Otolith chemistry as an indicator of movements of albacore (Thunnus alalunga) in the North Atlantic Ocean
Igaratza Fraile A D , Haritz Arrizabalaga A , Josu Santiago A , Nicolas Goñi A , Igor Arregi A , Sonia Madinabeitia B , R. J. David Wells C and Jay R. Rooker CA AZTI Tecnalia, Marine Research Division, Herrera Kaia, Portualdea z/g, E-20110 Pasaia, Gipuzkoa, Spain.
B Department of Mineralogy and Petrology, University of the Basque Country (EHU), E-48080 Bilbao, Spain.
C Department of Marine Biology, Texas A&M University, 1001 Texas Clipper Road, Galveston, TX 77553, USA.
D Corresponding author. Email: ifraile@azti.es
Marine and Freshwater Research 67(7) 1002-1013 https://doi.org/10.1071/MF15097
Submitted: 5 March 2015 Accepted: 7 January 2016 Published: 29 April 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Albacore (Thunnus alalunga) in the North Atlantic Ocean is currently managed as a single well-mixed stock, although this assumption remains contentious. We measured stable isotopes (δ13C and δ18O) and trace elements (Mg, Mn, Sr, Ba) in otoliths of albacore collected from two feeding grounds, namely the Bay of Biscay and Atlantic offshore waters, and compared them among sampling locations and life history stages. Measurements in otolith core, post-core and edge were used to determine whether albacore from these two regions have the same nursery origin and migratory patterns. We found no clear evidence of distinct nursery grounds based on otolith core chemistry, but Sr : Ca and Mg : Ca were different in the post-core portions of albacore from the two locations, suggesting residency in different regions during the early juvenile stage. Otolith edge chemistry, particularly stable isotopes and Sr : Ca, proved to be a valuable tool for classifying individuals to their capture locations. Annual cycles of Sr : Ca ratios were visible along life history transects, likely reflecting migratory patterns between water masses of differing salinity, but the timing of Sr : Ca cycles differed between the two groups. Differentiation in trace element concentrations in the otolith post-core and the timing of Sr : Ca cycles suggest the occurrence of two migratory contingents of albacore in the north-east Atlantic Ocean.
Additional keywords: migratory contingents, population structure.
Introduction
Albacore (Thunnus alalunga) is a highly migratory species that is widely distributed throughout the Atlantic, Pacific and Indian oceans. In the North Atlantic Ocean, the occurrence of three genetically distinct stocks is assumed: northern and southern Atlantic stocks (separated at 5°N) and the Mediterranean stock (Arrizabalaga et al. 2004; ICCAT 2012; Montes et al. 2012). Albacore is a temperate to subtropical tuna species and the distribution of the North Atlantic stock ranges between 0 and 50°N (Lehodey et al. 2014). Albacore are primarily exploited by surface fisheries operating in the Bay of Biscay and adjacent waters during the summer, and around the Azores Islands during summer and autumn (ICCAT 2011, 2012). In addition, Chinese Taipei longliners target albacore in the central and western North Atlantic Ocean throughout the year. Past decades have seen significant decline in albacore fisheries in the Bay of Biscay region, partly because fleets targeting albacore have diminished (Santiago 2004) but also because the spawning stock size has declined significantly (ICCAT 2014). Although the declining trend of North Atlantic albacore catches can be explained by different biotic and abiotic factors, studies suggest environmental variability may influence the distribution and migratory patterns of albacore (Ortiz de Zárate et al. 1998; Bard 2001; Goñi and Arrizabalaga 2005; Dufour et al. 2010), which may explain recently observed changes in fisheries, such as the lack of albacore within the Bay of Biscay in some years. These episodes have raised important population questions about stock structure and movements, which are critical for stock assessment and management of the fishery. It is essential to include population structuring during stock assessments, otherwise overexploitation and eventual collapse of less-productive subpopulations may occur, with underexploitation of more productive populations (Hilborn et al. 2004). For North Atlantic albacore, it is unknown whether declines within the Bay of Biscay are linked to environmental changes or whether a subpopulation is being depleted. Unfortunately, the life cycle and population structure of albacore in the North Atlantic Ocean is poorly understood (Lehodey et al. 2014).
The albacore fishery in the North Atlantic Ocean is currently managed as a single well-mixed stock, although the population structure of this species remains contentious. Studies carried out in the late 1960s based on catches of mature albacore and the presence of larvae indicated that spawning of albacore occurs in the Sargasso Sea and northern Venezuela (Koto 1969; Ueyanagi 1971). More recent investigations have reported spawning activity in the eastern tropical Atlantic indicated by the presence of several mature female albacore at the spawning stage (Ortiz de Zárate et al. 2004). Nevertheless, the sample size of that study was limited, and the reproductive biology of North Atlantic albacore remains largely unknown. A recent study of South Pacific albacore has found synchronised reproductive activity over a broad tropical area, with spawning peaking during the austral spring and summer months (Farley et al. 2013). In the North Atlantic, the spawning area and time remains uncertain, and the occurrence of possible subgroups spawning in different regions or with different behavioural traits is still not resolved. Several approaches have been used to address the population structure of albacore in the North Atlantic Ocean, including molecular genetics, blood group analyses and electronic and conventional tagging (Arrizabalaga et al. 2004; Cosgrove et al. 2010). However, there has been no agreement about whether North Atlantic albacore exist as a single well-mixed stock or whether this group comprises several subpopulations or contingents. Population genetic structure investigations using microsatellite markers have revealed some insights into genetic heterogeneity with the potential presence of three subpopulations within the North Atlantic Ocean stock (Davies et al. 2011), whereas single nucleotide polymorphisms did not detect significant heterogeneity (Albaina et al. 2013; Montes et al. 2012). Furthermore, there is some evidence to suggest that different groups with a different migration pattern may also exist within the North Atlantic Ocean (Bard 1981; Fonteneau 2010).
During the summer, juvenile, subadult (ages-1–4) and some larger adult specimens (age-5+) migrate towards the eastern North Atlantic Ocean, where recreational and bait boat fisheries operate along the coastal shelf of the Bay of Biscay, and a trolling fishery catches albacore in the open ocean zone. It is still unknown whether the albacore found in these two regions represent discrete subpopulations, migratory contingents or have age-specific distribution patterns influenced by environmental conditions. Consequently, a more refined understanding of the movement and stock structure is needed to effectively manage the species.
Recent studies using otolith chemical signatures as natural markers have shown to be a valuable tool for fishery ecologists in understanding the spatial ecology of tuna species (e.g. Wang et al. 2009; Wells et al. 2012, 2015; Rooker et al. 2014; Fraile et al. 2015). Otoliths are calcified structures that grow continuously throughout the life of the fish, and their chemical composition can provide continuous chronological records of the environment, allowing discrimination between groups of fish with different origins as well as reconstruction of life history and dispersal trajectories between habitats (Campana et al. 1994). Recent work by Macdonald et al. (2013) applying otolith trace element chemistry to albacore in the South Pacific Ocean provided some insights into connectivity between larval sources and adult populations of albacore across this region.
