The ‘Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests
Scott Bennett A B I , Thomas Wernberg A , Sean D. Connell C D , Alistair J. Hobday E , Craig R. Johnson F and Elvira S. Poloczanska G HA UWA Oceans Institute and School of Plant Biology, University of Western Australia, Crawley, WA 6009, Australia.
B Department of Environment and Agriculture, Curtin University, Bentley, WA 6102, Australia.
C Southern Seas Ecology Laboratories, The University of Adelaide, Adelaide, SA 5005, Australia.
D The Environment Institute and School of Biological Sciences, The University of Adelaide, Adelaide, SA 5005, Australia.
E CSIRO Oceans and Atmosphere Flagship, Hobart, Tas. 7000, Australia.
F Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, Tas. 7001, Australia.
G CSIRO Oceans and Atmosphere Flagship, Ecosciences Precinct, Brisbane, Qld 4001, Australia.
H Global Change Institute, The University of Queensland, Brisbane, Qld 4072, Australia.
I Corresponding author. Email: scott.bennett1@curtin.edu.au
Marine and Freshwater Research 67(1) 47-56 https://doi.org/10.1071/MF15232
Submitted: 17 June 2015 Accepted: 31 July 2015 Published: 27 August 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Kelp forests define >8000 km of temperate coastline across southern Australia, where ~70% of Australians live, work and recreate. Despite this, public and political awareness of the scale and significance of this marine ecosystem is low, and research investment miniscule (<10%), relative to comparable ecosystems. The absence of an identity for Australia’s temperate reefs as an entity has probably contributed to the current lack of appreciation of this system, which is at odds with its profound ecological, social and economic importance. We define the ‘Great Southern Reef’ (GSR) as Australia’s spatially connected temperate reef system. The GSR covers ~71 000 km2 and represents a global biodiversity hotspot across at least nine phyla. GSR-related fishing and tourism generates at least AU$10 billion year–1, and in this context the GSR is a significant natural asset for Australia and globally. Maintaining the health and ecological functioning of the GSR is critical to the continued sustainability of human livelihoods and wellbeing derived from it. By recognising the GSR as an entity we seek to boost awareness, and take steps towards negotiating the difficult challenges the GSR faces in a future of unprecedented coastal population growth and global change.
Additional keywords: ecosystem services, ecosystem values, temperate reef.
The Great Southern Reef – the case for an identity
Temperate reefs are hard-bottom marine ecosystems found in cool waters between the tropics and the poles. Temperate reef ecosystems are diverse, spanning supralittoral lichen-encrusted boulders to sponge gardens on rocky outcrops in the deep oceans. Worldwide, where enough light penetrates to the seafloor, healthy temperate reefs are usually dominated by ‘kelp forests’ – highly productive, structurally complex communities of canopy-forming seaweeds of the orders Laminariales, Desmarestiales and Fucales (Bolton 2010; Steneck and Johnson 2013). Depending on light, waves and grazers, kelp forests are found from the shoreline down to depths of 30–40 m (Marzinelli et al. 2015), but can be found at depths >60 m in some regions (e.g. Graham et al. 2007). Here, we recognise the diversity of temperate reef ecosystems, but focus primarily on kelp forests as they prevail in shallow and coastal waters, where most human attention and activity is concentrated.
In Australia, shallow (<30 m) temperate reefs are defined largely by the distribution of Ecklonia radiata kelp forests (Fig. 1), which span more than 8000 km of coastline from the subtropical waters of northern New South Wales (~28.5°S), down the east coast of mainland Australia, around Tasmania, along Australia’s southern coastline and north as far as Kalbarri (27.7°S) in Western Australia (Table S1) (Underwood et al. 1991; Connell and Irving 2008; Wernberg et al. 2011; Marzinelli et al. 2015). Most of Australia’s kelp-dominated temperate reefs lie within the ‘coastal zone’ under state jurisdiction (3 nautical miles or 5.5 km from shore), and are therefore managed independently by the five states in which they occur (Fig. 1). Perhaps as a consequence, Australia’s temperate reefs have long been perceived and managed in local contexts, without broader recognition of their significance as an interconnected system. This lack of identity stifles public and political awareness about their importance, not only of their ecology per se, but also their importance to the livelihoods of millions of Australians who live, work and engage in recreation around them, and in providing valuable ecosystem services to Australians.
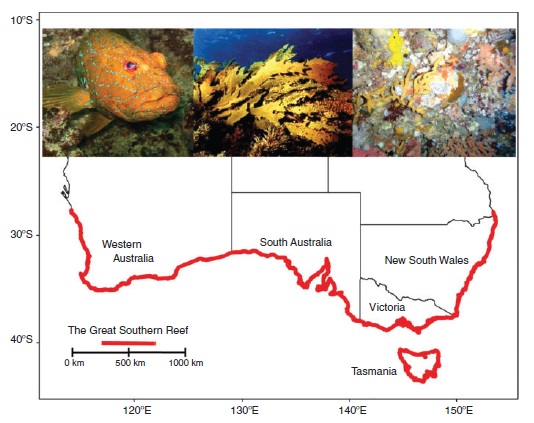
|
Just as the Great Barrier Reef (GBR) is recognised as an entity made up of more than 2900 individual reefs dominated by corals (Day 2002), Australia’s temperate reefs should be considered collectively as an entity made up of thousands of kilometres of rocky temperate reefs, dominated by kelp forests and interconnected through oceanographic (Coleman et al. 2011; Wernberg et al. 2013a), ecological (Irving and Connell 2006; Connell and Irving 2008; Vanderklift and Wernberg 2008) and evolutionary (Phillips 2001) processes – truly a Great Southern Reef (GSR). Here we show that lack of awareness and investment in research focussed on temperate reefs is at odds with the social, ecological and economic significance of the GSR. Australians ignore the GSR and its contribution to our society at our peril, but current levels of scientific engagement and public awareness threaten the health and future function of this significant integrated marine ecosystem. A multiscale view of the GSR as an integrated system would help advance management approaches that recognise both regional differences (Connell 2007), connectivity and ecosystem values (Magris et al. 2014), and assist the marine planning process based on the principles of Comprehensiveness, Adequacy and Representativeness (Day et al. 2003; Lourie and Vincent 2004) for Australian waters.
The ecological setting
The Great Southern Reef is a global biodiversity hotspot for multiple taxa including seaweeds, sponges, crustaceans, chordates, bryozoans, echinoderms and molluscs (Tables 1, S2) (Bolton 1994; O’Hara and Poore 2000; Kerswell 2006; Barnes and Griffiths 2008; Shenkar and Swalla 2011; Poore and Bruce 2012; Stöhr et al. 2012; Van Soest et al. 2012). In addition it is estimated that tens of thousands of species are yet to be discovered and described (Butler et al. 2010; Appeltans et al. 2012; Van Soest et al. 2012), many of which could play important functional roles within the GSR (e.g. Knudsen and Clements 2013). Importantly, many of the species found on the GSR make use of both the temperate reef habitat itself and adjoining inter-reef habitats such as seagrass meadows in shallow waters and the sponge ‘gardens’ that characterise deeper rocky reef habitats. As well as being important assemblages in their own right, these intermediary habitats facilitate connectivity among reefs (Vanderklift and Wernberg 2008) and act as nursery grounds for many species (Jenkins and Wheatley 1998).
|
A remarkable feature of the biodiversity of the GSR is the high rate of endemism within many taxa. For seaweeds, for example, the GSR is a global hotspot of endemism at the genus level (Kerswell 2006), indicating deep evolutionary isolation since the Oligocene that has resulted in unique and diverse biota (Hommersand 1986). An estimated 77% of the 565 species of red seaweed found on the GSR are not found anywhere else on Earth (Phillips 2001), whereas the rates of endemism for most other phyla range between 30 and 60% of the total species pool (Tables 1, S2).
