and abundance of Macrobrachium petersi (Hilgendorf) in the Keiskamma River and Estuary, South Africa.Crossref | GoogleScholarGoogle Scholar |
Rolim, G. S., Camargo, M. B. P., Lania, D. G., and Moraes, J. F. L. (2007). Classificação climática de Köppen e de Thornthwaite e sua aplicabilidade na determinação de zonas Agroclimáticas para o estado de São Paulo. Bragantia 66, 711–720.
| Classificação climática de Köppen e de Thornthwaite e sua aplicabilidade na determinação de zonas Agroclimáticas para o estado de São Paulo.Crossref | GoogleScholarGoogle Scholar |
Román-Contreras, R., and Campos-Lince, L. S. (1993). Aspectos reproductivos y aproximación a un modelo de crecimiento para una población de Macrobrachium acanthurus (Wiegmann, 1836) en el Rio Palizada, Campeche, México. Anales del Instituto de Ciencias del Mar y Limnología 20, 55–65.
Rome, N., Conner, S. L., and Bauer, R. T. (2009). Delivery of hatching larvae to estuaries by anamphidromous river shrimp: tests of hypotheses based on larval moulting and distribution. Freshwater Biology 54, 1924–1932.
| Delivery of hatching larvae to estuaries by anamphidromous river shrimp: tests of hypotheses based on larval moulting and distribution.Crossref | GoogleScholarGoogle Scholar |
Romero, R., and Silva, M. E. (2008). Crecimiento de Macrobrachium michoacanus con relación al tipo de alimento y densidad de cultivo. Naturaleza y Desarrollo 6, 15–25.
Sampaio, C. M. S., and Valenti, W. C. (1996). Growth curves for Macrobrachium rosenbergii in semi-intensive culture in Brazil. Journal of the World Aquaculture Society 27, 353–358.
| Growth curves for Macrobrachium rosenbergii in semi-intensive culture in Brazil.Crossref | GoogleScholarGoogle Scholar |
Sánchez-Palacios, J., Beltrán, R., and Ramírez, J. P. (2008). Crecimiento y reproducción del camarón Atya margaritacea (Decapoda: Atyidae) en el Río Presidio, Sinaloa, México. Revista de Biologia Tropical 56, 513–522.
| 19256424PubMed |
Silliman, R. P. (1969). Comparison between Gompertz and von Bertalanffy curves for expressing growth in weight of fishes. Journal of the Fisheries Research Board of Canada 26, 161–165.
| Comparison between Gompertz and von Bertalanffy curves for expressing growth in weight of fishes.Crossref | GoogleScholarGoogle Scholar |
Silva, M. N., Frédou, F. L., and Rosa-Filho, J. S. (2007). Estudo do crescimento do camarão Macrobrachium amazonicum (Heller, 1863) da Ilha de Combu, Belém, Estado do Pará. Amazônia. Ciência & Desenvolvimento 2, 85–104.
Sparre, P., and Venema, S. C. (1995). Introducción a la evaluación de recursos pesqueros tropicales: Documento Técnico de Pesca No. 306/1. FAO: Rome, Italy.
Sparre, P., and Venema, S. C. (1997). Introduction to tropical fish stock assessment: Parte 1 – manual. Roma: FAO (Fish. Paper, 306/1).
Valenti, W. C. (1985). ‘Cultivo de Camarões de Água Doce.’ (Nobel: São Paulo.)
Valenti, W. C., Mello, J. T. C., and Lobão, V. L. (1986). Dinâmica da reprodução de Macrobrachium acanthurus (Wiegmann, 1836) e Macrobrachium carcinus (Linnaeus, 1758) do Rio Ribeira de Iguape (Crustacea, Decapoda, Palaemonidae). Ciencia e Cultura 38, 349–355.
Valenti, W. C., Mello, J. T. C., and Lobão, V. L. (1987). Crescimento de Macrobrachium acanthurus (Wiegmann, 1836) do Rio Ribeira de Iguape (Crustacea, Decapoda, Palaemonidae). Revista Brasileira de Biologia 47, 349–355.
Wenner, A. M. (1972). Sex ratio as a function of size in marine Crustacea. American Naturalist 106, 321–350.
| Sex ratio as a function of size in marine Crustacea.Crossref | GoogleScholarGoogle Scholar |
Wenner, E. L., Coon, W. P., III, Sandifer, P. A., and Shealy, M. H., Jr (1991). A comparison of species composition and abundance of decapod crustaceans and fishes from the North and South Edisto Rivers in South Carolina. Marine Resources Center Technical Report 78, Marine Resources Division, South Carolina Wildlife and Marine Resources Department, Charleston, South Carolina, USA.
Wilson, K., and Hardy, I. C. W. (2002). Statistical analysis of sex ratios: an introduction. In ‘Sex Ratios: Concepts and Research Methods’. (Ed. I. C. W. Hardy.) pp. 48–92. (Cambridge University Press: Cambridge.)
Willson, M. F., and Pianka, E. R. (1963). Sexual selection, sex-ratio and mating system. American Naturalist 97, 405–407.
| Sexual selection, sex-ratio and mating system.Crossref | GoogleScholarGoogle Scholar |