In the present study we use both stable isotopes and trace elements in otoliths of albacore to improve the current understanding of the North Atlantic Ocean population structure and movement of individuals within this region. Our aim was to assess the feasibility of otolith chemistry as a tool to study movement patterns of albacore and to provide insights into its population substructure. Specifically, we combined otolith stable isotope (δ13C and δ18O) and trace element : Ca ratios (Mg : Ca, Mn : Ca, Sr : Ca, Ba : Ca) in the otolith edge to determine whether fish captured within the Bay of Biscay and those caught in offshore waters of the North Atlantic Ocean have inhabited different water masses before their capture. Then, the nursery origin of these two groups was explored by analysing chemical composition of otolith core and near-core areas. Finally, trace element chemistry was analysed along the otolith growth axis to retrospectively detect movements along the life history of the fish.
Materials and methods
Study location
The Bay of Biscay is located in the eastern North Atlantic Ocean, bounded by the western coast of France and the northern coast of the Iberian Peninsula, and is connected with the Celtic Sea in the north (Fig. 1). The Iberian margin is characterised by a narrow continental shelf, whereas the eastern margin of the Bay of Biscay has a wide shelf, with significant freshwater input. The oceanography of the Bay of Biscay is characterised by a variety of mesoscale features varying according to climatic and seasonal conditions. These characteristics have a strong effect on the biological production of the area and likely affect the distribution, abundance, and movement of albacore (Sagarminaga and Arrizabalaga 2014).

|
Sample collection
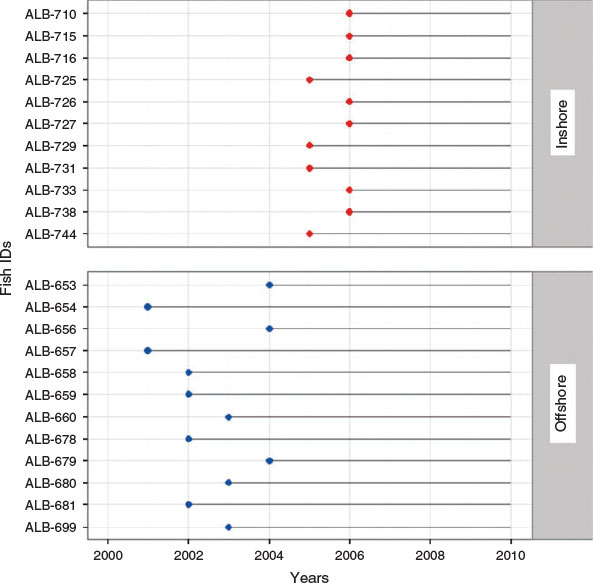
Albacore (n = 167) were collected in the eastern North Atlantic Ocean by commercial bait boat and trolling fleets over a 3-year period, between 2009 and 2011, during their summer migration (June–October) to the Bay of Biscay and surrounding waters. Samples were divided into two regions: (1) Bay of Biscay; and (2) offshore waters of the eastern North Atlantic Ocean, including the Celtic Sea, hereafter termed ‘offshore’ (Fig. 1). The division of these two regions was based on the lack of albacore within the Bay of Biscay in some recent years compared with a regular presence of albacore around the Azores Islands and southern Celtic Sea. This observation has raised the question of whether these two units comprise different subpopulations that differ in size or migratory patterns. Both Bay of Biscay samples (n = 69) and offshore samples (n = 98) were obtained in collaboration with on-board observers, distributors and fish processing manufacturers. Spatial and temporal distribution of catches depended on the activity of the commercial fleet over the study period, which varied among regions. Continuous sampling occurred from June to October in offshore waters, whereas albacore within the Bay of Biscay were only captured during August and October. The straight fork length (FL) of each fish was measured to the nearest centimetre (Table 1). The otolith collection used in the present study comprises albacore from different cohorts and collection dates in order to obtain a representative sample from each region.

|
Analytical method
Sagittal otoliths of albacore were extracted for chemical analysis. After removing the adhering organic tissues, whole otoliths were briefly soaked in nitric acid (1%) to eliminate any remaining biological material and cleaned with deionised water (Rooker et al. 2008). One otolith per fish was embedded in epoxy resin and sectioned using a Buehler low-speed Isomet saw (Buehler Ltd, Lake Bluff, IL, USA) fitted with a diamond-edged blade to obtain a transverse section that included the core. Otolith sections were washed with nitric acid (1%) and deionised water to remove any post-mortem and handling contamination (Davies et al. 2010). Transverse sections were cut for stable isotope and trace element analyses (1.5 and 0.5 mm respectively). Transverse sections were first polished with 1200-grit paper moistened with distilled water, and were further polished with a micro cloth and 0.3-µm alumina powder to ensure a smooth surface. Sections were glued in a sample plate using Crystalbond thermoplastic glue (Crystalbond 509, Buehler Ltd). Otoliths of 144 individuals caught in 2009, 2010 and 2011 were used for stable isotope analysis, whereas those from 23 individuals caught in 2010 were used for trace element studies.
For stable isotope analysis, we followed a similar procedure to that used by Wells et al. (2015) for North Pacific albacore. The portion of the otolith corresponding to the first translucent zone (approximately first 6 months of life, hereafter called the ‘core’) and the most posterior margin of each otolith (which represents the fish’s most recent growth, hereafter referred to as the ‘edge’) were milled using a high-resolution New Wave Research MicroMill System (New Wave Research, Fremont, CA, USA) consisting of a microscope and imaging system controlled by computer software. A series of 14 drill passes of 55-µm depth were run over a programmed drill path using a 300-µm diameter carbide bit. Carbon (δ13C) and oxygen (δ18O) stable isotopes of otolith powder were analysed using an automated carbonate preparation device (KIEL-III, Thermo Fisher Scientific, Waltham, MA, USA) coupled with a gas-ratio mass spectrometer (Finnigan MAT 252, Thermo Fisher Scientific) at the Environmental Isotope Laboratory of the University of Arizona. All isotope values are reported according to standards of the International Atomic Energy Agency in Vienna (Vienna Pee Dee Belemnite, VPDB).