The physical driver behind the GSR’s high diversity and endemism is thought to be the stable climatic and geological history it has experienced over the past 50 million years (cf. the biodiversity hotspot in the tropical Indo-Pacific: Bellwood and Meyer 2009) along with its geographical isolation (Phillips 2001; Langlois et al. 2012). The ecology of the GSR is shaped in large part by boundary currents that flow poleward along both coasts of Australia, transporting warm nutrient-poor water across Australia’s temperate coastline (e.g. Wernberg et al. 2013a). The Leeuwin Current flows southwards down the west coast and wraps along the southern coastline of the continent, whereas the East Australian Current extends down the east coast and penetrates along the east coast of Tasmania during summer (Condie and Dunn 2006). These currents play an important role in the connectivity of larvae and propagules throughout the GSR (Condie et al. 2005; Coleman et al. 2011) and maintain relatively stable climatic conditions over seasonal and evolutionary time scales (McGowran et al. 1997; Condie and Dunn 2006). Interestingly, however, although many species are shared between the eastern and western GSR, such as the dominant habitat-forming kelp Ecklonia radiata (Connell and Irving 2008; Marzinelli et al. 2015), these regions have been connected through the Bass Strait for only ~10 000 years (Waters 2008) and still have low genetic connectivity (Waters 2008; Coleman et al. 2013) and distinct biogeographical affinities (Waters et al. 2010).
The biological engine of the GSR are the kelp forests, which provide both the habitat and trophic foundation to support the system. Kelp forests are among the most productive ecosystems on the planet, with rates of productivity often exceeding that of the most intensively managed agricultural systems (Mann 1973). For example, the kelp forests of the GSR produce as much as 65 tonnes of biomass per hectare per year (de Bettignies et al. 2013), which is over 16 times more than Australia’s most fertile wheat fields (http://www.ausgrain.com.au, accessed 3 December 2014). This biological powerhouse then feeds directly into coastal ecosystems as food and detritus (Bustamante and Branch 1996; Wernberg et al. 2006; Vanderklift and Wernberg 2010; Krumhansl and Scheibling 2012), critical for the energy and nutrient cycles supporting the rich marine life throughout the GSR and out into the wider ocean beyond shelf waters (Thompson et al. 2011). The high diversity and endemism of the GSR make it globally unique and intrinsically fascinating both aesthetically and scientifically. In addition, its sheer scale and close proximity to more than two-thirds of the Australian population (ABS 2001) means that the GSR forms an integral part of Australian culture and society. In turn, the GSR plays an important role in Australia’s national economy, supporting a range of tourism ventures, and recreational and commercial fisheries.
Ecosystem services and the economics of the GSR
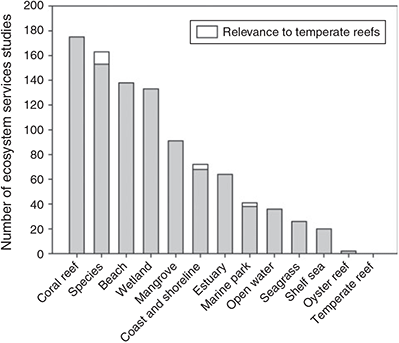
Ecosystem goods and services (herein referred to collectively as ecosystem services) are the direct (e.g. food production) or indirect (e.g. climate regulation, nutrient cycling, coastal protection) benefits that humans derive from ecosystems (Costanza et al. 1997). Quantifying the value of ecosystem services provided by the Great Southern Reef is critical as one measure of its importance to society, and to enable comparisons with other natural systems. Remarkably, analysis of 961 studies from a global database revealed that the value of ecosystem services provided by temperate reefs or kelp forest have scarcely been quantified for any region, let alone in Australia (Marine Ecosystem Services Partnership (MESP), see http://www.marineecosystemservices.org/, accessed 2 December 2014) (Fig. 2). As a result, major global and regional assessments of the value of ecosystem services do not recognise temperate reefs as distinct ecosystems but instead aggregate them with other shelf habitats (Costanza et al. 1997, 2014; de Groot et al. 2012). On the basis of these global assessments, the economic value of habitats that include temperate reefs is estimated at US$26 226–28 917 ha–1 year–1 based on 2007 dollar values (de Groot et al. 2012; Costanza et al. 2014). These estimates inevitably misrepresent the value of temperate reefs for several reasons, not least of which is that without data on ecosystem services specifically provided by temperate reefs, their contribution cannot be included in the overall valuation. The profound implications of this are illustrated for coral reefs, where the estimated value of ecosystem services increased by a factor of 42 between 1997 and 2012, primarily due to new research on the value of coral reefs (Costanza et al. 2014). Similar valuations would likely apply to temperate reefs; however, currently there are insufficient studies to accurately estimate their value. For example, kelp forests are capable of rapid biomass accumulation and represent globally significant standing stocks of carbon. Information on the fraction that is sequestered into long-term carbon sinks such as marine sediments and deep ocean, thus contributing to climate change mitigation, is unknown (Laffoley and Grimsditch 2009). Harvesting and appropriate use of seaweeds from cultivated and wild sources (e.g. biofuels) could play a significant role in greenhouse gas mitigation (Chung et al. 2011), but does not factor into current valuations of temperate reef ecosystems. Similarly, the indirect positive effect of kelp forests enhancing fisheries (Bertocci et al. 2015) is unaccounted for.
|
Although they are conservative, existing estimates of the value of ecosystem services from temperate reefs highlight the important contribution that they make to human welfare, particularly for services that do not directly contribute to the economy. The value of seaweed and seagrass habitats estimated by Costanza et al. (1997) was almost entirely attributable to nutrient cycling within these ecosystems. On reefs shallower than 30 m where seaweed canopies dominate, the GSR covers ~7.14 × 106 ha (Table 1), equating to as much as AU$187 billion year–1 of nutrient cycling services that are critical to human welfare but currently do not feature in Australia’s gross domestic product (GDP). Other more tangible ecosystem services that directly contribute to GDP, such as food production and recreation (e.g. tourism, fishing and surfing) are also missing from this valuation even if they make a significant contribution to the value of temperate reef ecosystems. Here we evaluate the ecosystem services provided by the GSR that directly contribute to the Australian economy and provide tens of thousands of jobs in industries such as commercial fishing, retail (e.g. surfing and fishing supply businesses), hospitality and tourism.
There are many commercial fisheries operating along the GSR. The two most valuable are the rock lobster and abalone fisheries, which together contribute ~AU$500 million year–1 to the Australian economy (Table 1, Fig. 3). The Western Australian rock lobster fishery is the most valuable single-species fishery in the country and is worth ~AU$185 million year–1 (Table S3). Similarly, wild-caught abalone in Tasmania alone are worth almost AU$100 million year–1 (Table S3). By way of comparison, the combined gross value of commercial fisheries catches from the GBR region is ~AU$120 million year–1 (Deloitte Access Economics 2013), less than one-quarter of the value of rock lobster and abalone on the GSR. Standardised by area, rock lobster and abalone fishing on the GSR is more than seven times more valuable than all commercial fishing on the GBR combined.