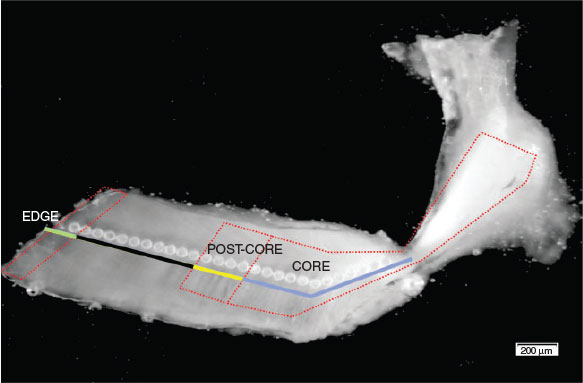
Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) was used to determine the trace element chemistry of the otolith. The system consisted of a laser ablation system (Nd : Yag 213 nm; New Wave Research) coupled to a mass spectrometer (X Series 2 ICP-MS; Thermo Electron Corporation, Cheshire, UK,). Otolith sections were placed in a sealed chamber and viewed through a microscope connected to MicroMill-specific software. Ablations were performed using a 40-µm spot size with a 10.00-Hz pulse rate at 70% energy (6.5 J cm–2). Each otolith was analysed using individual spots along the ventral arm of the otolith, beginning in the region corresponding to the early life stage of the fish and moving outward to the outer margin of the otolith until the edge (Fig. 2). The time frame in the life of the tuna represented by each laser spot varies from a few weeks, when the spot is close to the core, to several months for spots at the edge (Wells et al. 2013; Renck et al. 2014). Age determination for each ablation point was done on the same otolith used for elemental analysis by examining polished otolith sections under a microscope with transmitted light, and counting the annual formation of narrow opaque and wide translucent bands along the ventral arm. The methodology outlined by Wells et al. (2013) was applied to sectioned otoliths for the reading and interpretation of the growth increments. Otoliths from 23 individuals collected in 2010 and ranging in age from 4 to 10 years provided elemental signatures recorded between the hatch year and collection year (Fig. 3). To account for instrumental drift and sensitivity and to determine the precision of trace element measurements, a glass reference standard (National Institute of Standards and Technologies, NIST 612 SRM, US Department of Commerce) with known elemental concentrations was analysed prior and after otolith analysis. Mean values measured by the instrument have been compared with the recommended values (Jochum et al. 2011). In addition, the accuracy of the measurements was monitored with repeat measurements of the US Geological Survey (USGS) carbonate reference sample (MACS-3 pressed powder pellet). The measurements and the known values of Ca were used to correct for differences in ablation yields between standards and unknown samples. Calcium was used as an internal standard to account for variation in ablation efficiency, which is caused by variations in the mass of the material ablated (Campana et al. 1994). Four isotopes (25Mg, 88Sr, 55Mn and 138Ba) were measured from the core through all the layers to the outer edge of each otolith using the LA-ICP-MS system. Readings below detection limits were not used in analyses; in addition, ablation spots with a standard error >20% were discarded from the analysis. The selection of elements was based on their occurrence in concentrations above the detection limit and their stability within a growth band. Elemental concentrations (ppm) were calculated based on relative abundance of the isotopes. Data reduction and processing were performed using the software package Iolite version 2.2 (School of Earth Sciences, The University of Melbourne, Melbourne, Vic., Australia; available from http://www.lolite.org.au, accessed 12 December 2012), an application that operates within IGOR Pro ver. 6.2 (WaveMetrics, Inc., Lake Oswego, OR, USA, see http://www.wavemetrics.com, accessed 5 December 2012; Paton et al. 2011; Paul et al. 2012). Finally, calcium concentration was assumed from the stoichiometry of aragonitic calcium carbonate (400 000 µg Ca g–1 otolith), and the concentrations of other elements (ppm, µg g–1) were expressed as the element : Ca ratio (Ludsin et al. 2006). Ablation spots that resulted within the first translucent zone and first opaque zone (hereafter referred to as ‘core’ and ‘post-core’ respectively) were averaged to obtain a mean elemental concentration for each of the periods. The ‘edge’ was defined as the last ablation spot at the outer margin of the ventral arm of the otolith.
|
|
Otolith δ18O prediction
Summer otolith δ18O values were predicted using sea surface temperature (SST) and salinities (SSS) of the Sargasso Sea between the 2001 and 2006 time frame that corresponds with the birth year of the sampled individuals. Summer SST and SSS of the Sargasso Sea were derived from GLORYS2V3 global reanalysis using Copernicus Marine Environment Monitoring Service (CMEMS, see http://marine.copernicus.eu, accessed December 2015) products, and spatially averaged for the entire Sargasso Sea, which is considered the nursery ground of the North Atlantic albacore. Seawater oxygen isotope ratio (δ18Ow) was predicted from SSS using the equation provided by Harwood et al. (2008) and corrected to the VPDB scale using the relationship from Friedman and O’Neil (1977). The effect of temperature on the fractionation of oxygen isotopes was calculated with the inorganic aragonite relationship from Kim et al. (2007).
Statistical analysis
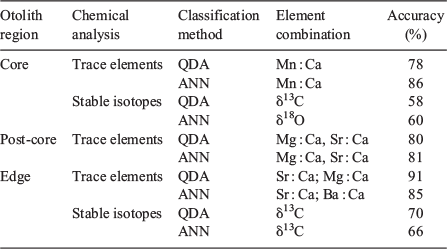
Normality of data was tested in both otolith core and edge measurements using a Shapiro–Wilk test, and log transformation was applied to trace element data to approach normality and stabilise variance. Interannual and monthly variability was assessed by multivariate analysis of variance (MANOVA) and Kruskal–Wallis tests. Similarly, one-way analysis of variance (ANOVA) was used to determine whether interannual differences on predicted otolith δ18O values for the Sargasso Sea between 2001 and 2006 were significant. Ontogenetic variability on stable isotopic composition was analysed using the general linear model (GLM) with FL as an explanatory variable on otolith edge measurements for each of the predefined groups. Different statistical methods were applied stepwise to define the variation in the chemical composition of otoliths from each environment tested (Bay of Biscay and offshore waters). In a first exploratory analysis, comparisons of otolith chemical composition between the albacore caught within the Bay of Biscay and those caught in offshore waters were made with the null hypothesis of no difference, and the Mann–Whitney–Wilcoxon test was used as the test statistic. In a second step, habitat discrimination capacity between these two groups based on otolith chemistry was evaluated by statistical classification methods. Recent work comparing the classification accuracy of different statistical methods has shown that machine learning methods often have greater classification efficiency with fewer assumptions than discriminant function analyses (Mercier et al. 2011). In the present study, two different statistical methodologies were selected: (1) a classical classification method, namely quadratic discriminant analysis (QDA); and (2) a data-mining technique capable of machine learning and pattern recognition, namely artificial neural network (ANN). Cross-validation was used to determine the accuracy of each classification method in order to select the best methodology and reduce subsequent misclassification errors when applying this classifier to predict fish movements. To get reliable results, we measured prediction efficiency over 1000 replicates and calculated a mean accuracy for each combination of elements. For each of the methods, all element combinations were tested and the optimal list(s) of elements for fish classification were selected. Prediction efficiency between all element combination and classification methods was measured by cross-validation. A detailed description of the statistical methodology is given in Mercier et al. (2011).
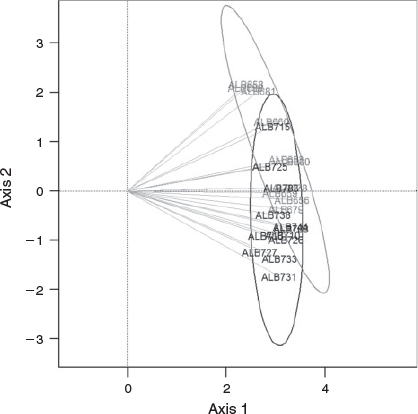
In order to compare migratory patterns between the two groups, core to edge transects were transformed into equally spaced observations using linear interpolation and generating a regular time series of elemental concentration from 0 to 4 years. Principal component analysis (PCA) was used to examine the leading patterns and temporal variations of the Sr : Ca ratio in albacore from the Bay of Biscay and offshore waters during the juvenile period (0–4 years). Data transformation and ordination analysis were performed using ‘zoo’ (Zeileis and Grothendieck 2005) and ‘vegan’ (J. Oksanen, F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. Henry, H. Stevens, and H. Wagner, see http://CRAN.R-project.org/package=vegan, accessed September 2015) packages implemented in the R CRAN environment (R Foundation for Statistical Computing, Vienna, Austria, see http://www.R-project.org/, accessed September 2015).