The value of tourism directly associated with the GSR has not been measured for any local or regional sections of reef, as far as we are aware. Nevertheless, total tourism expenditure in coastal municipalities immediately adjacent to the GSR provides an approximate indication of its value. Tourism along the GSR represents a multibillion dollar industry (AU$38 billion year–1, based on 2007–08 figures: Tables 1, S4) (Tourism Research Australia 2011) and is particularly vital to regional economies along the GSR. Of course, not all tourism expenditure can be directly attributed to the GSR and these figures inevitably overestimate direct, reef-related values, particularly in major cities where tourists are attracted for many reasons. Nevertheless, in regional coastal communities alone (i.e. excluding the cities of Sydney, Newcastle, Wollongong, Melbourne, Adelaide and Perth), where reef-related tourism such as fishing, scuba-diving, surfing ‘reef breaks’, whale watching and other ecotourism ventures are a major drawcard, total tourism expenditure is estimated at ~AU$9.8 billion year–1, representing ~15% of the total economic activity in some regional areas (e.g. Philip Island = 18.7%, Tasmanian west coast = 16.2%, Kangaroo Island and Tasmanian east coast = 14%: Tourism Research Australia 2011). The GSR is therefore a major contributor to the socio-economic fabric of Australia and particularly that of regional coastal communities (Metcalf et al. 2014).
Recreational activities on the GSR are similarly large, primarily due to the spatial scale and concentration of population centres along its shores. Approximately 67% of the Australian population live within 50 km of the GSR (ABS 2001), which if scaled to Australia’s 2014 population, equates to 15.9 million people. Of those, it is estimated that ~5.3 million regularly participate in recreational fishing. On the basis of 2001 values, recreational fishing along the GSR is estimated to be worth ~AU$500 million year–1, with over 6 million person-fishing-days conducted each year (Tables 1, S5). Nationally, coastal-water fishing effort is the highest among all waterbody-types (41%), with 9.5 million fishing events per year, compared with 35% in estuaries, 11% in rivers, 8% in lakes and dams, and 4% offshore (Henry and Lyle 2003). Owing to their size, the highest numbers of recreational fishers in Australia are concentrated adjacent to the major cities of Sydney, Melbourne and Perth, which are also experiencing the highest rates of population growth in the nation. Therefore, although more recent participation rates are not available, the economic value of recreational fishing on the GSR will inevitably have grown over the past decade. The economic value of other forms of recreation on the GSR is difficult to quantify; however, a range of other activities take advantage of the wild natural beauty, pristine waters and healthy ecosystems of the GSR even if not directly associated with its kelp forests. For example, most of the estimated 2.7 million surfers in Australia (www.surfingaustralia.com.au) live adjacent to the GSR. Much of the recreation and tourism in iconic places such as Phillip Island and Torquay in Victoria, or Margaret River in Western Australia, are based around surfing ‘reef breaks’ that form on the GSR and attract millions of visitors each year (Tourism Research Australia 2011).
Public perception and research investment
Despite its intrinsic and economic value, public perceptions of the Great Southern Reef are low compared with other comparable natural assets in Australia such as the GBR. Similarly, or perhaps as a consequence, rates of research investment into understanding the ecology that underpins the GSR ecosystem are also low.
Quantifying perceptions of the GSR by the Australian public is challenging. We use news reports as a proxy for public awareness but acknowledge that the relationship between news reports and public awareness is not necessarily a simple one (Duarte et al. 2008). We searched the online archives of 15 major news outlets from across Australia (Table S6) to investigate the public awareness of temperate reefs, and we compared this to coral reefs, the most comparable major ecosystem in Australia. Following the methods of Duarte et al. (2008), the electronic archives were searched during November 2014 to compare the number of news hits for (1) ‘temperate reef’ and ’coral reef’, and (2) ‘kelp’ and ‘coral’. The electronic archives all covered at least two years but did not all cover the same period. Therefore a rigorous analysis of temporal trends in reporting on the different ecosystems was not possible.
The media search revealed that for both comparisons (kelp v. coral and temperate reef v. coral reef), news reporting was more than an order of magnitude higher for coral reefs than for temperate reefs (Table S6). ‘Kelp’ accounted for 2.9% ± 0.6 (mean ± s.e.) of the total number of news reports on ‘kelp’ and ‘coral’ in Australian media. Similarly ‘temperate reefs’ accounted for only 5.6% ± 1.8 (mean ± s.e.) of all media reports on ‘temperate reef’ and ‘coral reef’. This finding was consistent irrespective of the geographical location of the media outlet. That is, despite their target audiences living thousands of kilometres away from the nearest coral reef, and immediately adjacent to the GSR, 81–99% of all reef-related stories reported in Tasmanian, Victorian and South Australian newspapers focused on coral or coral reefs.
To ascertain any patterns in public investment in research funding we searched the funding outcomes of all major funding schemes announced by the Australian Research Council since 2010 (project codes DP10, LP10 etc.) (http://www.arc.gov.au/applicants/fundingoutcomes.htm, accessed 29 March 2015) for all projects in ecology (FoR code 0602) as well as projects returned using the search words ‘coral’ and ‘kelp’. From the project description it was ascertained whether the project had a coral reef or temperate reef focus. This approach focused the search on the major habitat-formers (corals and kelps) but did not exclude other groups of organisms (e.g. barnacles, fish, anemones) or distinguish between subtidal and intertidal habitats.
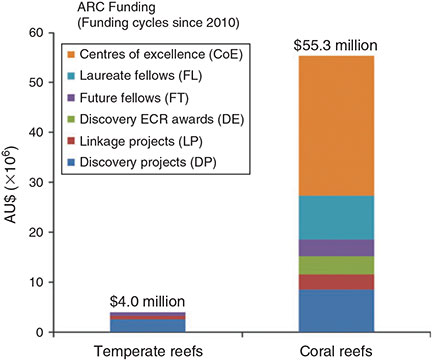
The imbalance in news reporting was also reflected in public investment in research funding within Australia, a pattern seen across all major funding schemes. Over the past five years (2010–14), coral reefs received AU$55.3 million in total research funding from the Australian Research Council, compared with only AU$4.0 million allocated to temperate reef research (Fig. 4). Australia is a world leader in coral reef research, reflecting decades of investment and the recognition of the importance of coral reef systems. The social, ecological and economic importance of kelp forests in Australia justifies a similar commitment to temperate reefs. The current funding imbalance creates a research vacuum on the GSR, and undermines the continued development of sustainable industries and knowledge-based management of the region.
|
Status and threats
Like many of Australia’s natural assets, the Great Southern Reef is relatively healthy and well managed by global standards. However, with growing pressures from climate change, population growth and coastal urban development, the GSR is at increasing risk, with many areas already showing severe signs of stress and degradation (Connell et al. 2008; Department of Sustainability, Environment, Water, Population and Communities 2011; Johnson et al. 2011; Wernberg et al. 2011b). Warming is currently occurring 2–4 times the global average in the western and south-eastern GSR respectively (Pearce and Feng 2007; Ridgway 2007; Hobday and Pecl 2014). Warming in the south-eastern GSR has been largely due to increased frequency and size of eddies of the East Australian Current propagating poleward towards Tasmania (Johnson et al. 2011). The increased influence of warm nutrient-poor water from the East Australian Current in eastern Tasmania has been associated with dramatic losses of giant kelp (Macrocystis pyrifera) forests (Johnson et al. 2011). As a consequence, in August 2012 the Australian giant kelp forests became the first marine community to be listed as endangered under the Environment Protection and Biodiversity Conservation Act (http://www.environment.gov.au/resource/giant-kelp-marine-forests-south-east-australia, accessed 4 June 2015). In 2011, gradual warming on the western GSR was punctuated by an unprecedented marine heatwave that saw summer temperatures reach 2–6°C above long-term maxima across almost 2000 km of coastline (Pearce and Feng 2013; Wernberg et al. 2013b). The marine heatwave had catastrophic impacts on the western GSR, resulting in a dramatic loss of kelp forests across more than 960 000 ha of reef in the north-western GSR (T. Wernberg and S. Bennett, unpub. data) and >100 km range contraction of other dominant canopy-forming seaweeds (Smale and Wernberg 2013). Range contractions present a serious risk of species extinction on the GSR because of the combination of lack of suitable habitat for retreat farther south and high rates of endemism (e.g. Wernberg et al. 2011a). In addition to range contractions and habitat losses, warming is also facilitating the addition of new species as warm temperate and tropical species increase in abundance and expand their ranges poleward (i.e. tropicalisation) into previously cooler environments (Wernberg et al. 2013b; Vergés et al. 2014; Bennett et al. 2015a). Observed changes include expansion of a subtidal sea urchin (Ling et al. 2009), a range of intertidal invertebrates (Pitt et al. 2010), zooplankton (Johnson et al. 2011), and coastal fishes (Johnson et al. 2011; Last et al. 2011; Bennett et al. 2015a; Robinson et al. 2015). In the case of the sea urchin (Centrostephanus rodgersii) in the south-eastern GSR, range extension and population expansion has led to overgrazing of kelp forests, resulting in barren formation and reduced fisheries productivity (Johnson et al. 2011). Similarly, in the south-western GSR tropical herbivorous fishes are now preventing the recovery of kelp forests lost during the 2011 marine heat wave (Bennett et al. 2015a).
Local stressors are also having an important impact on the health of the GSR. Kelp forests on the eastern (Coleman et al. 2008) and central (Connell et al. 2008) GSR have undergone decline and loss adjacent to intense coastal development as a result of localised pollution including nitrogen enrichment from discharge of sewage and storm water (Gorman et al. 2009). Such losses are likely to increase over the next century as the human population increases along the GSR, and as local declines accumulate they eventually coalesce to manifest as regional impacts. Furthermore, there are synergistic impacts between local nitrogen enrichment and global enrichment of carbon dioxide (i.e. ocean acidification: Connell et al. 2013) that causes a switch in the competitive dominance between perennial kelp and opportunistic seaweed turfs (Connell et al. 2014), favouring the establishment of resilient turf-dominated habitats. Not all areas of the GSR will suffer kelp loss because of the inherent stabilising processes of ecosystems (Bennett and Wernberg 2014; Bennett et al. 2015b; Connell and Ghedini 2015) that compensate for increasing effects of multiple disturbances (Ghedini et al. 2015) and broad spatial variation in the importance of particular or multiple stressors (Wernberg et al. 2011b). Because impacts ultimately depend on interactions between global and local stressors that might vary from place to place both in nature and in strength (e.g. Wernberg et al. 2010; Bennett and Wernberg 2014; Bennett et al. 2015b), understanding the current and future threats faced by the GSR from a local to continental scale is essential to enable appropriate management of the system and ensure the sustainability of the intrinsic value and ecosystem services derived from it (Connell and Irving 2009; Wernberg et al. 2011b).
Management of the GSR in a rapidly changing environment
The fastest rates of population growth in Australia are adjacent to the Great Southern Reef, and the current 16 million people within 50 km of the coast is projected to increase by another 3 million in the next decade and double in size by 2060 (based on a median growth rate 1.6%: Pink 2013). Given the associated increases in urbanisation and coastal development, maintaining and enhancing the social–ecological resilience of the GSR will be difficult in some regions, and losses of some GSR habitats are inevitable. The additional pressures from ongoing climate change will result in a range of existing and novel challenges for management of temperate reefs and associated industries along the southern coast of Australia. Importantly, management of local conditions can alleviate, if not reverse, global stressors (Wernberg et al. 2011b; Falkenberg et al. 2013) and there is substantial local impetus for participation in addressing the problems. For example, a study from South Australia found that households in the immediate area of marine habitat loss were willing to pay up to AU$67.1 million for improvements to waste-water management to reduce impacts (MacDonald et al. 2015).
Increased recognition of ecological linkages across southern Australia and the GSR as an entity will strengthen the potential for cooperative management among the federal and state governments. As a starting point, greater understanding of the functioning of the GSR entity will be required, with new ‘big picture’ research focusing on linkages and contrasts across the GSR system. Consistent governance through spatially flexible approaches will likely provide the most ecologically sensible and cost-effective management strategies. For example, currently variable fisheries regulations both within and among states (e.g. licensing, gear restrictions, seasonal closures and bag limits) may need to be harmonised for highly mobile and widespread species whereas locally appropriate management targets (e.g. nitrogen loads: Gorman et al. 2009) will be required where regional differences in, for example, sensitivity to water pollution and fishing are recognised (Connell 2007). Novel industries that exploit the GSR may arise, including bioprospecting for, and harvesting of, unique seaweed and sponge species, which will require new or cooperative legislation. Recall that our forbearers effectively fished-out native oyster reefs before the formation of the Australian parliament, representing the wholesale loss of thousands of kilometres of temperate habitat needed to maintain fish production and water quality (Alleway and Connell 2015). By recognising the GSR as a holistic and connected system, piecemeal decision-making can be avoided, and the importance of integrated planning and cumulative risk recognised, such that Australia’s temperate reefs continue to support and deliver valuable socio-economic and ecological services into the future.
Conclusions
There is currently a paradoxical (and concerning) mismatch between the low public awareness and investment in Australia’s temperate reef ecosystem and its high ecological and economic value for Australian society. By recognising the ‘Great Southern Reef’ as an entity and defining the benefits derived from it as a whole, we have highlighted the profound importance of this system. This process is only a first step towards a broader discussion about, and greater scrutiny of, the values of the GSR that will engender relationships and perceptions of Australian society towards this system. Given the rapid historical rates of environmental change throughout temperate Australia (Lough and Hobday 2011) and projected changes (Hobday and Lough 2011; Oliver et al. 2014), sustainable and adaptive management of the GSR over the coming decades will require a strong knowledge-base and generation of a public and political will to look after the system, and a commitment that reflects the immense ecological social and economic benefits we derive from the Great Southern Reef.
Acknowledgements
This work arose from discussions during the 10th International Temperate Reefs Symposium (ITRS) in Perth 2014. The ITRS was sponsored by The University of Western Australia, CSIRO, the Government of Western Australia Department of Fisheries, BMT Oceanica, CSIRO Publishing and Edith Cowan University. S. Bennett and T. Wernberg convened the public forum ‘The Forgotten Coast – the State and Future of Australia’s Temperate Reefs’ and wrote the paper with inputs from all authors. T. Wernberg and S. D. Connell were supported by Australian Research Council Future Fellowships; A. J. Hobday was supported by a travel grant to the ITRS.