Results
Interannual variability of δ13C and δ18O values measured at the otolith edge were not significantly different (P > 0.05, MANOVA; P > 0.05, Kruskal–Wallis). Moreover, otolith δ18O values predicted using SST and SSS fields from the Sargasso Sea did not differ from 2001 to 2006, and were therefore not considered in further analysis. GLM for otolith edge δ13C and δ18O values with FL as the explanatory variable indicated that ontogenetic influence was not significant for either isotope. Otolith δ13C values for albacore from the North Atlantic Ocean ranged from –9.17 to –6.35‰, whereas δ18O of the same otoliths ranged from –1.67 to 0.11‰.
Capture locations
Collection locations in the Bay of Biscay and offshore waters of the North Atlantic Ocean were clearly separated based on otolith edge chemistry when all year-classes were pooled. Separation between the two locations was based primarily on stable isotope composition and Sr : Ca ratio (P < 0.05, Mann–Whitney–Wilcoxon test). Otolith edge δ13C and δ18O were more enriched for albacore captured in offshore waters compared with those captured within the Bay of Biscay (Table 2; Figs 4, 5). A significant month effect was detected for otolith edge δ18O, indicating that δ18O values across capture months (from June to October) were not consistent. In contrast, no significant differences in otolith edge δ13C were observed by month (P > 0.05, Kruskal–Wallis). However, given that the discriminatory power of δ18O was not significant (Table 3), discrimination of the two capture locations was not affected by month-to-month variability of δ18O. Among the trace element analyses at the otolith edge, significant spatial differences were present only for Sr : Ca, with albacore captured in offshore waters having higher mean values than those captured in the Bay of Biscay (mean ± s.d. 5.63 ± 0.36 v. 4.82 ± 0.23 mmol mol–1 respectively). Differences for the remaining elements measured at the edge were not significant (Table 2). Classification accuracy of albacore to their capture locations based on otolith edge chemistry varied between 66 and 90% depending on the statistical classification method and chemical elements included (Table 3). Capture locations were discriminated with a moderate accuracy (66 and 69% using ANN and QDA approaches respectively) based on δ13C measurements, whereas trace element chemistry was more efficient in discriminating between the Bay of Biscay and Atlantic offshore waters. QDA was identified as the best classification method for trace element signatures, because fish caught from the two different geographic regions could be accurately classified to their capture locations (90% of fish correctly classified). The ANN method was also successful in classifying otolith edge signature, with 85% accuracy. In both cases, element combination displaying the greatest accuracy (selected by a stepwise variable selection procedure) included the Sr : Ca ratio.
Error1: Incorrect filename or format (MF15097_F4.gif). Please check out Fig. 4.
|
|
Otolith core and post-core chemistry
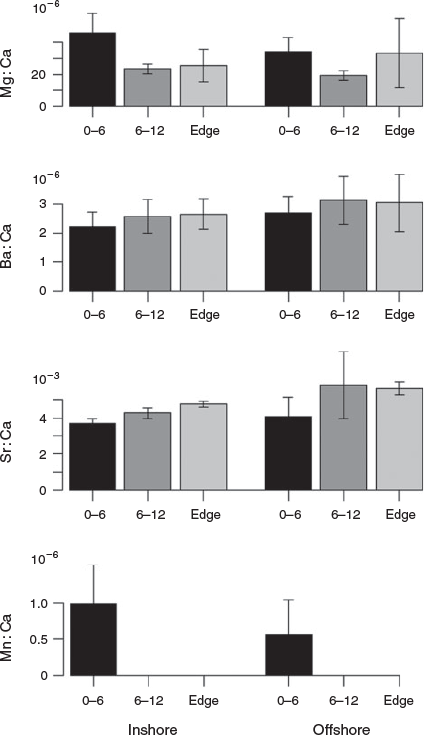
Chemical signatures in otolith cores were analysed to identify nursery origin of albacore. Otolith core δ18O values were significantly higher in otoliths of albacore caught in the Bay of Biscay (P < 0.05, Mann–Whitney–Wilcoxon test), whereas no significant difference in otolith core δ13C was found between the two groups (Table 2; Fig. 4). Among the elements that occurred above instrument detection limits within the core, Mn : Ca ratios were significantly different between the two groups, with higher ratios of this element found in albacore from the Bay of Biscay (P < 0.05, Mann–Whitney–Wilcoxon test; Table 2; Fig. 5). The remaining elements were undifferentiated across the two capture locations.
Otolith post-core values of Mg : Ca and Sr : Ca ratios were significantly different between the two groups (P < 0.05, Mann–Whitney–Wilcoxon test; Table 2; Fig. 5). Mean Sr : Ca was lower and Mg : Ca higher for fish captured within the Bay of Biscay. Statistical classification techniques using δ13C and δ18O values measured at the otolith core showed low evidence of differentiation between the two groups (58–60%). Based on trace element chemistry, optimal discrimination was attained from Mn : Ca, with classification accuracy ranging from 78 to 86% using QDA and ANN techniques respectively. Classification analyses, based on otolith post-core element : Ca ratios produced noticeable separation between albacore caught in the two regions (cross-validated classification success was between 80 and 81% depending on the statistical method selected). Regardless of the method used for prediction, habitat discrimination was maximised when the combination of Sr : Ca and Mg : Ca was used to separate the two regions. Details about the optimal combination of elements and the classification success of each classification method are given in Table 3.