References
ABS (2001). Regional population growth. 3218.0. Australian Bureau of Statistics, Canberra.Alleway, H. K., and Connell, S. D. (2015). Loss of an ecological baseline through the eradication of oyster reefs from coastal ecosystems and human memory. Conservation Biology 29, 795–804.
| Loss of an ecological baseline through the eradication of oyster reefs from coastal ecosystems and human memory.Crossref | GoogleScholarGoogle Scholar | 25588455PubMed |
Appeltans, W., Ahyong, S. T., Anderson, G., Angel, M. V., Artois, T., Bailly, N., Bamber, R., Barber, A., Bartsch, I., and Berta, A. (2012). The magnitude of global marine species diversity. Current Biology 22, 2189–2202.
| The magnitude of global marine species diversity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs12gtb7K&md5=fc9ae0d150c12546cc684525e8fa6a91CAS | 23159596PubMed |
Barnes, D. K., and Griffiths, H. J. (2008). Biodiversity and biogeography of southern temperate and polar bryozoans. Global Ecology and Biogeography 17, 84–99.
Bellwood, D. R., and Meyer, C. P. (2009). Searching for heat in a marine biodiversity hotspot. Journal of Biogeography 36, 569–576.
| Searching for heat in a marine biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Bennett, S., and Wernberg, T. (2014). Canopy facilitates seaweed recruitment on subtidal temperate reefs Journal of Ecology 102, 1462–1470.
| Canopy facilitates seaweed recruitment on subtidal temperate reefsCrossref | GoogleScholarGoogle Scholar |
Bennett, S., Wernberg, T., Harvey, E. S., Santana-Garcon, J., and Saunders, B. (2015a). Tropical herbivores provide resilience to a climate mediated phase-shift on temperate reefs. Ecology Letters 18, 714–723.
| Tropical herbivores provide resilience to a climate mediated phase-shift on temperate reefs.Crossref | GoogleScholarGoogle Scholar | 25994785PubMed |
Bennett, S., Wernberg, T., Anderson, R. J., Bolton, J. J., Bettignies, T. D., Kendrick, G. A., Rodgers, K. L., Shears, N. T., Leclerc, J. C., Lévêque, L., Davoult, D., and Christie, H. C. (2015b). Canopy interactions and physical stress gradients in subtidal communities Ecology Letters 18, 677–686.
| Canopy interactions and physical stress gradients in subtidal communitiesCrossref | GoogleScholarGoogle Scholar | 25975532PubMed |
Bertocci, I., Araújo, R., Oliveira, P., and Sousa-Pinto, I. (2015). Potential effects of kelp species on local fisheries. Journal of Applied Ecology 52, 1216–1226.
| Potential effects of kelp species on local fisheries.Crossref | GoogleScholarGoogle Scholar |
Bolton, J. (1994). Global seaweed diversity: patterns and anomalies. Botanica Marina 37, 241–246.
| Global seaweed diversity: patterns and anomalies.Crossref | GoogleScholarGoogle Scholar |
Bolton, J. J. (2010). The biogeography of kelps (Laminariales, Phaeophyceae): a global analysis with new insights from recent advances in molecular phylogenetics. Helgoland Marine Research 64, 263–279.
| The biogeography of kelps (Laminariales, Phaeophyceae): a global analysis with new insights from recent advances in molecular phylogenetics.Crossref | GoogleScholarGoogle Scholar |
Bustamante, R. H., and Branch, G. M. (1996). The dependence of intertidal consumers on kelp-derived organic matter on the west coast of South Africa. Journal of Experimental Marine Biology and Ecology 196, 1–28.
| The dependence of intertidal consumers on kelp-derived organic matter on the west coast of South Africa.Crossref | GoogleScholarGoogle Scholar |
Butler, A. J., Rees, T., Beesley, P., and Bax, N. J. (2010). Marine biodiversity in the Australian region. PLoS One 5, e11831.
| Marine biodiversity in the Australian region.Crossref | GoogleScholarGoogle Scholar | 20689847PubMed |
Chung, I. K., Beardall, J., Mehta, S., Sahoo, D., and Stojkovic, S. (2011). Using marine macroalgae for carbon sequestration: a critical appraisal. Journal of Applied Phycology 23, 877–886.
| Using marine macroalgae for carbon sequestration: a critical appraisal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFymtbzJ&md5=8d76d2024e4ba924d8019c49c9d11448CAS |
Coleman, M. A., Kelaher, B. P., Steinberg, P. D., and Millar, A. J. K. (2008). Absence of a large brown macroalga on urbanized rocky reefs around Sydney, Australia, and evidence for historical decline. Journal of Phycology 44, 897–901.
| Absence of a large brown macroalga on urbanized rocky reefs around Sydney, Australia, and evidence for historical decline.Crossref | GoogleScholarGoogle Scholar |
Coleman, M. A., Roughan, M., MacDonald, H. S., Connell, S. D., Gillanders, B. M., Kelaher, B. P., and Steinberg, P. D. (2011). Variation in the strength of continental boundary currents determines continent-wide connectivity in kelp. Journal of Ecology 99, 1026–1032.
| Variation in the strength of continental boundary currents determines continent-wide connectivity in kelp.Crossref | GoogleScholarGoogle Scholar |
Coleman, M., Feng, M., Roughan, M., Cetina-Heredia, P., and Connell, S. D. (2013). Temperate shelf water dispersal by Australian boundary currents: implications for population connectivity. Limnology and Oceanography: Fluids and Environments 3, 295–309.
Condie, S. A., and Dunn, J. R. (2006). Seasonal characteristics of the surface mixed layer in the Australasian region: implications for primary production regimes and biogeography. Marine and Freshwater Research 57, 569–590.
| Seasonal characteristics of the surface mixed layer in the Australasian region: implications for primary production regimes and biogeography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovVeksr8%3D&md5=fed7a0ec478203fe663fd95cb9163ce8CAS |
Condie, S. A., Waring, J., Mansbridge, J. V., and Cahill, M. L. (2005). Marine connectivity patterns around the Australian continent. Environmental Modelling & Software 20, 1149–1157.
| Marine connectivity patterns around the Australian continent.Crossref | GoogleScholarGoogle Scholar |
Connell, S. D. (2007). Water quality and loss of coral reefs and kelp forests: alternative states and the influence of fishing. In ‘Marine Ecology’. (Eds S. D. Connell and B. M. Gillanders.) pp. 556–568. (Oxford University Press: Melbourne.)
Connell, S. D., and Ghedini, G. (2015). Resisting regime-shifts: the stabilising effect of compensatory processes. Trends in Ecology & Evolution 30, 513–515.
| Resisting regime-shifts: the stabilising effect of compensatory processes.Crossref | GoogleScholarGoogle Scholar |
Connell, S. D., and Irving, A. D. (2008). Integrating ecology with biogeography using landscape characteristics: a case study of subtidal habitat across continental Australia. Journal of Biogeography 35, 1608–1621.
| Integrating ecology with biogeography using landscape characteristics: a case study of subtidal habitat across continental Australia.Crossref | GoogleScholarGoogle Scholar |
Connell, S. D., and Irving, A. D. (2009). The subtidal ecology of rocky coasts: local–regional–biogeographic patterns and their experiental analysis. In ‘Marine Macroecology’. (Eds J. D. Witman, R. Roy.) pp. 392–424. (University of Chicago Press: Chicago, IL.)
Connell, S. D., Russell, B. D., Turner, D. J., Shepherd, A. J. S., Kildea, T. N., Miller, D., Airoldi, L., and Cheshire, A. (2008). Recovering a lost baseline: missing kelp forests from a metropolitan coast. Marine Ecology Progress Series 360, 63–72.
| Recovering a lost baseline: missing kelp forests from a metropolitan coast.Crossref | GoogleScholarGoogle Scholar |
Connell, S. D., Kroeker, K. J., Fabricius, K. E., Kline, D. I., and Russell, B. D. (2013). The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368, 20120442.
| The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance.Crossref | GoogleScholarGoogle Scholar | 23980244PubMed |
Connell, S., Foster, M., and Airoldi, L. (2014). What are algal turfs? Towards a better description of turfs. Marine Ecology Progress Series 495, 299–307.
| What are algal turfs? Towards a better description of turfs.Crossref | GoogleScholarGoogle Scholar |
Costanza, R., d’Arge, R., de Groot, R., Farber, S., Grasso, M., Hannon, B., Limburg, K., Naeem, S., O’Neill, R. V., Paruelo, J., Raskin, R. G., Sutton, P., and van den Belt, M. (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260.
| The value of the world’s ecosystem services and natural capital.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtlShtbs%3D&md5=25828f5b7a831e14a9c671e6ca22d945CAS |
Costanza, R., de Groot, R., Sutton, P., van der Ploeg, S., Anderson, S. J., Kubiszewski, I., Farber, S., and Turner, R. K. (2014). Changes in the global value of ecosystem services. Global Environmental Change 26, 152–158.
| Changes in the global value of ecosystem services.Crossref | GoogleScholarGoogle Scholar |
Day, J. C. (2002). Zoning – lessons from the Great Barrier Reef Marine Park. Ocean and Coastal Management 45, 139–156.
| Zoning – lessons from the Great Barrier Reef Marine Park.Crossref | GoogleScholarGoogle Scholar |
Day, J., Fernandes, L., Lewis, A., and Innes, J. (2003). RAP – an ecosystem level approach to biodiversity protection planning. In ‘Proceedings of the Second International Tropical Marine Ecosystems Management Symposium. Great Barrier Reef Marine Park Authority’, Townsville, Qld, Australia. pp. 251–265. (Great Barrier Reef Marine Park Authority: Townsville, Qld.)
de Bettignies, T., Wernberg, T., Lavery, P. S., Vanderklift, M. A., and Mohring, M. B. (2013). Contrasting mechanisms of dislodgement and erosion contribute to production of kelp detritus. Limnology and Oceanography 58, 1680–1688.
| Contrasting mechanisms of dislodgement and erosion contribute to production of kelp detritus.Crossref | GoogleScholarGoogle Scholar |
de Groot, R., Brander, L., van der Ploeg, S., Costanza, R., Bernard, F., Braat, L., Christie, M., Crossman, N., Ghermandi, A., and Hein, L. (2012). Global estimates of the value of ecosystems and their services in monetary units. Ecosystem Services 1, 50–61.
| Global estimates of the value of ecosystems and their services in monetary units.Crossref | GoogleScholarGoogle Scholar |
Deloitte Access Economics (2013). Economic contribution of the Great Barrier Reef, Great Barrier Reef Marine Park Authority, Townsville.
Department of Sustainability, Environment, Water, Population and Communities (2011). State of the Environment 2011 – Marine Environment. Independent report to the Australian Government Minister for Sustainability, Environment, Water, Population and Communities. Department of Sustainability, Environment, Water, Population and Communities, Canberra.
Duarte, C. M., Dennison, W. C., Orth, R. J., and Carruthers, T. J. (2008). The charisma of coastal ecosystems: addressing the imbalance. Estuaries and Coasts 31, 233–238.
| The charisma of coastal ecosystems: addressing the imbalance.Crossref | GoogleScholarGoogle Scholar |
Falkenberg, L. J., Connell, S. D., and Russell, B. D. (2013). Disrupting the effects of synergies between stressors: improved water quality dampens the effects of future CO2 on a marine habitat. Journal of Applied Ecology 50, 51–58.
| Disrupting the effects of synergies between stressors: improved water quality dampens the effects of future CO2 on a marine habitat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmslWju7s%3D&md5=78ce3227390f5d05129f329c9b23287eCAS |
Ghedini, G., Russell, B. D., and Connell, S. D. (2015). Trophic compensation reinforces resistance: herbivory absorbs the increasing effects of multiple disturbances. Ecology Letters 18, 182–187.
| Trophic compensation reinforces resistance: herbivory absorbs the increasing effects of multiple disturbances.Crossref | GoogleScholarGoogle Scholar | 25581377PubMed |
Gorman, D., Russell, B. D., and Connell, S. D. (2009). Land-to-sea connectivity: linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts. Ecological Applications 19, 1114–1126.
| Land-to-sea connectivity: linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts.Crossref | GoogleScholarGoogle Scholar | 19688920PubMed |
Graham, M. H., Kinlan, B. P., Druehl, L. D., Garske, L. E., and Banks, S. (2007). Deep-water kelp refugia as potential hotspots of tropical marine diversity and productivity. Proceedings of the National Academy of Sciences of the United States of America 104, 16576–16580.
| Deep-water kelp refugia as potential hotspots of tropical marine diversity and productivity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1WgsrnK&md5=3e34620606480dac72dfd62eec31e8bcCAS | 17913882PubMed |
Henry, G. W., and Lyle, J. M. (2003). The national recreational and Indigenous fishing survey. Fisheries Research and Development Corporation Project 1999/158. Australian Government Department of Agriculture, Fisheries and Forestry, Canberra.
Hobday, A. J., and Lough, J. M. (2011). Projected climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 1000–1014.
| Projected climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Hobday, A. J., and Pecl, G. T. (2014). Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Reviews in Fish Biology and Fisheries 24, 415–425.
| Identification of global marine hotspots: sentinels for change and vanguards for adaptation action.Crossref | GoogleScholarGoogle Scholar |
Hommersand, M. (1986). The biogeography of the South African marine red algae: a model. Botanica Marina 29, 257–270.
| The biogeography of the South African marine red algae: a model.Crossref | GoogleScholarGoogle Scholar |
Irving, A. D., and Connell, S. D. (2006). Predicting understorey structure from the presence and composition of canopies: an assembly rule for marine algae. Oecologia 148, 491–502.
| Predicting understorey structure from the presence and composition of canopies: an assembly rule for marine algae.Crossref | GoogleScholarGoogle Scholar | 16502000PubMed |
Jenkins, G. P., and Wheatley, M. J. (1998). The influence of habitat structure on nearshore fish assemblages in a southern Australian embayment: comparison of shallow seagrass, reef-algal and unvegetated sand habitats, with emphasis on their importance to recruitment. Journal of Experimental Marine Biology and Ecology 221, 147–172.
| The influence of habitat structure on nearshore fish assemblages in a southern Australian embayment: comparison of shallow seagrass, reef-algal and unvegetated sand habitats, with emphasis on their importance to recruitment.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. R., Banks, S. C., Barrett, N. S., Cazassus, F., Dunstan, P. K., Edgar, G. J., Frusher, S. D., Gardner, C., Haddon, M., Helidoniotis, F., Hill, K. L., Holbrook, N. J., Hosie, G. W., Last, P. R., Ling, S. D., Melbourne-Thomas, J., Miller, K., Pecl, G. T., Richardson, A. J., Ridgway, K. R., Rintoul, S. R., Ritz, D. A., Ross, D. J., Sanderson, J. C., Shepherd, S. A., Slotwinski, A., Swadling, K. M., and Taw, N. (2011). Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. Journal of Experimental Marine Biology and Ecology 400, 17–32.
| Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania.Crossref | GoogleScholarGoogle Scholar |
Kerswell, A. P. (2006). Global biodiversity patterns of benthic marine algae. Ecology 87, 2479–2488.
| Global biodiversity patterns of benthic marine algae.Crossref | GoogleScholarGoogle Scholar | 17089657PubMed |
Knudsen, S. W., and Clements, K. D. (2013). Kyphosus gladius, a new species of sea chub from Western Australia (Teleostei: Kyphosidae), with comments on Segutilum klunzingeri Whitley. Zootaxa 3599, 1–18.
| Kyphosus gladius, a new species of sea chub from Western Australia (Teleostei: Kyphosidae), with comments on Segutilum klunzingeri Whitley.Crossref | GoogleScholarGoogle Scholar | 24583811PubMed |
Krumhansl, K., and Scheibling, R. (2012). Production and fate of kelp detritus. Marine Ecology Progress Series 467, 281–302.
| Production and fate of kelp detritus.Crossref | GoogleScholarGoogle Scholar |
Laffoley, D., and Grimsditch, G. D. (2009). ‘The Management of Natural Coastal Carbon Sinks.’ (IUCN: Gland, Switzerland.)
Langlois, T. J., Radford, B. T., Van Niel, K. P., Meeuwig, J. J., Pearce, A. F., Rousseaux, C. S., Kendrick, G. A., and Harvey, E. S. (2012). Consistent abundance distributions of marine fishes in an old, climatically buffered, infertile seascape. Global Ecology and Biogeography 21, 886–897.
| Consistent abundance distributions of marine fishes in an old, climatically buffered, infertile seascape.Crossref | GoogleScholarGoogle Scholar |
Last, P. R., White, W. T., Gledhill, D. C., Hobday, A. J., Brown, R., Edgar, G. J., and Pecl, G. (2011). Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Global Ecology and Biogeography 20, 58–72.
| Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices.Crossref | GoogleScholarGoogle Scholar |
Ling, S., Johnson, C., Ridgway, K., Hobday, A., and Haddon, M. (2009). Climate‐driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics. Global Change Biology 15, 719–731.
| Climate‐driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics.Crossref | GoogleScholarGoogle Scholar |
Lough, J. M., and Hobday, A. J. (2011). Observed climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 984–999.
| Observed climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Lourie, S. A., and Vincent, A. C. (2004). Using biogeography to help set priorities in marine conservation. Conservation Biology 18, 1004–1020.
| Using biogeography to help set priorities in marine conservation.Crossref | GoogleScholarGoogle Scholar |
MacDonald, D. H., Ardeshiri, A., Rose, J. M., Russell, B. D., and Connell, S. D. (2015). Valuing coastal water quality: Adelaide, South Australia metropolitan area. Marine Policy 52, 116–124.
| Valuing coastal water quality: Adelaide, South Australia metropolitan area.Crossref | GoogleScholarGoogle Scholar |
Magris, R. A., Pressey, R. L., Weeks, R., and Ban, N. C. (2014). Integrating connectivity and climate change into marine conservation planning. Biological Conservation 170, 207–221.
| Integrating connectivity and climate change into marine conservation planning.Crossref | GoogleScholarGoogle Scholar |
Mann, K. H. (1973). Seaweeds – their productivity and strategy for growth. Science 182, 975–981.
| Seaweeds – their productivity and strategy for growth.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvlsFymtg%3D%3D&md5=be6d7c29d012db786f437fb78c02fc81CAS | 17833778PubMed |
Marzinelli, E. M., Williams, S. B., Babcock, R. C., Barrett, N. S., Johnson, C. R., Jordan, A., Kendrick, G. A., Pizarro, O. R., Smale, D. A., and Steinberg, P. D. (2015). Large-scale geographic variation in distribution and abundance of Australian deep-water kelp forests. PLoS One 10, e0118390.
| Large-scale geographic variation in distribution and abundance of Australian deep-water kelp forests.Crossref | GoogleScholarGoogle Scholar | 25693066PubMed |
McGowran, B., Li, Q., Cann, J., Padley, D., McKirdy, D. M., and Shafik, S. (1997). Biogeographic impact of the Leeuwin Current in southern Australia since the late middle Eocene. Palaeogeography, Palaeoclimatology, Palaeoecology 136, 19–40.
| Biogeographic impact of the Leeuwin Current in southern Australia since the late middle Eocene.Crossref | GoogleScholarGoogle Scholar |
Metcalf, S., van Putten, E., Frusher, S., Tull, M., and Marshall, N. (2014). Adaptation options for marine industries and coastal communities using community structure and dynamics. Sustainability Science 9, 247–261.
O’Hara, T. D., and Poore, G. C. (2000). Patterns of distribution for southern Australian marine echinoderms and decapods. Journal of Biogeography 27, 1321–1335.
| Patterns of distribution for southern Australian marine echinoderms and decapods.Crossref | GoogleScholarGoogle Scholar |
Oliver, E. C. J., Wotherspoon, S. J., Chamberlain, M. A., and Holbrook, N. J. (2014). Projected Tasman Sea extremes in sea surface temperature through the twenty-first century. Journal of Climate 27, 1980–1998.
| Projected Tasman Sea extremes in sea surface temperature through the twenty-first century.Crossref | GoogleScholarGoogle Scholar |
Pearce, A., and Feng, M. (2007). Observations of warming on the Western Australian continental shelf. Marine and Freshwater Research 58, 914–920.
| Observations of warming on the Western Australian continental shelf.Crossref | GoogleScholarGoogle Scholar |
Pearce, A. F., and Feng, M. (2013). The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011. Journal of Marine Systems 111–112, 139–156.
| The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011.Crossref | GoogleScholarGoogle Scholar |
Phillips, J. A. (2001). Marine macroalgal biodiversity hotspots: why is there high species richness and endemism in southern Australian marine benthic flora? Biodiversity and Conservation 10, 1555–1577.
| Marine macroalgal biodiversity hotspots: why is there high species richness and endemism in southern Australian marine benthic flora?Crossref | GoogleScholarGoogle Scholar |
Pink, B. (2013). Population projections, Australia, 2012 to 2101. 3222.0. Australian Bureau of Statistics, Canberra.
Pitt, N. R., Poloczanska, E. S., and Hobday, A. J. (2010). Climate-driven range changes in Tasmanian intertidal fauna. Marine and Freshwater Research 61, 963–970.
| Climate-driven range changes in Tasmanian intertidal fauna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Snt7nO&md5=b56be911853651737c6ea021d3b0c74cCAS |
Poore, G. C., and Bruce, N. L. (2012). Global diversity of marine isopods (except Asellota and crustacean symbionts). PLoS One 7, e43529.
| Global diversity of marine isopods (except Asellota and crustacean symbionts).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlSltrzN&md5=3b61f21068b8776b9157088df911465dCAS | 22952700PubMed |
Ridgway, K. (2007). Long‐term trend and decadal variability of the southward penetration of the East Australian Current. Geophysical Research Letters 34, L13613.
| Long‐term trend and decadal variability of the southward penetration of the East Australian Current.Crossref | GoogleScholarGoogle Scholar |
Robinson, L. M., Gledhill, D. C., Moltschaniwskyj, N. A., Hobday, A. J., Frusher, S., Barrett, N., Stuart-Smith, J., and Pecl, G. T. (2015). Rapid assessment of an ocean warming hotspot reveals ‘high’ confidence in potential species’ range extensions. Global Environmental Change 31, 28–37.