Trace element transects
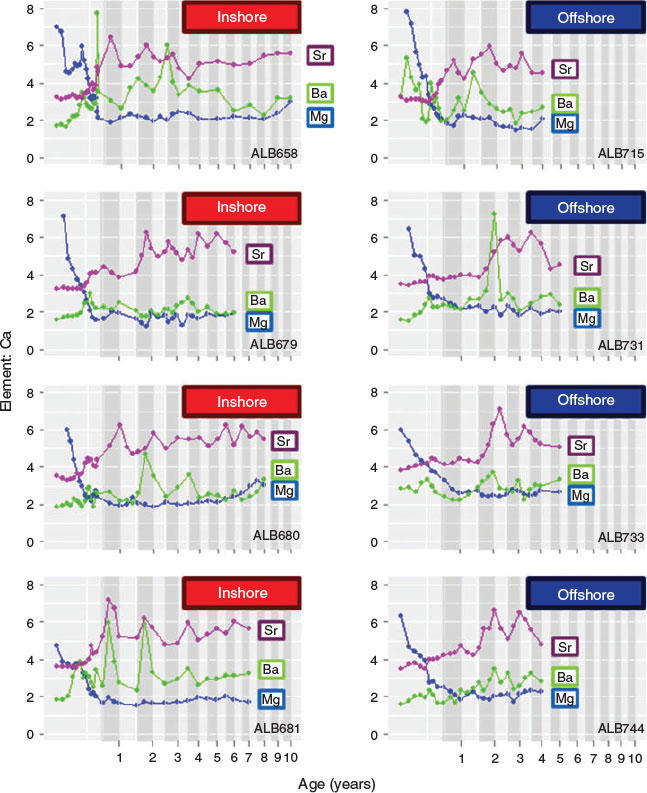
Three of the four trace elements (Mg, Sr and Ba) were well above the detection limits along the entire transect. Based on back-calculated harvested year, the time frame covered by the ablation transects ranged from 2001 to 2010 (Fig. 2). Maximum Mg : Ca was observed close to the otolith core and decreased rapidly during the age-0 period, showing little fluctuation along the rest of the otolith transect (Fig. 6). Otolith Sr : Ca was relatively low and stable during the first few months of life, and then fluctuated through the entire life history. Although the timing of the first Sr : Ca cycling varied among individuals, marked Sr : Ca cycling was visible during the juvenile stage (ages-1–4), with a high Sr : Ca ratio often corresponding with the opaque bands. In most of the otoliths, annual periodicity in Sr : Ca persisted through the adult stage (age-4+), although in some cases concentrations remained relatively stable during the adult stage (e.g. ALB-658 in Fig. 6). The timing of the first peak in otolith Sr : Ca varied between groups: All albacore caught in the Bay of Biscay showed Sr : Ca peaks at age-1, whereas in the majority of the offshore samples (55%) first peaking in Sr : Ca occurred after the first year or it was less pronounced. PCA applied to the time series of otolith Sr : Ca during the juvenile stage (0–4 years) showed that individuals captured in the Bay of Biscay and offshore waters were grouped into two distinct clusters (Fig. 7). The first two axes of the PCA explained 78% of the total variation, and the scores of these two axes were significantly different between albacore caught in the Bay of Biscay and offshore waters (P < 0.05, MANOVA). Otolith Ba : Ca fluctuated considerably along the transects and the patterns were not consistent among individuals. A significant positive correlation between Sr : Ca and Ba : Ca was found in 43% of otoliths (e.g. ALB-681), but in most otoliths no clear relationship was found between cycles of these two elements. Otolith Mg : Ca was inversely correlated with both Sr : Ca and Ba : Ca concentrations (e.g. ALB-715) for 76 and 57% of samples respectively.
|
Discussion
Improving our understanding of population structure of commercially exploited fish stocks is needed to define appropriate management units for stock assessments. Otolith chemistry can provide valuable information on the life history, dispersal and stock characteristics of fish, and the present study demonstrates the potential of otolith chemical analyses to provide important information about the stock structure and connectivity of albacore in the eastern North Atlantic Ocean. The otolith edge chemistry indicated that albacore captured within the Bay of Biscay and offshore waters of the North Atlantic Ocean have inhabited different water masses before capture. Among the trace elements that routinely occurred above instrument detection limits, otolith Sr : Ca was considered the most reliable marker as a geographic proxy for recent regional habitat use within the eastern North Atlantic Ocean, most likely generated by the salinity gradient of the study area (Fig. 1). QDA performed slightly better than ANN in discriminating the different water masses of the eastern North Atlantic Ocean. We found a lack of evidence of discrete nursery grounds for albacore from the North Atlantic Ocean (revealed by relatively invariant δ13C, Mg : Ca, Sr : Ca and Ba : Ca in the core across the two capture locations), although otolith δ18O and Mn : Ca were significantly different between the two groups. The low classification accuracy attained using both QDA and ANN methods (expected classification is 50% based on random assignment) indicates similarity in the core chemical signature of the two groups. Differences in Mn : Ca found in otolith core could be explained by physiological or structural factors rather than environmental drivers. Mn is introduced into the water column through different sources, such as terrestrial input, upwelling of Mn-rich waters or microbial oxidation, among others (Landing and Bruland 1980; Johnson et al. 1992; Klinkhammer and McManus 2001), but previous studies found variable and no conclusive effects of temperature and salinity on otolith Mn : Ca (Elsdon and Gillanders 2002). Elevated Mn concentration is often found in otolith primordia because maternal transfer appears to be the principal mechanism of incorporation (Brophy et al. 2004; Martin and Thorrold 2005; DiMaria et al. 2010). Thus, differences in otolith Mn : Ca, although statistically significant, would likely be due to proximity of the ablation spot to the primordium rather than environmentally driven.
We found no evidence that tuna captured in offshore waters and those found in the Bay of Biscay originated in different geographic regions, but differences in Sr : Ca and Ba : Ca in the post-core portion of the otolith suggest that the two groups resided in habitats with distinct physicochemical properties during the early juvenile stage (coinciding with the first winter). Alternatively, differences in the post-core could be explained by interannual variability in environmental conditions, because albacore captured in the two geographic areas were sampled from different cohorts (Fig. 3). However, if differences in otolith Sr : Ca and Ba : Ca were linked to interannual variability, differences would also be reflected in the core signature. Given that the core signature of albacore from the two regions were statistically similar, we conclude that differences in the post-core portion are linked to migrations to different habitats. Similarly, Wells et al. (2015) found little evidence to support separate production zones of albacore for the eastern North Pacific, but found different recent environmental history of two subgroups of juvenile albacore inhabiting the California Current.
Differences in Sr : Ca along the otolith transects were observed between the two groups. Sr is incorporated into the otolith in proportion to that of ambient seawater composition (Secor and Rooker 2000) and, given its positive relationship with water salinity (Secor and Rooker 2000; Martin et al. 2004), otolith Sr : Ca is often used to reconstruct the environmental salinity history (Limburg et al. 2001; Jessop et al. 2002; Wang et al. 2009). Otolith Sr : Ca fluctuations in our samples were interpreted as annual migratory movements between water masses of differing salinities. Generally, peaks of Sr concentration coincided with narrow opaque bands (slow growth zone generally associated with winter), suggesting that during winter the albacore migrate to high-salinity regions, such as tropical and subtropical regions of the North Atlantic Ocean. The central Atlantic Ocean is considered the main wintering area for albacore (Bard 1981), although there are major uncertainties given the scarcity of fisheries data on juvenile albacore during the winter months. Migration to high-salinity waters is visible during the first winter for the individuals captured in the Bay of Biscay (100%). In contrast, most fish captured in offshore waters (55%) do not reflect a migration signal until age-2, or the amplitude of the otolith Sr : Ca cycle at age-1 is smaller, suggesting that the magnitude of movements that may take place at age-1 is more limited. PCA applied to otolith Sr : Ca transects from 0 to 4 years reinforces the partitioning of albacore migratory patterns during the juvenile stage into two units.
Based on the differences in otolith post-core Sr : Ca and Ba : Ca and the timing of Sr : Ca cycles, albacore in the North Atlantic Ocean could be classified into two different migratory contingents: (1) a group captured within the Bay of Biscay, with early life movements between different water masses (starting at age-1+); and (2) a group formed by individuals captured in offshore waters, with limited movements during the early life or annual migration starting at later stages.
Davies et al. (2011) described the potential presence of three different subpopulations within the eastern North Atlantic Ocean, which fits with our findings based on movement patterns. Furthermore, Bard (1981) reported that albacore captured in the Bay of Biscay (Cantabrian Sea) and offshore waters (Azores Islands) present different morphometric characteristics and hypothesised that different subpopulations may follow a different migration route in the eastern North Atlantic Ocean. Despite the fact that we found no evidence of different nursery areas, our results support the occurrence of two migratory contingents of albacore in the eastern North Atlantic Ocean. Further research is needed to determine whether the two contingents follow different migration routes or differences in elemental chemistry are generated by the timing of the movements.
Observed differences in otolith chemistry between the two locations suggest that both classes of natural markers are useful, and further development and application of the approach for understanding the population structure of albacore in the North Atlantic Ocean is warranted. The combination of stable isotopes and trace elements in otoliths of albacore shows great potential for studying the variability in migration patterns, physiology and life histories among groups. Assessing interannual variations in stable isotopes and trace elements, as well as extending this work to the broader North Atlantic Ocean, will help clarify the highly complex population structure of albacore, which is crucial for sustainable management and exploitation of the fishery.
Acknowledgements
The authors thank fisheries observers, L. Naval and the staff from ‘Nardin’ and ‘Batteleku’ for their assistance collecting tuna samples, and X. Salaberria for his contribution to otolith preparation. The authors also thank B. Morales-Nin for helpful comments on a previous version of the manuscript. This work was supported by grant 251BI20090047 from the Basque Government. This is article number 735 from AZTI-Tecnalia.
References
Albaina, A., Iriondo, M., Velado, I., Laconcha, U., Zarraonaindia, I., Arrizabalaga, H., Pardo, M. A., Lutcavage, M., Grant, W. S., and Estonba, A. (2013). Single nucleotide polymorphism discovery in albacore and Atlantic bluefin tuna provides insights into worldwide population structure. Animal Genetics 44, 678–692.| Single nucleotide polymorphism discovery in albacore and Atlantic bluefin tuna provides insights into worldwide population structure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs12mtbzL&md5=87d24997949b532f9c98bd45cf78fc75CAS | 23668670PubMed |
Antonov, J. I., Seidov, D., Boyer, T. P., Locarnini, R. A., Mishonov, A. V., García, H. E., Baranova, O. K., Zweng, M. M., and Johnson, D. R. (2010). ‘World Ocean Atlas 2009 Volume 2: Salinity.’ (Ed. S. Levitus.) NOAA Atlas NESDIS 69. (US Government Printing Office: Washington, DC.) Available at http://www.nodc.noaa.gov/OC5/indprod.html [Verified 18 January 2016].
Arrizabalaga, H., Costas, E., Juste, J., González-Garcés, A., Nieto, B., and Victoria, L. R. (2004). Population structure of albacore Thunnus alalunga inferred from blood groups and tag–recapture analyses. Marine Ecology Progress Series 282, 245–252.
| Population structure of albacore Thunnus alalunga inferred from blood groups and tag–recapture analyses.Crossref | GoogleScholarGoogle Scholar |
Bard, F. X. (1981). Le thon germon (Thunnus alalunga Bonnaterre 1788) de l’Océan Atlantique. Ph.D. Thesis, Université de Paris 6.
Bard, F. X. (2001). Extension of the geographical and vertical habitat of albacore (T. alalunga) in the North Atlantic. Possible consequences on true rate of exploitation of this stock. ICCAT Collective Volume of Scientific Papers 52, 1447–1456.
Brophy, D., Jeffries, E., and Danilowicz, S. (2004). Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural origins. Marine Biology 144, 779–786.
| Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural origins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXis1Ghurw%3D&md5=f7a81a61a0f77c9064c590db2393964dCAS |
Brown, M. (1998). Ocean Data View 4.0. Oceanography 11, 19–21.
| Ocean Data View 4.0.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., Fowler, A. J., and Jones, C. M. (1994). Elemental fingerprinting for stock identification of Atlantic cod (Gadus morhua) using laser ablation ICPMS. Canadian Journal of Fisheries and Aquatic Sciences 51, 1942–1950.
| Elemental fingerprinting for stock identification of Atlantic cod (Gadus morhua) using laser ablation ICPMS.Crossref | GoogleScholarGoogle Scholar |
Cosgrove, R., Arregi, I., Brophy, D., Arrizabalaga, H., Ortiz de Zárate, V., and Griffin, N. (2010). A simulated archival tagging programme for albacore (Thunnus alalunga) in the Northeast Atlantic, including an analysis of factors affecting tag recovery. ICES Journal of Marine Science 67, 1216–1221.
| A simulated archival tagging programme for albacore (Thunnus alalunga) in the Northeast Atlantic, including an analysis of factors affecting tag recovery.Crossref | GoogleScholarGoogle Scholar |
Davies, C. A., Brophy, D., Jeffries, T., and Gosling, E. (2010). Trace elements in the otoliths and dorsal spines of albacore tuna (Thunnus alalunga, Bonnaterre, 1788): an assessment of the effectiveness of cleaning procedures at removing postmortem contamination. Journal of Experimental Marine Biology and Ecology 296, 162–170.
Davies, C. A., Gosling, E. M., Was, A., Brophy, D., and Tysklind, N. (2011). Microsatellite analysis of albacore tuna (Thunnus alalunga): population genetic structure in the north-east Atlantic Ocean and Mediterranean Sea. Marine Biology 158, 2727–2740.
| Microsatellite analysis of albacore tuna (Thunnus alalunga): population genetic structure in the north-east Atlantic Ocean and Mediterranean Sea.Crossref | GoogleScholarGoogle Scholar |
DiMaria, R. A., Miller, J. A., and Hurst, T. P. (2010). Temperature and growth effects on otolith elemental chemistry of larval Pacific cod, Gadus macrocephalu. Environmental Biology of Fishes 89, 453–462.
| Temperature and growth effects on otolith elemental chemistry of larval Pacific cod, Gadus macrocephalu.Crossref | GoogleScholarGoogle Scholar |
Dufour, F., Arrizabalaga, H., Irigoien, X., and Santiago, J. (2010). Climate impacts on albacore and bluefin tunas migrations phenology and spatial distribution. Progress in Oceanography 86, 283–290.
| Climate impacts on albacore and bluefin tunas migrations phenology and spatial distribution.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., and Gillanders, B. M. (2002). Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish. Canadian Journal of Fisheries and Aquatic Sciences 59, 1796–1808.
| Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtlKhs70%3D&md5=9c62e75165ef1551930f65f7273f6c50CAS |
Farley, J. H., Williams, A. J., Hoyle, S. D., Davies, C. R., and Nicol, S. J. (2013). Reproductive dynamics and potential annual fecundity of South Pacific albacore tuna (Thunnus alalunga). PLoS One 8, e60577.
| Reproductive dynamics and potential annual fecundity of South Pacific albacore tuna (Thunnus alalunga).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmtVehtL8%3D&md5=ef3931bdc4ce1d7601a0a37a90fc0319CAS | 23565258PubMed |
Fonteneau, A. (2010). On the North Atlantic albacore stock and on its potential sub-populations. ICCAT Collective Volume of Scientific Papers 65, 1282–1290.
Fraile, I., Arrizabalaga, H., and Rooker, J. (2015). Origin of Atlantic bluefin tuna (Thunnus thynnus) in the Bay of Biscay. ICES Journal of Marine Science 72, 625–634.
| Origin of Atlantic bluefin tuna (Thunnus thynnus) in the Bay of Biscay.Crossref | GoogleScholarGoogle Scholar |
Friedman, I., and O’Neil, J. R. (1977). Compilation of stable isotope fractionation factors of geochemical interest. In ‘Data of Geochemistry’. (Ed. M. Fleischer.) pp. 1–12. (United States Government Printing Office: Washington, DC.).
Goñi, N., and Arrizabalaga, H. (2005). Analysis of juvenile North Atlantic albacore (Thunnus alalunga) catch per unit effort by surface gears in relation to environmental variables. ICES Journal of Marine Science 62, 1475–1482.
| Analysis of juvenile North Atlantic albacore (Thunnus alalunga) catch per unit effort by surface gears in relation to environmental variables.Crossref | GoogleScholarGoogle Scholar |
Harwood, A. J. P., Dennis, P. F., Marca, A. D., Pilling, G. M., and Millner, R. S. (2008). The oxygen isotope composition of water masses within the North Sea. Estuarine, Coastal and Shelf Science 78, 353–359.
| The oxygen isotope composition of water masses within the North Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtVKltbc%3D&md5=9570238cbb0b3f2920cf903aeda3924cCAS |
Hilborn, R., Punt, A. E., and Orenasanz, J. (2004). Beyond band-aids in fisheries management: fixing world fisheries. Bulletin of Marine Science 74, 493–507.
ICCAT (2011). Albacore. In ‘ICCAT Manual’. ICCAT Technical Report. (International Commission for the Conservation of Atlantic Tunas.) Available at http://www.iccat.es/Documents/SCRS/Manual/CH2/2_1_4_ALB_ENG.pdf [Verified 27 February 2016].
ICCAT (2012). Report of the Standing Committee on Research and Statistics (SCRS), Madrid, Spain, 1–5 October, executive summary ALB, 60–80. (International Commission for the Conservation of Atlantic Tunas: Madrid, Spain.)
ICCAT (2014). Report of the 2013 ICCAT Albacore Stock Assessment Session. ICCAT Collective Volume of Scientific Papers 70, 830–995.
| Report of the 2013 ICCAT Albacore Stock Assessment Session.Crossref | GoogleScholarGoogle Scholar |
Jessop, B. M., Shiao, J. C., Iizuka, Y., and Tzeng, W. N. (2002). Migratory behaviour and habitat use by American eels Anguilla rostrata as revealed by otolith microchemistry. Marine Ecology Progress Series 233, 217–229.
| Migratory behaviour and habitat use by American eels Anguilla rostrata as revealed by otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Jochum, K. P., Weis, U., Stoll, B., Kuzmin, D., Yang, Q., Raczek, I., Jacob, D. E., Stracke, A., Birbaum, K., Frick, D. A., Günther, D., and Enzweiler, J. (2011). Determination of reference values for NIST SRM 610617 glasses following ISO guidelines. Geostandards and Geoanalytical Research 35, 397–429.
| Determination of reference values for NIST SRM 610617 glasses following ISO guidelines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XisFGlu78%3D&md5=86cdeeb3311e4795a2a10b669006da82CAS |
Johnson, K. S., Bereison, W. M., Coale, K. H., Coley, T. L., Elrod, V. A., Fairey, R. W., Iams, H. D., Kilgore, T. E., and Nowicki, J. L. (1992). Manganese flux from continental margin sediments in a transect through the oxygen minimum. Science 257, 1242–1245.
| Manganese flux from continental margin sediments in a transect through the oxygen minimum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XlslOrtrk%3D&md5=78c0d8e6b03aec47352e4838577d060eCAS | 17742757PubMed |
Kim, S. T., O’Neil, J. R., Hillaire-Marcel, C., and Mucci, A. (2007). Oxygen isotope fractionation between synthetic aragonite and water: influence of temperature and Mg2+ concentration. Geochimica et Cosmochimica Acta 71, 4704–4715.
| Oxygen isotope fractionation between synthetic aragonite and water: influence of temperature and Mg2+ concentration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFyku7jM&md5=31b0a061215f6263e01f9d280b93af0eCAS |
Klinkhammer, G. P., and McManus, J. (2001). Dissolved manganese in the Columbia River estuary: production in the water column. Geochimica et Cosmochimica Acta 65, 2835–2841.
| Dissolved manganese in the Columbia River estuary: production in the water column.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtVOmu7w%3D&md5=3e1bc88679174bbd0fea44dde8e2e2b4CAS |
Koto, T. (1969). Distribution and movement of the albacore in the Indian and the Atlantic oceans based on the catch statistics of Japanese tuna long-line fishery. Bulletin of Far Seas Fisheries Research Laboratory 1, 115–129.
Landing, W. M., and Bruland, K. W. (1980). Manganese in the North Pacific. Earth and Planetary Science Letters 49, 45–56.
| Manganese in the North Pacific.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXlt1Ckug%3D%3D&md5=0de56540c53ae262c5144ffe48810e2fCAS |
Lehodey, P., Senina, I., Dragon, A. C., and Arrizabalaga, H. (2014). Spatially explicit estimates of stock size, structure and biomass of North Atlantic albacore tuna (Thunnus alalunga). Earth System Science Data 6, 317–329.
| Spatially explicit estimates of stock size, structure and biomass of North Atlantic albacore tuna (Thunnus alalunga).Crossref | GoogleScholarGoogle Scholar |
Limburg, K. E., Landergren, P., Westin, L., Elfman, M., and Kristiansson, P. (2001). Flexible modes of anadromy in Baltic sea trout: making the most of marginal spawning streams. Journal of Fish Biology 59, 682–695.
| Flexible modes of anadromy in Baltic sea trout: making the most of marginal spawning streams.Crossref | GoogleScholarGoogle Scholar |
Ludsin, S. A., Fryer, B. J., and Gagnon, J. E. (2006). Comparison of solution-based versus laser-ablation ICPMS for analysis of larval fish otoliths. Transactions of the American Fisheries Society 135, 218–231.
| Comparison of solution-based versus laser-ablation ICPMS for analysis of larval fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Macdonald, J. I., Farley, J. H., Clear, N. P., Williams, A. J., Carter, T. I., Davies, C. R., and Nicol, S. J. (2013). Insights into mixing and movement of South Pacific albacore Thunnus alalunga derived from trace elements in otoliths. Fisheries Research 148, 56–63.
| Insights into mixing and movement of South Pacific albacore Thunnus alalunga derived from trace elements in otoliths.Crossref | GoogleScholarGoogle Scholar |
Martin, G. B., and Thorrold, S. R. (2005). Temperature and salinity effects on magnesium, manganese, and barium incorporation in otoliths of larval and early juvenile spot Leiostomus xanthurus. Marine Ecology Progress Series 293, 223–232.
| Temperature and salinity effects on magnesium, manganese, and barium incorporation in otoliths of larval and early juvenile spot Leiostomus xanthurus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXps1CqtLw%3D&md5=364a12eec67799367dac243881ae0d9fCAS |
Martin, G. B., Thorrold, S. R., and Jones, C. M. (2004). Temperature and salinity effects on strontium incorporation in otoliths of larval spot (Leiostomus xanthurus). Canadian Journal of Fisheries and Aquatic Sciences 61, 34–42.
| Temperature and salinity effects on strontium incorporation in otoliths of larval spot (Leiostomus xanthurus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjslylurw%3D&md5=b843292a4e1d4e25006cb7facf07d27dCAS |
Mercier, L., Darnaude, A. M., Bruguier, O., Vasconcelos, R. P., Cabral, H. N., Costa, M. J., Lara, M., Jones, D. L., and Mouillot, D. (2011). Selecting statistical models and variable combinations for optimal classification using otolith microchemistry. Ecological Applications 21, 1352–1364.
| Selecting statistical models and variable combinations for optimal classification using otolith microchemistry.Crossref | GoogleScholarGoogle Scholar | 21774435PubMed |
Montes, I., Iriondo, M., Manzano, C., Arrizabalaga, H., Jimnez, E., Pardo, M. A., Goñi, N., Davies, C. A., and Estonba, A. (2012). Worldwide genetic structure of albacore Thunnus alalunga revealed by microsatellite DNA markers. Marine Ecology Progress Series 471, 183–191.
| Worldwide genetic structure of albacore Thunnus alalunga revealed by microsatellite DNA markers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltFChsrk%3D&md5=65a9db1564574d23e62e9a828bdd4c80CAS |
Ortiz de Zárate, V., Lavin, A., and Moreno-Ventas, X. (1998). Is there a relationship between environmental variables and the surface catch of albacore (Thunnus alalunga, Bonnaterre, 1788) in the North Atlantic? ICCAT Collective Volume of Scientific Papers 48, 250–252.
Ortiz de Zárate, V., Macías, D., Satoh, K., and Saito, H. (2004). Information on the reproduction of albacore (Thunnus alalunga) in the central and tropical North Atlantic in 2002. ICCAT Collective Volume of Scientific Papers 56, 1450–1462.
Paton, C., Hellstrom, J., Paul, B., Woodhead, J., and Hergt, J. (2011). Iolite: freeware for the visualization and processing of mass spectrometric data. Journal of Analytical Atomic Spectrometry 26, 2508–2518.
| Iolite: freeware for the visualization and processing of mass spectrometric data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVKhs7rM&md5=08cee72054fa398cc91ede5cc1b36a3dCAS |
Paul, B., Paton, C., Norris, A., Woodhead, J., Hellstrom, J., Hergt, J., and Greig, A. (2012). CellSpace: a module for creating spatially registered laser ablation images within the Iolite freeware environment. Journal of Analytical Atomic Spectrometry 27, 700–706.
| CellSpace: a module for creating spatially registered laser ablation images within the Iolite freeware environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjslalu7w%3D&md5=0b07e1cb2ba8d74474946ca91e62ab3dCAS |
Renck, C. L., Wells, R. J. D., and Dewar, H. (2014). Regional growth patterns of juvenile albacore (Thunnus alalunga) in the eastern North Pacific. California Cooperative Oceanic Fisheries Investigations Reports 55, 135–143.
Rooker, J. R., Secor, D. H., DeMetrio, G., Kaufman, A. J., Rios, A. B., and Ticina, V. (2008). Evidence of trans-Atlantic movement and natal homing of bluefin tuna from stable isotopes in otoliths. Marine Ecology Progress Series 368, 231–239.
| Evidence of trans-Atlantic movement and natal homing of bluefin tuna from stable isotopes in otoliths.Crossref | GoogleScholarGoogle Scholar |
Rooker, J. R., Arrizabalaga, H., Fraile, I., Secor, D. H., Dettman, D. L., Abid, N., Addis, P., Deguara, S., Karakulak, S., Kimoto, A., Sakai, O., Macías-López, A. D., and Neves-Santos, M. (2014). Crossing the line: migratory and homing behaviours of Atlantic bluefin tuna. Marine Ecology Progress Series 504, 265–276.
| Crossing the line: migratory and homing behaviours of Atlantic bluefin tuna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtlKju7rI&md5=6ce3675bac303716c4594cec9343b10fCAS |
Sagarminaga, Y., and Arrizabalaga, H. (2014). Relationship of Northeast Atlantic albacore juveniles with upper surface thermal and chlorophyll-a fronts. Deep-sea Research. Part II, Topical Studies in Oceanography 107, 54–63.
| Relationship of Northeast Atlantic albacore juveniles with upper surface thermal and chlorophyll-a fronts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1Ors7jO&md5=2c4502b9380ab061b874995de4a35071CAS |
Santiago, J. (2004). Dinámica de la población de atún blanco (Thunnus alalunga Bonaterre 1788) del Atlántico norte. Ph.D. Thesis, Servicio de Publicaciones del Gobierno Vasco, University of the Basque Country, Leioa, Spain.
Santiago, J., and Arrizabalaga, H. (2005). An integrated growth study for North Atlantic albacore (Thunnus alalunga Bonn. 1788). ICES Journal of Marine Science 62, 740–749.
| An integrated growth study for North Atlantic albacore (Thunnus alalunga Bonn. 1788).Crossref | GoogleScholarGoogle Scholar |
Secor, D. H., and Rooker, J. R. (2000). Is otolith strontium a useful scalar of life cycles in estuarine fishes? Fisheries Research 46, 359–371.
Ueyanagi, S. (1971). Larval distribution of tunas and billfishes in the Atlantic Ocean. FAO Fisheries Report 71, 297–305.
Wang, C. H., Lin, Y. T., Shiao, J. C., You, C. F., and Tzeng, W. N. (2009). Spatiotemporal variation in the elemental compositions of otoliths of southern bluefin tuna Thunnus maccoyii in the Indian Ocean and its ecological implication. Journal of Fish Biology 75, 1173–1193.
| Spatiotemporal variation in the elemental compositions of otoliths of southern bluefin tuna Thunnus maccoyii in the Indian Ocean and its ecological implication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1SltbfL&md5=7ca0ae577b94c92fb6a8c703fd57f34bCAS | 20738607PubMed |
Wells, R. J. D., Rooker, J. R., and Itano, D. G. (2012). Nursery origin of yellowfin tuna in the Hawaiian Islands. Marine Ecology Progress Series 461, 187–196.
| Nursery origin of yellowfin tuna in the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFeru7%2FJ&md5=76004ddc3ab3e4ec410dad51d84be7cbCAS |
Wells, R. J. D., Kohin, S., Teo, S. L. H., Snodgrass, O. E., and Uosaki, K. (2013). Age and growth of North Pacific albacore (Thunnus alalunga): implications for stock assessment. Fisheries Research 147, 55–62.
| Age and growth of North Pacific albacore (Thunnus alalunga): implications for stock assessment.Crossref | GoogleScholarGoogle Scholar |
Wells, R. J. D., Kinney, M. J., Kohin, S., Dewar, H., Rooker, J. R., and Snodgrass, O. E. (2015). Natural tracers reveal population structure of albacore (Thunnus alalunga) in the eastern North Pacific. ICES Journal of Marine Science 72, 2118–2127.
| Natural tracers reveal population structure of albacore (Thunnus alalunga) in the eastern North Pacific.Crossref | GoogleScholarGoogle Scholar |
Zeileis, A., and Grothendieck, G. (2005). zoo: S3 infrastructure for regular and irregular time series. Journal of Statistical Software 14, 1–27.
| zoo: S3 infrastructure for regular and irregular time series.Crossref | GoogleScholarGoogle Scholar |