| Rapid assessment of an ocean warming hotspot reveals ‘high’ confidence in potential species’ range extensions.Crossref | GoogleScholarGoogle Scholar |
Shenkar, N., and Swalla, B. J. (2011). Global diversity of Ascidiacea. PLoS One 6, e20657.
| Global diversity of Ascidiacea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotVKlu7k%3D&md5=b40950188117d4b0e3485ddeb6d5f3f6CAS | 21701684PubMed |
Smale, D. A., and Wernberg, T. (2013). Extreme climatic event drives range contraction of a habitat-forming species. Proceedings of the Royal Society of London – B. Biological Sciences 280, 20122829.
| Extreme climatic event drives range contraction of a habitat-forming species.Crossref | GoogleScholarGoogle Scholar |
Steneck, R. S., and Johnson, C. R. (2013). Kelp forests: dynamic patterns, processes, and feedbacks. In ‘Marine Community Ecology’. (Eds M. D. Bertness, J. Bruno, B. R. Silliman and J. J. Stachowicz.) pp. 315–336. (Sinauer Associates: Sunderland, MA.)
Stöhr, S., O’Hara, T. D., and Thuy, B. (2012). Global diversity of brittle stars (Echinodermata: Ophiuroidea). PLoS One 7, e31940.
| Global diversity of brittle stars (Echinodermata: Ophiuroidea).Crossref | GoogleScholarGoogle Scholar | 22396744PubMed |
Thompson, P., Bonham, P., Waite, A., Clementson, L., Cherukuru, N., Hassler, C., and Doblin, M. (2011). Contrasting oceanographic conditions and phytoplankton communities on the east and west coasts of Australia. Deep-sea Research. Part II, Topical Studies in Oceanography 58, 645–663.
| Contrasting oceanographic conditions and phytoplankton communities on the east and west coasts of Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXit1aqsbo%3D&md5=f4efec35c0f96c2462c266075207f17bCAS |
Tourism Research Australia (2011). The economic importance of tourism in Australia’s regions. Tourism Research Australia, Canberra.
Underwood, A. J., Kingsford, M. J., and Andrew, N. L. (1991). Patterns in shallow subtidal marine assemblages along the coast of New South Wales Australia. Australian Journal of Ecology 16, 231–249.
| Patterns in shallow subtidal marine assemblages along the coast of New South Wales Australia.Crossref | GoogleScholarGoogle Scholar |
Van Soest, R. W., Boury-Esnault, N., Vacelet, J., Dohrmann, M., Erpenbeck, D., De Voogd, N. J., Santodomingo, N., Vanhoorne, B., Kelly, M., and Hooper, J. N. (2012). Global diversity of sponges (Porifera). PLoS One 7, e35105.
| Global diversity of sponges (Porifera).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XntVemtrs%3D&md5=c144b5ad4272fe65cd39164224160a28CAS | 22558119PubMed |
Vanderklift, M. A., and Wernberg, T. (2008). Detached kelps from distant sources are a food subsidy for sea urchins. Oecologia 157, 327–335.
| Detached kelps from distant sources are a food subsidy for sea urchins.Crossref | GoogleScholarGoogle Scholar | 18491144PubMed |
Vanderklift, M., and Wernberg, T. (2010). Stable isotopes reveal a consistent consumer–diet relationship across hundreds of kilometres. Marine Ecology Progress Series 403, 53–61.
| Stable isotopes reveal a consistent consumer–diet relationship across hundreds of kilometres.Crossref | GoogleScholarGoogle Scholar |
Vergés, A., Steinberg, P. D., Hay, M. E., Poore, A. G. B., Campbell, A. H., Ballesteros, E., Heck, K. L., Booth, D. J., Coleman, M. A., Feary, D. A., Figueira, W., Langlois, T., Marzinelli, E. M., Mizerek, T., Mumby, P. J., Nakamura, Y., Roughan, M., van Sebille, E., Gupta, A. S., Smale, D. A., Tomas, F., Wernberg, T., and Wilson, S. K. (2014). The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proceedings of the Royal Society of London – B. Biological Sciences 281, 20140846.
| The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts.Crossref | GoogleScholarGoogle Scholar |
Waters, J. M. (2008). Marine biogeographical disjunction in temperate Australia: historical landbridge, contemporary currents, or both? Diversity & Distributions 14, 692–700.
| Marine biogeographical disjunction in temperate Australia: historical landbridge, contemporary currents, or both?Crossref | GoogleScholarGoogle Scholar |
Waters, J. M., Wernberg, T., Connell, S. D., Thomsen, M. S., Zuccarello, G. C., Kraft, G. T., Sanderson, J. C., West, J. A., and Gurgel, C. F. D. (2010). Australia’s marine biogeography revisited: Back to the future? Austral Ecology 35, 988–992.
| Australia’s marine biogeography revisited: Back to the future?Crossref | GoogleScholarGoogle Scholar |
Wernberg, T., Vanderklift, M. A., How, J., and Lavery, P. S. (2006). Export of detached macroalgae from reefs to adjacent seagrass beds. Oecologia 147, 692–701.
| Export of detached macroalgae from reefs to adjacent seagrass beds.Crossref | GoogleScholarGoogle Scholar | 16323014PubMed |
Wernberg, T., Thomsen, M. S., Tuya, F., Kendrick, G. A., Staehr, P. A., and Toohey, B. D. (2010). Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecology Letters 13, 685–694.
| Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future.Crossref | GoogleScholarGoogle Scholar | 20412279PubMed |
Wernberg, T., Thomsen, M. S., Tuya, F., and Kendrick, G. A. (2011). Biogenic habitat structure of seaweeds change along a latitudinal gradient in ocean temperature. Journal of Experimental Marine Biology and Ecology 400, 264–271.
| Biogenic habitat structure of seaweeds change along a latitudinal gradient in ocean temperature.Crossref | GoogleScholarGoogle Scholar |
Wernberg, T., Russell, B., Thomsen, M., Gurgel, C., Bradshaw, C., Poloczanska, E., and Connell, S. (2011a). Seaweed communities in retreat from ocean warming. Current Biology 21, 1828–1832.
| Seaweed communities in retreat from ocean warming.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVKmurjF&md5=c7a4f1c8d8670d08e27174c98c63b3a0CAS | 22036178PubMed |
Wernberg, T., Russell, B. D., Moore, P. J., Ling, S. D., Smale, D. A., Campbell, A., Coleman, M. A., Steinberg, P. D., Kendrick, G. A., and Connell, S. D. (2011b). Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. Journal of Experimental Marine Biology and Ecology 400, 7–16.
| Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming.Crossref | GoogleScholarGoogle Scholar |
Wernberg, T., Thomsen, M. S., Connell, S. D., Russell, B. D., Waters, J. M., Zuccarello, G. C., Kraft, G. T., Sanderson, C., West, J. A., and Gurgel, C. F. D. (2013a). The footprint of continental-scale ocean currents on the biogeography of seaweeds. PLoS One 8, e80168.
| The footprint of continental-scale ocean currents on the biogeography of seaweeds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslOrtbrO&md5=1618555ec79b3f61ca5c1d08ab4b9b96CAS | 24260352PubMed |
Wernberg, T., Smale, D. A., Tuya, F., Thomsen, M. S., Langlois, T. J., de Bettignies, T., Bennett, S., and Rousseaux, C. S. (2013b). An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nature Climate Change 3, 78–82.
| An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |



