Potential effects of climate change on Australian estuaries and fish utilising estuaries: a review
Bronwyn M. Gillanders A F , Travis S. Elsdon B , Ian A. Halliday C , Gregory P. Jenkins D , Julie B. Robins C and Fiona J. Valesini EA Southern Seas Ecology Laboratories, DX650 418, School of Earth and Environmental Sciences, University of Adelaide, SA 5005, Australia.
B Sinclair Knight Merz, Adelaide, SA 5000, Australia.
C Sustainable Fisheries Unit; Fisheries and Aquaculture; Animal Science; Science, Agriculture, Food and Regional Services; Department of Employment, Economic Development and Innovation; Ecosciences Precinct, GPO Box 46, Brisbane, Qld 4001, Australia.
D Fisheries Research Branch, and Department of Zoology, University of Melbourne, Department of Primary Industries Queenscliff Centre, PO Box 114, Queenscliff, Vic. 3225, Australia.
E Centre for Fish and Fisheries, Murdoch University, South Street, Murdoch, Perth, WA 6150, Australia.
F Corresponding author. Email: bronwyn.gillanders@adelaide.edu.au
Marine and Freshwater Research 62(9) 1115-1131 https://doi.org/10.1071/MF11047
Submitted: 23 February 2011 Accepted: 28 July 2011 Published: 21 September 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Estuaries are especially vulnerable to the impacts of climate change because changes in climatic and hydrologic variables that influence freshwater and marine systems will also affect estuaries. We review potential impacts of climate change on Australian estuaries and their fish. Geographic differences are likely because southern Australian climates are predicted to become warmer and drier, whereas northern regions may see increased precipitation. Environmental factors, including salinity gradients, suspended sediment, dissolved oxygen and nutrient concentrations, will be influenced by changing freshwater input and other climate variables. Potential impacts will vary depending on the geomorphology of the estuary and the level of build-up of sand bars across estuarine entrances. Changes to estuarine fish assemblages will depend on associated changes to salinity and estuarine-mouth morphology. Marine migrants may be severely affected by closure of estuarine mouths, depending on whether species ‘must’ use estuarine habitat and the level of migratory v. resident individuals. Depending on how fish in coastal waters locate estuaries, there may be reduced cues associated with estuarine mouths, particularly in southern Australia, potentially influencing abundance. In summary, climate change is expected to have major consequences for Australian estuaries and associated fish, although the nature of impacts will show significant regional variation.
Additional keywords: estuarine mouth, freshwater, marine, salinity.
Introduction
Estuaries are critical transition zones linking land, freshwater and marine habitats, and are spatially and temporally complex because of mixing of saltwater and freshwater. They are also highly productive systems utilised by a range of organisms (see Beck et al. 2001). Despite their ecological significance, estuaries are also some of the most anthropogenically degraded habitats on Earth (Edgar et al. 2000; Blaber 2002). Numerous definitions of an estuary exist, most of which have been derived from a northern hemisphere perspective (Potter et al. 2010). Recently, a definition that encompasses the main characteristics of all estuaries was proposed, as follows: ‘an estuary is a partially enclosed coastal body of water that is either permanently or periodically open to the sea and which receives at least periodic discharge from a river(s), and thus, while its salinity is typically less than that of natural sea water and varies temporally and along its length, it can become hypersaline in regions when evaporative water loss is high and freshwater and tidal inputs are negligible’ (Potter et al. 2010: 499). This definition is strongly applicable to Australian estuaries where freshwater input can be reduced, particularly during summer, and where their entrances to the ocean may be permanently, periodically, intermittently or occasionally open.
Estuaries are geologically young; however, they are complex, dynamic and variable environments changing with input of sediment from land and sea (Roy et al. 2001). There have been multiple phases of excavation and infilling over tens of millions of years (Roy et al. 2001). Superimposed on these changes are potential impacts from changing climate. Major impacts include changes in hydrology; for example, freshwater inflows from both surface- and groundwater sources are expected to change substantially, because these flows depend primarily on precipitation, evapotranspiration and the ability of the ground to store water. Moreover, changes in sea level will affect the extent of the marine influence on these systems.
Australia lies in the mid-latitude high-pressure belt of the southern hemisphere and, therefore, its climate is dominated by eastward-moving anticyclonic cells with a low moisture content (Nix 1981). Australia yields the world’s lowest percentage of rainfall as continental runoff (mean of 12% for Australia cf. 33% for North America) (Arthington and Pusey 2003). Rainfall and runoff in Australia are also spatially and temporally more variable than on any other continent (Arthington and Pusey 2003). Spatially, the greatest proportion of total runoff occurs in the northern and north-eastern coastal areas (Arthington and Pusey 2003).
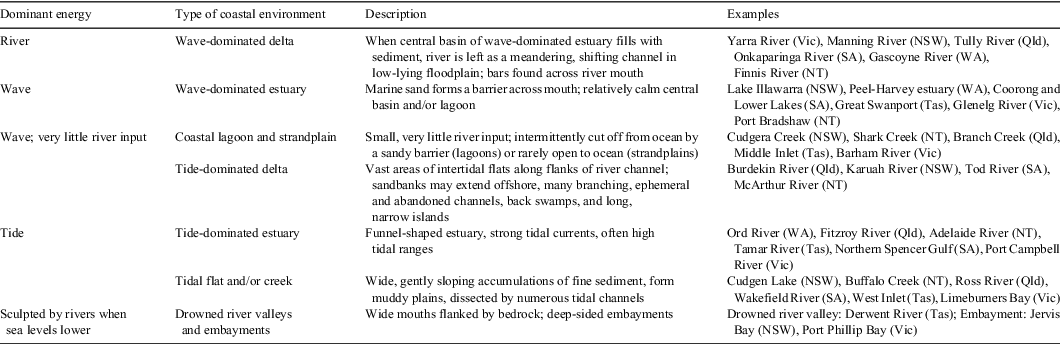
Australia has a diverse array of estuaries influenced by coastal wave action, the movement of tides and river flow. The position of estuaries along a coast is ultimately dictated by topography and sea level (Kench 1999), whereas the relative strength of the key physical energy sources dictates the form of the estuary. Because climate will influence the key physical energy sources, different types of estuaries may be differentially affected. Estuarine form then influences water flow, nutrient cycling and ecosystem processes within the estuary (Turner et al. 2004). Six to seven main types of estuary are found in Australia, depending on whether drowned river valleys and embayments are included (Table 1). In general, wave-dominated estuaries predominate in southern Australia and tide-dominated systems are common in northern Australia, where they grade into deltas with increasing sediment input (Heap et al. 2004).
|
Considerable research has focussed on the fish species utilising estuaries, as well as their seasonal and spatial variation. Although estuaries support resident species, many other species utilise estuaries during juvenile development, or for migration routes and refuge areas (Elliott et al. 2007). Guilds or groups of species can be categorised on the basis of, for example, estuarine use, feeding and/or reproductive mode. Here, we use the functional groups of estuarine use proposed by Elliott et al. (2007). The range of functional groups encompasses the different groups of fish found in estuaries and their links with either fresh or marine waters (Elliott et al. 2007). Briefly, the following 10 categories of fish that use estuaries are recognised: freshwater stragglers and migrants, marine stragglers and migrants (the latter of which are divided into marine estuarine-opportunist and marine estuarine-dependent species), estuarine species (further divided into estuarine residents and migrants) and five categories of diadromous fish (anadromous, semi-anadromous, catadromous, semi-catadromous and amphidromous) (see Elliott et al. 2007 for definitions). Given the differences among these functional groups in their environmental uses and tolerances, climate change is likely to have differential impacts on these different groups of fish.
In the present paper, we review the potential impacts of climate change on estuaries and fish utilising estuarine waters, with a focus on Australia. We investigate climatic and hydrological processes that may affect Australian estuaries, before discussing likely changes to their environmental features and entrance structures. Potential impacts on fish species and assemblages utilising estuaries are then discussed. We provide several case studies that illustrate some of the potential differences associated with different regions and types of estuaries, and acknowledge that climate is not the only variable likely to have an impact on these systems. We do not discuss potential climate impacts on offshore systems from estuarine material. For example, material exported from estuaries may subsidise coastal food webs (e.g. Connolly et al. 2009), and freshwater discharge from estuarine plumes may stimulate phytoplankton and subsequently zooplankton assemblages and fisheries offshore (e.g. Schlacher et al. 2008).
Future scenarios of climate change in estuaries
Climatic and hydrologic processes affecting Australian estuaries
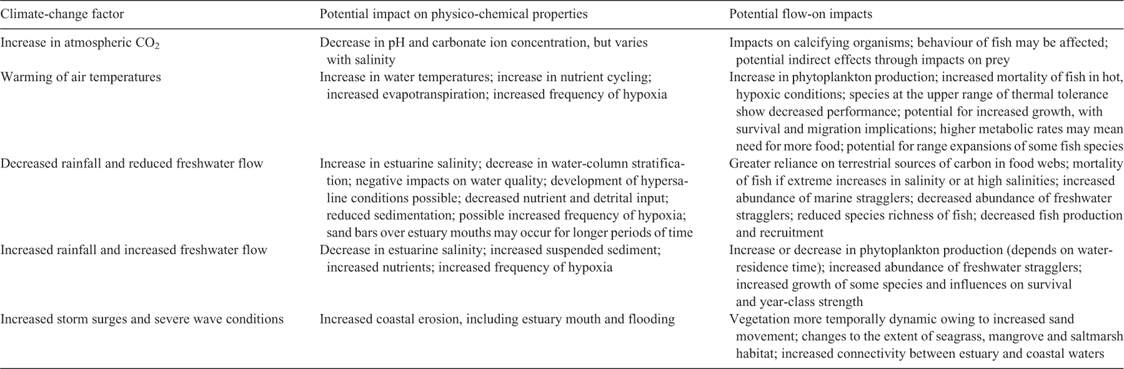
Estuaries are likely to be affected by several interacting climatic and hydrologic (including flow) variables that influence both freshwater and marine systems (see Lough et al. 2011 for details). These include the quality and quantity of riverine and groundwater flow, air–water fluxes of CO2, air and thus water temperatures, momentum (i.e. wind stress) and fluctuations in sea level and other ocean properties (Najjar et al. 2010). An understanding of how these variables may affect estuarine waters will allow the impacts of climate change on estuaries and their assemblages to be assessed (Table 2).
|
Climate models generally provide plausible estimates of change at continental scales, although there is greater confidence in temperature than precipitation (Randall et al. 2007). On the basis of such models, it was predicted that all of Australia was likely to warm this century, although the warming would be less in the south, especially in winter (Christensen et al. 2007). Further, it was predicted that precipitation would be likely to decrease in southern Australia (Christensen et al. 2007; Perkins and Pitman 2009), and increase over northern and central Australia (Perkins and Pitman 2009). However, downscaling anticipated changes in climate from continental- and regional-scale models to those that may be applicable to specific estuaries is more challenging, although necessary (but see Timbal et al. 2009).
Predictions suggest that atmospheric concentrations of CO2 will continue to increase throughout the 21st century, although there is a lot of uncertainty about CO2 trajectories because they depend on the actions of nations around the world, and there is a paucity of information on future CO2 emissions (Najjar et al. 2010). Surface-water CO2 changes will lead to a decrease in pH and carbonate-ion concentrations (Orr et al. 2005; Najjar et al. 2010). Research on pH changes has focussed on marine systems, although, recently, Mosley et al. (2010) developed an estuarine-equilibrium model using the composition of the river and seawater end members to model pH across salinities (see also Hunter 2007). Using this model, pH initially declined at low salinities (<2), then increased rapidly, before stabilising at higher salinities (>15) (Mosley et al. 2010).
Inputs of CO2 to estuaries may differ between river- and marine-dominated systems (Jiang et al. 2008; Hunt et al. 2011). In river-dominated estuaries, freshwater runoff from land may be an important source of dissolved CO2. In such estuaries, the relative importance of the riverine and estuarine zones to CO2 levels may be a function of the water-residence time in the estuary and river discharge rates (Borges et al. 2006). Inputs of nutrients and organic matter from rivers may also influence concentrations of dissolved CO2 (Feely et al. 2010). In contrast, the air–water CO2 fluxes in marine-dominated estuaries are likely to be considerably lower than those in river-dominated estuaries (Ortega et al. 2005; Jiang et al. 2008). The air–water CO2 fluxes also depend on salinity, such that brackish (<20) zones of the estuary may act as a source of CO2 to the atmosphere, whereas more saline zones (>30) may act as a sink (Ortega et al. 2005). We are not aware of studies of air–water CO2 fluxes in Australian estuaries. Australian estuaries are generally small by global scales (e.g. in comparison to Chesapeake Bay and Puget Sound, USA); therefore, research from other countries may not be applicable to our systems. For a regional understanding of CO2 fluxes and given the diversity of estuarine types and climates in Australia, further research is needed.
Australia’s air temperatures over land and oceans have already increased and this warming is faster than the global average (Lough et al. 2011). There are modelled records of daily maximum and minimum air temperature, including daily and monthly averages at spatial resolutions of 0.05° × 0.05° (~5 km × 5 km) (Jones et al. 2009), but few long-term data for estuarine water temperatures. Estuarine waters are expected to warm; such warming may be further accelerated in Australian estuaries because many are shallow (exceptions include the central coast of New South Wales and some parts of Tasmania) and small in water area.
Precipitation influences potential runoff and therefore the input of freshwater to estuarine environments. Besides the amount of precipitation, the intensity (annual mean precipitation divided by the number of days with rain) is also important, particularly where there is high inter-annual rainfall variability. Trends in rainfall suggest drying in areas of southern Australia where rainfall tends to arise from mid-latitude westerlies (south-west of Western Australia, western Tasmania, highlands of Victoria and New South Wales) (Jones et al. 2009). Most northern Australian estuaries are characterised by a tropical wet–dry climate with marked seasonality, with most rainfall (and flow) occurring over a few months of the year. There is high rainfall and flow variability between years and usually some intermittency during the dry season (Warfe et al. 2011).
River flow will be driven by water abstraction and precipitation, and influenced by evapotranspiration. Evapotranspiration will be affected by air temperature, CO2 changes, net radiation, vapour pressure and wind speed, all of which may change with changing climate (Donohue et al. 2010). Other factors that may influence river flow include changes in catchment vegetation or other land cover, groundwater recharge and freshwater abstraction. Reduced freshwater inflow is likely to have a negative impact on water quality, particularly for intermittently closed estuaries (Nicholson et al. 2008), and may lead to development of hypersaline conditions. Nutrient and detrital input may also change with flow, which may alter ecological functioning of estuarine areas (see below).
Sea level is expected to rise and directly affect shorelines and cause flooding. Not only will the mean sea level be affected, but also the intensity of extreme sea-level events (e.g. tropical cyclones and mid-latitude storms) (Church et al. 2006). Storm surges and severe wave conditions will lead to increased coastal erosion and flooding (Church et al. 2006). Estimates of sea-level rise are generally based on tide-gauge data, for which long-term datasets are lacking for most of Australia (Church et al. 2006). These data suggest that the rate of sea-level rise around Australia is ~1.2 mm per year (Church et al. 2006). Within estuarine systems, the impact of sea-level rise is likely to vary depending on the magnitude and frequency of offshore waves, water depth, the morphology of the estuary and coastline topography (Zhong et al. 2008). Both tides and winds will contribute to sea-level fluctuations within estuaries (Zhong et al. 2008). These factors, along with increased evaporation within estuaries and altered freshwater-flow regimes, make it difficult to determine whether changes in sea level as a result of climate change have occurred in individual estuaries (see also Lough et al. 2011). If sea level does increase in estuarine areas, then critical habitat for some species may be lost, especially where coastal development prevents landward migration of habitat-forming vegetation, such as mangrove and saltmarsh (Drinkwater et al. 2010).
The dynamic nature of estuarine systems complicates predictions of climatic impacts on estuaries, especially given the lack of time-series data for many variables. Overall, changes to dissolved-CO2 concentrations, temperature, precipitation and sea level associated with climate change will affect the circulation, levels of salinity, suspended sediments, dissolved oxygen and biogeochemistry of estuaries (Fig. 1).
|
Changes to environmental factors within estuaries
Impacts of climate change on estuarine circulation are likely to be based on changes to water temperature, salinity and flow, which will affect the stratification of the water column (Fig. 1). We are not aware of any studies that consider the long-term impact of climate change on estuarine circulation in Australia.
Well defined salinity gradients characterise many estuaries where salinity is low near the river mouth, increasing to seawater salinity near the connection to the sea or beyond the estuarine plume (‘positive estuaries’). In addition, the waters may also be vertically stratified, with the surface salinity lower than the bottom salinity (see fig. 6 in Gillanders and Kingsford 2002). Variations in estuarine salinity are generally linked to freshwater input. In areas where precipitation and freshwater input are likely to decrease and evaporation is likely to increase with climate change, estuarine salinity may increase and produce ‘negative estuaries’. Australia already has several large negative or inverse estuaries (e.g. Shark Bay, Western Austarlia (Burling et al. 1999); Gulf St Vincent, South Australia (Samarasinghe and Lennon 1987; Samarasinghe et al. 2003); Spencer Gulf, South Australia (Nunes and Lennon 1986)) and several smaller ones (e.g. small estuaries in South Australia and Western Australia; dry tropical estuaries of Queensland, wetland pools of the dry tropical–subtropical zone (Sheaves et al. 2007; Sheaves and Johnston 2009)). In South Australia, about one-third of the 33 estuaries sampled by B. M. Gillanders and T. S. Elsdon (unpubl. data) showed inverse salinity gradients during summer when precipitation and freshwater input declined relative to winter. The classic example of extreme inverse salinity gradients with reduced freshwater input is the Coorong estuary (see below).
In areas of Australia with a Mediterranean climate (areas of south-western West Australia and South Australia, Klausmeyer and Shaw 2009), increased numbers of estuaries are likely to become inverse, and for longer periods of time. Recent studies have also suggested that Hervey Bay (Queensland) has inverse salinity throughout most of the year and that such conditions may persist with drought (Ribbe 2006; Gräwe et al. 2010). Inverse salinity in northern Australia is likely only during the dry season and will depend on rainfall changes associated with climate change. Effects of changes in salinity on primary production are linked to freshwater input and the level of nutrients, as well as whether the water column is stratified and therefore phytoplankton retained in estuaries (Mallin et al. 1993; Snow et al. 2000). Maximum phytoplankton (as measured via Chlorophyll a) is generally found under low-salinity conditions (Malone et al. 1988; Mallin et al. 1993).
Suspended sediment in estuaries is controlled by several factors that are sensitive to climate, including freshwater input through stream flow, shoreline erosion, in situ biological production and decomposition, resuspension of particulate matter, advection and mixing, and rate of sedimentation (Najjar et al. 2010). Estuaries receiving considerable freshwater inputs or large flooding events, such as those in tropical regions, may be expected to have more suspended sediment. This greater sedimentation may increase light attenuation, and in turn limit primary productivity of seagrasses and benthic and pelagic algae. Contrary to this, temperate estuaries that experience drought conditions and longer periods of low flows may have reduced sedimentation. Consequently, these estuaries may experience increases in photic depth and enhanced growth of photosynthetic algae and seagrasses.
Low dissolved oxygen (<2.0 mL L–1 oxygen) or hypoxia can occur naturally where there is low water flow or limited water–atmosphere gas exchange and can also be exacerbated by vertical stratification of the water column because mixing will be inhibited (Hassell et al. 2008a). Several variables influenced by climate change, including reduced river flow and increased temperatures, may lead directly to increased frequency of hypoxia. There are also likely to be indirect effects; for example, increased temperatures may increase nutrient recycling and phytoplankton production and deposition (Justic et al. 2007). Similarly, increased nutrient concentrations via increased freshwater inputs may increase the frequency and distribution of hypoxic conditions (Justic et al. 2007). These examples demonstrate the complexity of links between climate and hypoxia in estuarine waters (Fig. 1).
Predicting how climate variability influences estuarine eutrophication and thereby hypoxia is challenging and likely to vary from one system to another (Justic et al. 2005). Opposite responses were predicted for two river-dominated systems in North America, namely the northern Gulf of Mexico and the Hudson River estuary (Justic et al. 2005). Increased freshwater flow stimulated phytoplankton growth, thereby increasing eutrophication in the northern Gulf of Mexico, whereas it decreased water residence time in the Hudson River estuary, leading to reduced phytoplankton growth and a less eutrophic system (Justic et al. 2007). In seasonally open estuaries where there is low flow relative to coastal wave energy and stratification of the water column, hypoxic conditions may occur in deeper waters (Nicholson et al. 2008). Artificial opening of these estuaries may exacerbate the extent and severity of hypoxic conditions because the surface waters flow out to sea, leaving behind hypoxic deeper waters (Becker et al. 2009). However, deeper, hypoxic water may also be flushed out if the opening is large enough.
Nutrients in estuaries are derived from natural (e.g. upwelling, litter fall, storm events, weathering) and anthropogenic (e.g. sewage outfalls, leaching of nitrogen and phosphorus from cleared land, fertiliser runoff, industrial and agricultural effluents) sources. Estuaries are important for the processing of nutrients, with the degree of nutrient modification being largely dependent on estuarine flushing time (Eyre and Twigg 1997). Flushing time is regulated by river discharge and tidal movements. Hence, climate change may have an impact not only on nutrients coming into the estuary, but also on the length of time they remain within the estuary. A recent study (Abrantes and Sheaves 2010) provided evidence to suggest that the importance of autochthonous and allochthonous sources of productivity for fish may change with climate change. Abrantes and Sheaves (2010) found that the importance of autochthonous and allochthonous productivity sources changed between pre- and post-wet seasons, such that there was a greater reliance on terrestrial carbon after the wet season, in an intermittently connected estuary of the Australian dry tropics. Therefore, reduced runoff into estuaries may lead to a greater reliance on non-terrestrial sources of carbon in food webs.
Nutrients and light are the two key factors controlling phytoplankton growth (Eyre 2000). Phytoplankton biomass and productivity, timing and intensity of seasonal blooms and spatial and temporal composition and diversity are all also linked to freshwater inflow (Schlacher et al. 2008). The abundance, distribution and assemblage structure of zooplankton and other organisms are in turn influenced by the characteristics of phytoplankton assemblages (Schlacher et al. 2008; Brown et al. 2010). The distribution and abundance of primary production in oceanic systems has already been linked to climate change, leading to changes in higher trophic levels (Richardson and Schoeman 2004; Beaugrand et al. 2008). Thus, indirect effects on estuarine food webs are likely. Brown et al. (2010) simulated the effects of changes in primary production on marine ecosystems in Australia, including several bays and harbours (e.g. Darwin Harbour, Port Phillip Bay). In general, climate change results in increased primary production in Australian marine ecosystems and is likely to benefit fisheries (Brown et al. 2010). A recent study, however, suggested that drought may lead to reduced phytoplankton productivity and a reduction in food availability for higher trophic levels (Wetz et al. 2011).
Changes to entrance structure
Geomorphology of estuaries is affected by river discharge and the extent of marine influence, both of which will be affected by climate change and variability. Drowned river valleys and embayments typically have open entrances and climate-change impacts to such estuaries are likely to affect, among other influences, the size of the offshore estuarine plume. Wave-dominated estuaries and deltas often have sand bars that form barriers across the entrance that may completely close and have variable opening regimes (Rustomji 2007). Under reduced rainfall conditions, particularly in southern Australia, these sand bars may occur for longer periods of time, although inundation from the seaward side from high seas and storm surges may also influence mouth opening (Rustomji 2007). Both sand-bar formation and storm surges are likely to increase erosion at the estuary mouth and vegetation changes may be more temporally dynamic as a result of reduced sand stability. Because the geomorphology of estuaries also influences their flushing times (residence period), hydrological processes within estuaries may be further influenced by climate change.
Effects on fish species and assemblages
Estuarine fish assemblages show considerable spatial and temporal variability. Within an estuary, environmental parameters (e.g. salinity, temperature, turbidity and dissolved oxygen) may influence ecological patterns, whereas among-estuary patterns will also be affected by estuary-mouth configuration, estuarine water area, latitude and catchment-area hydrology (Nicolas et al. 2010). Considerable research has focussed on modelling species–environment relationships (reviews in Guisan and Zimmermann 2000; Austin 2007). A vast array of approaches is possible and Austin (2007) suggested that quantile regression (see below), and structural equation modelling are worth exploring further. A further approach was provided by Bond et al. (2011) who used boosted regression trees to link freshwater fish distributions to hydro-climatic and catchment predictors. These modelling approaches allow the consequences of environmental change on fish faunas to be explored.
Fish population dynamics (e.g. recruitment, growth, survival, abundance) and reproduction (e.g. maturity, fecundity, spawning) are influenced by a range of environmental factors (e.g. temperature, salinity, dissolved oxygen) that are highly likely to be affected by climate change (Table 2). In some cases, mobile fish and/or older life-history stages may be able to migrate away from adverse conditions; however, in other cases, this may not be possible. Here, we investigate the potential impacts of individual climatic variables on fish in estuaries, and note that multiple factors will interact with each other. For example, mortality of fish may be higher in hot, hypoxic conditions. Synergistic effects and complex interactions of climatic variables have rarely been examined for estuarine fish; however, see Munday et al. (2009a) and Donelson et al. (2010) for examples of coral-reef fish. In addition, changing environmental and hydrological factors will also affect habitats and food sources within estuaries, which are likely to have a range of indirect impacts on estuarine fish.
Salinity and freshwater-flow impacts on estuarine fish
Salinity is an important determinant of the distribution of fish, and the guild approach to categorising estuarine use by fish relates to salinity tolerances of species (Elliott et al. 2007). Salinity affects the metabolism of fish through influences on osmoregulation and oxygen consumption. Extremes and rapid changes in salinity can stress many species. The osmoregulatory ability of Australian estuarine fish over a wide salinity range has rarely been investigated. Wedderburn et al. (2008) compared osmoregulation of three atherinids, including the estuarine small-mouth hardyhead, Atherinosoma microstoma, and found that all were euryhaline. The small-mouth hardyhead was able to tolerate hypersaline conditions (85); laboratory tests have also found wide salinity tolerances for this species (LD50 3.3–108; Lui 1969). Similarly, another species in this genus, namely A. elongata, has been shown to be relatively abundant in salinities of 122 in the normally closed Wellstead estuary along the southern coast of Western Australia (Young and Potter 2002). Moreover, the progressive increase in salinity towards this extremely high salinity was accompanied by mortalities of other estuarine-spawning fish species, including the atherinid Leptatherina wallacei (Young and Potter 2002).
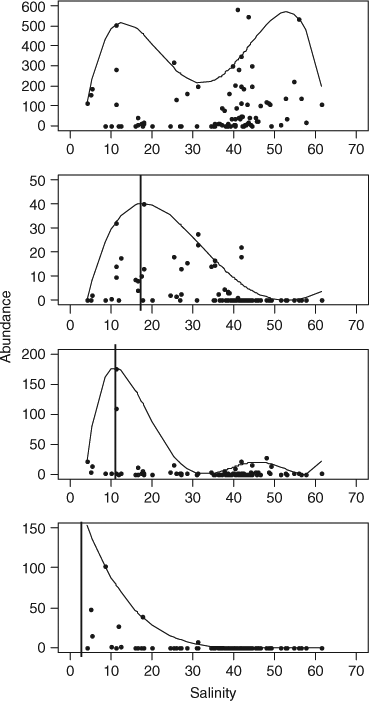
Studies of the spatial distribution of estuarine fish species in relation to salinity may indicate how different species will respond to changes in salinity, although other environmental and biological variables (e.g. reproduction, recruitment) may also influence their distribution and abundance, particularly in subtropical and tropical regions (Blaber 2002). Quantile regression models, which model the upper quantile rather than the mean (Anderson 2008), can indicate where the predicted density of fish achieves a maximum along a salinity gradient, thereby showing the optimum salinity for particular species. For example, such models clearly demonstrate the wide salinity range for small-mouth hardyhead, and the narrower range for several other species in estuaries in South Australia (Fig. 2).
|
Extreme increases in salinity have led to massive mortalities. For example, during 2001, an estimated 1.3 million black bream (A. butcheri) individuals died in the normally closed Culham Inlet along the southern coast of Western Australia (Hoeksema et al. 2006). Salinities increased to >60, which is where this species becomes osmotically stressed, and reached levels of at least 95 in some regions (Hoeksema et al. 2006). Black bream was prevented from moving to lower salinity waters further upstream by a rock bar in the tributary (Hoeksema et al. 2006).
Estuarine species occur in waters that vary daily and seasonally in salinity. These species are generally able to tolerate a wide range of salinities, although diadromous species also show wide salinity tolerances (Nordlie 2009). In contrast, stragglers are restricted to either the freshwater or seawater end of the salinity continuum and are typically found in low numbers (Elliott et al. 2007). Marine stragglers may increase in abundance in estuaries where there is reduced freshwater input and increases in salinity (southern Australia), whereas freshwater stragglers are likely to increase in abundance in estuaries where there is increased freshwater input and decreases in salinity (northern Australia). Freshwater migrants are regularly common in estuaries (Elliott et al. 2007). Although they may be found beyond the oligohaline sections of an estuary, their distribution under future climate-change scenarios is likely to depend on the amount of freshwater flow into the estuary. Morrongiello et al. (2011a) provided further information on likely impacts of climate change on Australian freshwater fish species. The extent of connectivity between marine and freshwater systems will also influence the proportion of fish that are spawned in freshwater v. marine systems and found in estuaries (see Sheaves et al. 2007; Sheaves and Johnston 2008).
Increases in salinity have led to reduced species richness and diversity of estuarine fish assemblages, with freshwater and diadromous species becoming less abundant (Zampatti et al. 2010). In extreme cases of hypersalinity, only a single atherinid species was found (Noell et al. 2009). There is also the possibility of an increase in estuarine fish assemblages (number of species and abundances) if conditions do not become hypersaline and the estuary maintains a connection with the sea. Many species move throughout the estuary in response to changes in salinity, associated with changes in factors such as freshwater discharge or tidal water movements. For example, acoustically tagged black bream in a small estuary on the eastern coast of Tasmania moved downstream with increases in freshwater discharge and returned upstream when salinities increased (Sakabe and Lyle 2010).
Decreased freshwater flows under climate change not only increase salinity but may also decrease water-column stratification or the difference between the surface and bottom salinity. Jenkins et al. (2010) found a consistent linear relationship between water-column stratification and recruitment of black bream in the Gippsland Lakes, Victoria. They suggested that there may be higher recruitment when waters were stratified if stratification provides a cue for spawning or supports higher survival of eggs or larvae.
Several studies have investigated relationships between river flow and catch of fish species in Australian estuaries, suggesting that increased freshwater enhances productivity (e.g. Loneragan and Bunn 1999; Meynecke et al. 2006; Gillson et al. 2009). Relationships with fish recruitment have also been found for tropical (e.g. Staunton-Smith et al. 2004; Halliday et al. 2008) and temperate estuaries (e.g. Ferguson et al. 2008; Jenkins et al. 2010; Zampatti et al. 2010), although these were not always linear. Decreased fish production and/or recruitment from reduced freshwater flow may be attributable to reduced food availability (and reduced growth rates at higher trophic levels, see below), deteriorating water quality, seaward migration of estuarine residents (e.g. Sakabe and Lyle 2010), increasing predation pressure from marine species or altered habitat availability (Gillanders and Kingsford 2002; Robins et al. 2005; Gillson et al. 2009). Although there may be a relationship between river flow and salinity in the estuary, river flow is also likely to affect other variables within the estuary; therefore, determining causal factors is difficult. Several reviews (Gillanders and Kingsford 2002; Robins et al. 2005) have examined the influence of freshwater flow on estuarine systems in Australia.
Relationships between freshwater flows and growth of fish have also been found (Robins et al. 2006). Hard parts of fish, such as otoliths, provide an opportunity to investigate climate–growth relationships. Analysis of annually formed growth increments in otoliths provides a lifetime record of fish growth, similar to that of tree rings. If climate variables fluctuate through time, this then influences the width of growth increments of fish and allows inferences to be drawn between growth rates and environmental conditions. Numerous freshwater (see Morrongiello et al. 2011b for an Australian example) and marine (e.g. Thresher et al. 2007; Black et al. 2008; Black 2009) examples can be found; however, such techniques have not been widely applied to estuarine fish, especially in Australian waters.
Temperature impacts on estuarine fish
Warming of estuarine waters will have less of an influence on guilds of estuarine fish than do salinity or freshwater flow, and will affect tropical and temperate species differentially, depending on whether they are at extremes of their distribution and temperature tolerance. Tolerance ranges will, for example, vary with latitude (Pörtner and Peck 2010). Species near the upper limits of thermal tolerance will show impaired growth, foraging, immune competence, behaviours and competitiveness (Pörtner and Farrell 2008). The tolerable thermal window of fish may also show ontogenetic shifts. For example, eggs, larvae and spawning adults may have narrow thermal windows because of developmental constraints or the need to provide oxygen to sperm or egg masses, respectively (Najjar et al. 2010; Pörtner and Peck 2010). Because eggs and larvae are generally most sensitive to environmental conditions, climate change may affect distribution and abundance of fish through recruitment (Nicholson et al. 2008; Pörtner and Peck 2010). Recruitment may also be influenced through impacts on adult reproductive performance (Pörtner and Peck 2010).
Increased temperature may also have positive impacts, especially for species within their tolerance limits. If individuals grow faster, they may gain survival advantages through reduced susceptibility to predation at early life-history stages (Drinkwater et al. 2010). Species that utilise estuaries as juvenile habitat (marine migrants) may show increased growth with warmer temperatures, leading to earlier migration of such fish out of the estuary. These potential benefits may depend on whether temperature also influences swimming speed and activity rates (Drinkwater et al. 2010) and whether there is sufficient food, because higher temperatures will raise metabolic rates.
Range expansions or contractions of fish species will see some species extend or reduce their use of estuarine waters. Changes to distribution are likely to be most clearly found at the southern or northern boundaries of the species geographic range (see examples in Last et al. 2011). The geographic range of estuarine species will also be affected by factors influencing connectivity between estuarine and marine waters (e.g. estuarine mouth closure) (Gillanders et al. 2012). Most studies in Australian waters have focussed on future predictions rather than demonstrating definitive evidence of a shift in range (Booth et al. 2011). Detecting range shifts for estuarine species is especially difficult, given the dynamic environment that such fish occupy (Booth et al. 2011).
Dissolved-oxygen impacts on estuarine fish
Dissolved oxygen in estuarine systems will be influenced through climate-related impacts on river flow and temperature, which subsequently affect salinity, nutrient concentrations and phytoplankton (Fig. 1), making the actual changes difficult to predict (see above). Other factors can co-vary with and sometimes counteract or exacerbate effects of low dissolved oxygen (Rose et al. 2009). Interactive effects between temperature and salinity may intensify the effects of low dissolved oxygen and thereby negatively affect fish assemblages. Population-level effects of hypoxia have been estimated from laboratory experiments, localised effects in nature and fish kills (Rose et al. 2009). Because fish are mobile, they may move from areas of low dissolved oxygen; however, if climate change leads to whole estuaries becoming hypoxic, then increases in fish mortality events may be expected.
Low concentrations of dissolved oxygen will affect fish growth, mortality, distribution and abundance, as well as food-web interactions of a range of organisms including fish. Differential effects on different life-history stages are also expected, such that early life-history stages will be most sensitive to reduced oxygen (Levin et al. 2009). Laboratory studies on black bream found deleterious effects of reduced oxygen, including delayed hatching, reduced hatch rates and survival and increased deformities of larvae in moderately hypoxic conditions (Hassell et al. 2008a, 2008b). Reduced recruitment and lowered abundance is therefore likely under hypoxic conditions (Hassell et al. 2008a). Reduced flushing of estuaries because of reduced freshwater input may mean that ‘old’ deoxygenated water is not replaced with ‘fresh’ marine water, thereby providing lower-quality conditions and affecting spawning success (e.g. black bream; Nicholson et al. 2008; Sakabe and Lyle 2010).
Impacts of elevated CO2 on estuarine fish
Marine organisms may be affected by elevated partial pressure of CO2 (pCO2) through impacts on metabolic physiology and calcification rates (Fabry et al. 2008). Few studies have investigated potential impacts of elevated pCO2 on estuarine organisms and these have generally been limited to calcifying organisms (Miller et al. 2009; Parker et al. 2009, 2010). Despite this, estuarine environments may be more susceptible to reduced pH because they are shallower, less saline and have lower alkalinity than do marine waters (Miller et al. 2009). They are also likely to have additional sources of CO2 such as via freshwater input (Miller et al. 2009).
Fish possess calcium-carbonate otoliths that aid in balance and hearing. Potential impacts of elevated pCO2 may depend on whether fish can regulate the acid–base balance in the endolymph surrounding the otoliths and other tissues (Fabry et al. 2008). Several studies have suggested that fish may be able to regulate the acid–base balance better than do invertebrates and thereby be less affected by enhanced CO2 alone (Munday et al. 2009c). Whether this prediction applies to estuarine fish is unknown. However, elevated CO2 may affect behaviour of fish, including sensory ability, thereby having consequences for replenishment and sustainability of populations (Munday et al. 2009b, 2010; Dixson et al. 2010). No research has investigated whether the sensory abilities of estuarine fish will be affected by elevated CO2, but given that fish are likely to use olfactory cues to find estuaries (see below), this is possible.
Acidification of estuarine waters currently occurs near acid-sulfate soils, severely reducing the pH for periods of time (Sammut et al. 1996) and leading to increased mortality and reduced growth of oysters (Dove and Sammut 2007), as well as fish. Rainfall is important in mobilising acidified water into estuaries. Reduction in the pH of estuarine waters through acidification has been widely studied, and the severity of pH reduction (e.g. pH <3.5) is much greater than that expected under climate-change scenarios (0.5 change in pH).
Indirect effects of elevated CO2 on estuarine fish are likely to occur through the effects on prey. Many estuarine fish feed on a variety of prey with calcified structures (e.g. crustaceans, molluscs) at some stage in their life history (Gillanders 1995; Sanchez-Jerez et al. 2002). Potential impacts on early life history and reproductive stages of marine calcifiers may affect population size, with flow-on effects to food-web interactions (Kurihara 2008).
Changes to entrance-channel openings and access for species using estuaries
Marine migrants include species that typically spawn at sea, but are found in large numbers in estuaries, particularly as juveniles (Elliott et al. 2007). These species often show seasonal changes in abundance through immigration or emigration, or associated with changing environmental conditions (e.g. changes in salinity). Marine migrants may be severely affected by closure of estuarine mouths because, depending on the timing and duration of estuarine closure, they may be unable to move from estuaries to the ocean to spawn or from the ocean to use estuaries for juvenile or nursery habitats. The longer the estuary mouth is open to the sea, the more likely marine species are able to enter (Griffiths 2001). There is, however, increasing recognition that not all individuals in a population may need to migrate to complete their life cycles (e.g. Elsdon and Gillanders 2005; Crook et al. 2008). Climate change may see increases in resident individuals within a population, especially if the benefits of remaining in estuarine waters outweigh those of migrating. To determine which marine migrant species are most likely affected by closure of estuarine mouths, it is important to ascertain which species ‘must’ use estuarine habitats v. those which ‘may’ use estuarine habitats (Elliott et al. 2007).
Estuaries also act as a migratory route for diadromous species, which move either between freshwater and marine waters or between marine waters and freshwaters (McDowall 1988). These movements are mostly linked to reproductive activities (Elliott et al. 2007). The closure of estuarine mouth will have an impact on diadromous species similar to that on marine and estuarine migrants. Because diadromous species rely on fresh, estuarine and marine waters, they face an uncertain future because each of these waters will experience a range of threats associated with climate change (Morrongiello et al. 2011a). Ultimately, whether migratory species can move between environments may depend on their energetic condition. This is likely to be influenced by transit time, metabolic rate and how non-energetic factors (e.g. disease, stress) interact (Rand et al. 2006).
Several studies have investigated fish assemblages in intermittently and permanently open estuaries (e.g. Pollard 1994; Jones and West 2005). Permanently open estuaries generally have more species (Pollard 1994), suggesting that if estuarine mouths close more frequently under climate-change scenarios, then there may be reductions in their number of species.
Case studies
The geographic spread of Australia and its climate regimes provides an opportunity to compare case studies of the likely impacts of climate change on fish that utilise estuaries across a variety of estuarine types and climates. Mediterranean-type ecosystems characterised by cool, wet winters and hot, dry summers are projected to be most affected by climate change (Klausmeyer and Shaw 2009; Yates et al. 2010). These areas occur in southern and south-western Australia where climates are predicted to be warmer and drier (CSIRO 2007). Australia has both wet and dry tropics in its northern regions, and in these areas, altered rainfall and river flow are predicted to occur. The strength of the wet season is likely to influence freshwater flow to estuaries and thereby affect fish populations. Below, we consider how climate change may affect several estuaries across Australia.
Temperate estuaries
The Coorong is the terminal estuary of Australia’s largest river system, the Murray–Darling. This estuary extends 110 km south-east from the Murray Mouth and is divided into North and South Lagoons. A series of barrages, constructed between 1935 and 1940 separate the Coorong from freshwater reaches of the River Murray. It is a Ramsar-listed wetland, important for many waterbirds and native fish. A decade-long drought and continued upstream water diversion provide an opportunity to examine how reduced freshwater input affects this system, and thus how these impacts may be exacerbated under future climate change (Chiew et al. 2009).
Runoff across the Murray–Darling Basin is predicted to decline by 15% in the southern regions by 2030 (Kingsford et al. 2011). Mean modelled flow at the barrages is currently a fraction of that under a scenario of no river regulation and abstraction (termed ‘natural’) and is more similar to dry years under the ‘natural’ scenario (Kingsford et al. 2011). There was a 34% decline in runoff between 2004 and 2006 (Lester and Fairweather 2009) and streamflow has been reduced by 61% between 1895 and 2006 (Kingsford et al. 2011). Low flows have led to the closure of the Murray Mouth, which first occurred in 1981. The Murray Mouth has been continually dredged since 2002, but dredging stopped in 2011 (Kingsford et al. 2011). Environmental conditions extend from estuarine–marine (a salinity of 30–43) around the Murray Mouth in the north to extremely hypersaline in the South Lagoon (a salinity of up to ~160) (Lester and Fairweather 2009; Noell et al. 2009). Water levels have also decreased, disconnecting the South Lagoon from the rest of the system, and there have been changes in nutrient concentrations (Lester and Fairweather 2009).
An ecological response model developed for the Coorong, that incorporated physical and biological characteristics of the system, identified eight ‘ecosystem states’ reflecting marine or hypersaline states (Lester and Fairweather 2009; Fairweather and Lester 2010; Lester et al. 2011). Two scenarios that incorporated climate change, along with two further scenarios representative of no water-resource development (apart from the barrages) and the current conditions were then investigated (Lester et al. 2011). Under the climate-change scenarios, water extraction had a major effect on freshwater flows into the estuary, especially during periods of low flow; salinity in the South Lagoon increased up to ~130–170% that of the current-conditions scenario, depending on flow conditions (Lester et al. 2011). Degraded ecosystem states occurred more frequently under one of the climate-change scenarios, but not under the climate-change scenario that incorporated an environmental water recovery (Lester et al. 2011).
The fish species richness decreases with increasing salinity, declining from 26 species near the Murray Mouth to 13 in the North Lagoon and just a single species (Atherinosoma microstoma) in the hypersaline regions of South Lagoon (Noell et al. 2009). No fish are found in the most hypersaline regions (salinity of >133.5). Closure of the Murray Mouth and subsequent loss of ocean connectivity will affect not only diadromous species, but also estuarine and marine migrants. The present ecological condition of the Coorong is not just a reflection of the drought, but also includes over-allocation and diversion of water throughout the Murray–Darling Basin (Kingsford et al. 2011). Nonetheless, this provides a stark example of the potential impacts on estuarine fish of reduced freshwater inflows as a result of climate change and anthropogenic pressures in a large, shallow, temperate estuary.
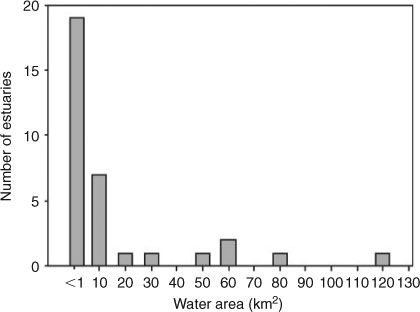
The temperate coast of southern and south-eastern Australia is dominated by small, shallow and often ephemeral estuaries, some of which may be only a few kilometres in length and <15 km2 in water-surface area (Fig. 3; see also Roy et al. 2001; Chuwen et al. 2009). Many of these systems are separated from the ocean by sand bars across their entrances which vary in the frequency with which they are breached, with some remaining closed for several years (e.g. Chuwen et al. 2009). In south-eastern Australia, intermittently open estuaries comprise 45% of all estuaries (Griffiths and West 1999). These systems are likely to be greatly influenced by small changes in climate. Specifically, changes in precipitation and evaporation that may occur with a drying climate are likely to reduce available habitat, limit connectivity between the estuary and ocean, and impair water quality. In small temperate estuaries, hydrological changes may result in the estuary drying completely or becoming disconnected from freshwater and marine waters. For example, the normally closed Hamersley and Culham inlets, located in a low-rainfall region along the southern coast of Western Australia, have been shown to reach salinities of 145 and 296, respectively (Chuwen et al. 2009). Such impacts may affect fish recruitment, especially for species that spawn within estuaries (e.g. black bream, an estuarine resident), effectively negating inputs of recruits to the population. Impacts on fish will depend on duration, frequency and timing of opening of estuaries (Griffiths and West 1999).
|
Insight into the impact of climate change on conditions within small ephemeral estuaries may be gained from examining physico-chemical properties in those systems during extensive long-term drought, when catchment runoff and associated impacts mimic conditions under climate-change scenarios. A recent drought in Australia resulted in rainfall ~20% below long-term averages from 2001 to 2007 (Australian Government Bureau of Meterology 2007). During this period, Elsdon et al. (2009) sampled water quality in several South Australian estuaries and found no differences in water quality (salinity, temperature, pH, oxygen, nutrients) among estuaries with different catchment uses, such as urban and agriculture. The conditions during drought are contrary to that when catchment runoff is high and estuaries receive different anthropogenic and natural sources of nutrients, and this may provide a glimpse of water quality expected in ephemeral estuaries under future climate-change conditions.
The Gippsland Lakes in south-eastern Australia are the country’s largest lagoonal estuarine system, with the chain of lakes extending 70 km and covering an area of ~600 km2. The largest lakes in the system, from west to east, are Lake Wellington, Lake Victoria and Lake King. The Lakes have a low (<30 cm) tidal range, and are connected to the open ocean at the eastern end of the system by an artificial channel that was cut across the beach at Lakes Entrance in 1889. Five major rivers enter the lakes, two feeding into Lake Wellington (the Avon and Latrobe Rivers) and three feeding the central basin of Lake King (the Mitchell, Nicholson and Tambo Rivers). The catchments of these rivers drain 10% of the freshwater flows in the State of Victoria. The salinity of the Lakes is generally lowest in the west (Lake Wellington) and highest near Lakes Entrance. A major water-storage dam for Melbourne was built in the Latrobe River catchment in the early 1980s.
Historically, the Gippsland Lakes have supported an important black bream fishery that has shown a long-term cycle in annual catches, with a peak ~1970 of nearly 600 t (Jenkins et al. 2010). Since the early 1980s, however, catches have been declining. This period has also seen declining river flows, increasing salinity and decreased stratification in the Lakes (Jenkins et al. 2010), among other variables (e.g. habitat modification). Research suggests that black bream eggs and larvae are associated with the halocline in stratified conditions (Nicholson et al. 2008), and the degree of stratification may be positively related to recruitment (Jenkins et al. 2010). The decline in the bream fishery may thus relate to the contraction of stratified conditions to the tributary rivers in recent years, greatly reducing the area of potential spawning habitat for black bream. There has also been an increase in the abundance of marine stragglers in the lakes since the early 1980s, leading to switching of target species in the fishery.
There is predicted to be a decrease in freshwater runoff in south-eastern Australia under climate change (CSIRO 2007; Chiew et al. 2009), and the drought conditions over the past decade have given a snap-shot of the possible conditions that will occur in the future. Proposals to construct additional dams on feeder rivers for human water supply, or create additional entrances to the lakes to increase marine flushing and reduce problems such as algal blooms, would exacerbate the impact of climate change on estuarine fish populations, including black bream.
Tropical Australian estuaries
The impact of climate change on tropical and subtropical estuaries will vary among regions, having differing levels of impacts on the fish species that utilise estuaries. In northern Australia, altered rainfall and river flows are suggested to be more important factors as a consequence of climate change than are increases in temperature (Hobday et al. 2008). Reduced river flows have already occurred in eastern Australia (Gräwe et al. 2010). The direct human use of water resources (e.g. dams and water abstraction) also reduces river flows to estuaries and places stress on estuaries additional to that of climate change (Vörösmarty et al. 2000).
Conditions in estuaries in subtropical and tropical Australia are dominated by wet and dry seasons. The Fitzroy River estuary (Queensland) (see Staunton-Smith et al. 2004 for full description) has the largest coastal drainage basin in eastern Australia (146 000 km2). Freshwater flows into this estuary have shown climatic variability that oscillates at decadal scales between wet and dry. This variability shows significant positive correlations with commercial catches of several tropical fisheries species dependent on estuaries, including barramundi (Robins et al. 2005; Balston 2009). Within fished populations, flow is significantly correlated with the survival of young-of-the-year barramundi, Lates calcarifer, and king threadfin, Polydactylus macrochir (Staunton-Smith et al. 2004; Halliday et al. 2008). The timing of flows plays a critical role in fish population dynamics because it is likely that Australian estuarine species have evolved to cope with the flood–drought regime of Australian climates. Therefore, changes to the timing of important flows should not be underestimated. Flows arriving either too early or too late have little positive effect on the survival of young-of-the-year, with spring and summer flows the most beneficial for recruitment of these species.
Relationships between growth of fish and freshwater flow have also been found (e.g. Robins et al. 2006). Analysis of tagged and recaptured barramundi from the Fitzroy River estuary showed that growth was seasonal and related to freshwater flow experienced by individuals (Robins et al. 2006). Faster growth during higher freshwater flows may lead to increased survival of young fish and enhanced year-class strength of particular cohorts (Staunton-Smith et al. 2004; Robins et al. 2006). The mechanism by which freshwater influences growth rates is likely to be complex, with potential changes to energy budgets being associated with osmoregulation and/or changes to trophic linkages through changes to primary and/or secondary production. Barramundi individuals that enter freshwater for at least some part of the life cycle also grow faster than those that remain solely in saltwater habitats (Milton et al. 2008). Population modelling of barramundi that incorporates the environmental effect of flows on recruitment and growth suggests that water-resource development and water abstraction negatively influence barramundi population size, to the same degree as that modelled under the driest climate-change projection (i.e. 90th percentile) for 2050. Therefore, if all current water-licence holders abstracted their full entitlement at the present time, the barramundi population could decrease by some 30% (M. Tanimoto, unpubl. data).
As air temperatures rise, so too will the estuarine and riverine water temperatures. In the Fitzroy River estuary, minimum monthly air temperatures significantly correlate with the estuarine water temperature. This indicates that for a 2°C increase in temperature in the region, water temperatures would increase by ~1.5°C. This would be well below the maximum threshold for barramundi survival and would most likely increase the length of the growing season (barramundi do not feed when water temperatures get below ~24°C), increasing the potential growth of the population. If, however, the timing of rainfall events changes from the current regime, then there is potential to see a disconnect between the peak spawning periods and the timing of the flow. In a worst-case scenario, this would mean that only small numbers of recruits would survive, even though average annual rainfall may not have altered.
Summary and future directions
Euryoecious species (i.e. those that have wide tolerances to several environmental variables) such as estuarine residents and marine migrants are likely to have the physiological capacity to tolerate and survive changing estuarine conditions. These fish species are presently exposed to fluctuating salinity, temperature and CO2 in estuaries, and therefore may have the adaptive capacity to cope with changing conditions. Whether estuarine residents and marine migrants are better able to tolerate changing climate than are freshwater or marine species remains to be seen, and may depend on how environmental conditions in estuaries change.
Life-history characteristics (e.g. age at maturity, fecundity, life span and natural mortality rates) are likely to influence a species’ ability to respond to climate change and recover, should populations become depleted. Basic biological and life-history information is lacking for many estuarine species. Despite this, short-lived species (e.g. some gobies) with reproductive strategies that optimise output over short periods may be able to survive in changing systems. Long-lived species that can successfully reproduce each year for many years may also be less vulnerable because there can be a storage effect in the population. Early life-history stages (eggs and larvae) are likely to be the ones most severely affected by climate change. For many species utilising estuaries, there is often insufficient information on factors affecting spawning and survival of eggs and larvae. Research has tended to focus on barramundi in subtropical and tropical regions and on black bream across southern Australia, particularly Victoria.
Estuaries represent discrete ecosystems separated by coastal waters that may differ in salinity, temperature, chemical and oceanographic properties (Gillanders et al. 2012). For various life-history stages of fish to move among estuaries, they are essentially moving among fragmented seascapes. How species respond to climate change, especially where estuarine habitats contract, may depend on the dispersal distances of eggs and larvae and the movement capabilities of older life-history stages (assuming that estuary mouths stay open). Similarly, connectivity of populations may change under climate-change scenarios. Populations range from those that are largely self-recruiting and independent in different estuaries through to those where complete mixing occurs, producing one large panmictic population. Continued connectivity of populations will depend, at least in part, on estuarine-mouth morphology. Wave-dominated estuaries and deltas may be expected to see increased occurrences of isolated populations, especially in southern Australia. Isolation of populations has implications for fitness, local adaptation and resilience of estuarine populations (Gillanders et al. 2012).
A recent modelling study of passive particles found that connectivity was not limited by distance among estuaries along the eastern coast of Australia, although adjacent estuaries tended to have the highest probabilities of connectivity (Roughan et al. 2011). Biological parameters (e.g. pelagic larval duration, larval behaviour, mortality) and available habitat for settlement also need consideration in such models, but have not yet been incorporated into estuarine connectivity models (Gillanders et al. 2012). Such models, combined with climate-change variables, may assist in determining how connectivity within and among estuaries may change.
We have not considered how estuarine habitats may change in response to climate change, or how food availability may change. Increased concentration of dissolved CO2 may, for example, enhance growth of autotrophs (e.g. seagrasses). In addition, the response of foundation (habitat-forming) species in estuaries to increased temperature and salinity is largely unknown (Koch et al. 2007). Sea-level rise and storm surges may also have an impact on habitats, especially where landward migration is not possible because of development (e.g. mangroves, samphire). Ultimately, estuarine systems will not just change in relation to climate. Land use within catchments and the use and management of groundwater and surface water will all influence potential flows into estuaries and thereby affect the ecology of the system.
Climate change will have an impact on estuaries; however, predicting impacts is difficult because changes will occur in freshwater and marine systems that subsequently influence estuaries. Differences are likely among different regions of Australia, as well as among different types of estuary. Few estuaries are pristine environments and other anthropogenic stressors, such as water abstraction, habitat modification, urbanisation and exploitation of resources, will interact with climate-related variables to profoundly influence estuarine waters and associated assemblages. Long-term datasets on environmental variables in estuaries are needed. We also lack basic life-history information for many species of fish that use estuaries, making predictions of potential climate-related impacts difficult. Assemblage-level responses are also likely to depend on habitat changes and trophic interactions. Potentially compounding climate-related impacts is significant urban development, especially because most people in Australia live on or near the coast.
Acknowledgements
This paper arose from the Australian Society for Fish Biology 2010 symposium ‘Climate change and the aquatic environment: the future for fish and fisheries’ at the meeting sponsored by the Museum of Victoria, the Department of Sustainability and Environment, Victoria and the Fisheries Research and Development Corporation. We thank the referees, guest editors and Editor in Chief, Andrew Boulton, for their comments. Gillanders and Elsdon acknowledge funding from an ARC linkage grant (LP0669378) for some of the unpublished data reported here. Gillanders acknowledges support from an ARC Future Fellowship (FT100100767) during revision of the manuscript.
References
Abrantes, K. G., and Sheaves, M. (2010). Importance of freshwater flow in terrestrial-aquatic energetic connectivity in intermittently connected estuaries of tropical Australia. Marine Biology 157, 2071–2086.| Importance of freshwater flow in terrestrial-aquatic energetic connectivity in intermittently connected estuaries of tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Anderson, M. J. (2008). Animal–sediment relationships re-visited: characterising species’ distributions along an environmental gradient using canonical analysis and quantile regression splines. Journal of Experimental Marine Biology and Ecology 366, 16–27.
| Animal–sediment relationships re-visited: characterising species’ distributions along an environmental gradient using canonical analysis and quantile regression splines.Crossref | GoogleScholarGoogle Scholar |
Arthington, A. H., and Pusey, B. J. (2003). Flow restoration and protection in Australian rivers. River Research and Applications 19, 377–395.
| Flow restoration and protection in Australian rivers.Crossref | GoogleScholarGoogle Scholar |
Austin, M. (2007). Species distribution models and ecological theory: a critical assessment and some possible new approaches. Ecological Modelling 200, 1–19.
| Species distribution models and ecological theory: a critical assessment and some possible new approaches.Crossref | GoogleScholarGoogle Scholar |
Australian Government Bureau of Meterology (2007). Six years of widespread drought in southern and eastern Australia. November 2001–October 2007. Special climate statement 14. Australian Government Bureau of Meterology, Melbourne.
Balston, J. (2009). Short-term climate variability and the commercial barramundi (Lates calcarifer) fishery of north-east Queensland, Australia. Marine and Freshwater Research 60, 912–923.
| Short-term climate variability and the commercial barramundi (Lates calcarifer) fishery of north-east Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Beaugrand, G., Edwards, M., Brander, K., Luczak, C., and Ibanez, F. (2008). Causes and projections of abrupt climate-driven ecosystem shifts in the North Atlantic. Ecology Letters 11, 1157–1168.
| Causes and projections of abrupt climate-driven ecosystem shifts in the North Atlantic.Crossref | GoogleScholarGoogle Scholar |
Beck, M. W., Heck, K. L., Able, K. W., Childers, D. L., Eggleston, D. B., Gillanders, B. M., Halpern, B., Hays, C. G., Hoshino, K., Minello, T. J., Orth, R. J., Sheridan, P. F., and Weinstein, M. R. (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51, 633–641.
| The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates.Crossref | GoogleScholarGoogle Scholar |
Becker, A., Laurenson, L. J. B., and Bishop, K. (2009). Artificial mouth opening fosters anoxic conditions that kill small estuarine fish. Estuarine, Coastal and Shelf Science 82, 566–572.
| Artificial mouth opening fosters anoxic conditions that kill small estuarine fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFOrsbk%3D&md5=0edf238a4b6afc27033bfd58cddb95bfCAS |
Blaber, S. J. M. (2002). ‘Fish in hot water’: the challenges facing fish and fisheries research in tropical estuaries. Journal of Fish Biology 61, 1–20.
Black, B. A. (2009). Climate-driven synchrony across tree, bivalve, and rockfish growth-increment chronologies of the northeast Pacific. Marine Ecology Progress Series 378, 37–46.
| Climate-driven synchrony across tree, bivalve, and rockfish growth-increment chronologies of the northeast Pacific.Crossref | GoogleScholarGoogle Scholar |
Black, B. A., Boehlert, G. W., and Yoklavich, M. M. (2008). Establishing climate–growth relationships for yelloweye rockfish (Sebastes ruberrimus) in the northeast Pacific using a dendrochronological approach. Fisheries Oceanography 17, 368–379.
| Establishing climate–growth relationships for yelloweye rockfish (Sebastes ruberrimus) in the northeast Pacific using a dendrochronological approach.Crossref | GoogleScholarGoogle Scholar |
Bond, N., Thomson, J., Reich, P., and Stein, J. (2011). Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Marine and Freshwater Research 62, 1043–1061.
| Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Booth, D. J., Bond, N., and Macreadie, P. (2011). Detecting range shifts among Australian fishes in response to climate change. Marine and Freshwater Research 62, 1027–1042.
| Detecting range shifts among Australian fishes in response to climate change.Crossref | GoogleScholarGoogle Scholar |
Borges, A. V., Schiettecatte, L. S., Abril, G., Delille, B., and Gazeau, E. (2006). Carbon dioxide in European coastal waters. Estuarine Coastal and Shelf Science 70, 375–387.
| Carbon dioxide in European coastal waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFSktrnN&md5=3ad82df9672f2fed7ebc91573f6c75e0CAS |
Brown, C. J., Fulton, E. A., Hobday, A. J., Matear, R. J., Possingham, H. P., Bulman, C., Christensen, V., Forrest, R. E., Gehrke, P. C., Gribble, N. A., Griffiths, S. P., Lozano-Montes, H., Martin, J. M., Metcalf, S., Okey, T. A., Watson, R., and Richardson, A. J. (2010). Effects of climate-driven primary production change on marine food webs: implications for fisheries and conservation. Global Change Biology 16, 1194–1212.
| Effects of climate-driven primary production change on marine food webs: implications for fisheries and conservation.Crossref | GoogleScholarGoogle Scholar |
Burling, M. C., Ivey, G. N., and Pattiaratchi, C. B. (1999). Convectively driven exchange in a shallow coastal embayment. Continental Shelf Research 19, 1599–1616.
| Convectively driven exchange in a shallow coastal embayment.Crossref | GoogleScholarGoogle Scholar |
Chiew, F. H. S., Teng, J., Vaze, J., Post, D. A., Perraud, J. M., Kirono, D. G. C., and Viney, N. R. (2009). Estimating climate change impact on runoff across southeast Australia: method, results, and implications of the modeling method. Water Resources Research 45, W10414.
| Estimating climate change impact on runoff across southeast Australia: method, results, and implications of the modeling method.Crossref | GoogleScholarGoogle Scholar |
Christensen, J. H., Hewitson, B., Busuioc, A., Chen, A., Gao, X., Held, I., Jones, R., Kolli, R. K., Kwon, W.-T., Laprise, R., Magaña Rueda, V., Mearns, L., Menéndez, C. G., Räisänen, J., Rinke, A. A. S., and Whetton, P. (2007). Regional climate projections. In ‘Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor and H. L. Miller) pp. 847–940. (Cambridge University Press: Cambridge, UK.)
Church, J. A., Hunter, J. R., McInnes, K. L., and White, N. J. (2006). Sea-level rise around the Australian coastline and the changing frequency of extreme sea-level events. Australian Meteorological Magazine 55, 253–260.
Chuwen, B. M., Hoeksema, S. D., and Potter, I. C. (2009). The divergent environmental characteristics of permanently-open, seasonally-open and normally-closed estuaries of south-western Australia. Estuarine Coastal and Shelf Science 85, 12–21.
| The divergent environmental characteristics of permanently-open, seasonally-open and normally-closed estuaries of south-western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlSjsLrE&md5=898fc7509d30b88bb4482cd1002cfa94CAS |
Connolly, R. M., Schlacher, T. A., and Gaston, T. F. (2009). Stable isotope evidence for trophic subsidy of coastal benthic fisheries by river discharge plumes off small estuaries. Marine Biology Research 5, 164–171.
| Stable isotope evidence for trophic subsidy of coastal benthic fisheries by river discharge plumes off small estuaries.Crossref | GoogleScholarGoogle Scholar |
Crook, D. A., Macdonald, J. I., and Raadik, T. A. (2008). Evidence of diadromous movements in a coastal population of southern smelts (Retropinninae: Retropinna) from Victoria, Australia. Marine and Freshwater Research 59, 638–646.
| Evidence of diadromous movements in a coastal population of southern smelts (Retropinninae: Retropinna) from Victoria, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovVKkurk%3D&md5=9b88c896aba3e95274cd5ee8645062a1CAS |
CSIRO (2007). Climate change in Australia: technical report 2007. CSIRO Marine and Atmospheric Research, Melbourne.
Dixson, D. L., Munday, P. L., and Jones, G. P. (2010). Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecology Letters 13, 68–75.
| Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues.Crossref | GoogleScholarGoogle Scholar |
Donelson, J. M., Munday, P. L., McCormick, M. I., Pankhurst, N. W., and Pankhurst, P. M. (2010). Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Marine Ecology Progress Series 401, 233–243.
| Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish.Crossref | GoogleScholarGoogle Scholar |
Donohue, R. J., McVicar, T. R., and Roderick, M. L. (2010). Assessing the ability of potential evaporation formulations to capture the dynamics in evaporative demand within a changing climate. Journal of Hydrology 386, 186–197.
| Assessing the ability of potential evaporation formulations to capture the dynamics in evaporative demand within a changing climate.Crossref | GoogleScholarGoogle Scholar |
Dove, M. C., and Sammut, J. (2007). Impacts of estuarine acidification on survival and growth of Sydney rock oysters Saccostrea glomerata (Gould 1850). Journal of Shellfish Research 26, 519–527.
| Impacts of estuarine acidification on survival and growth of Sydney rock oysters Saccostrea glomerata (Gould 1850).Crossref | GoogleScholarGoogle Scholar |
Drinkwater, K. F., Beaugrand, G., Kaeriyama, M., Kim, S., Ottersen, G., Perry, R. I., Portner, H. O., Polovina, J. J., and Takasuka, A. (2010). On the processes linking climate to ecosystem changes. Journal of Marine Systems 79, 374–388.
| On the processes linking climate to ecosystem changes.Crossref | GoogleScholarGoogle Scholar |
Edgar, G. J., Barrett, N. S., Graddon, D. J., and Last, P. R. (2000). The conservation significance of estuaries: a classification of Tasmanian estuaries using ecological, physical and demographic attributes as a case study. Biological Conservation 92, 383–397.
| The conservation significance of estuaries: a classification of Tasmanian estuaries using ecological, physical and demographic attributes as a case study.Crossref | GoogleScholarGoogle Scholar |
Elliott, M., Whitfield, A. K., Potter, I. C., Blaber, S. J. M., Cyrus, D. P., Nordlie, F. G., and Harrison, T. D. (2007). The guild approach to categorizing estuarine fish assemblages: a global review. Fish and Fisheries 8, 241–268.
| The guild approach to categorizing estuarine fish assemblages: a global review.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., and Gillanders, B. M. (2005). Alternative life-history patterns of estuarine fish: barium in otoliths elucidates freshwater residency. Canadian Journal of Fisheries and Aquatic Sciences 62, 1143–1152.
| Alternative life-history patterns of estuarine fish: barium in otoliths elucidates freshwater residency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXoslGqs7o%3D&md5=292ce5558ed539b3b0736bb9904562fcCAS |
Elsdon, T. S., De Bruin, M. B. N. A., Diepen, N. J., and Gillanders, B. M. (2009). Extensive drought negates human influence on nutrients and water quality in estuaries. Science of the Total Environment 407, 3033–3043.
| Extensive drought negates human influence on nutrients and water quality in estuaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivVegt70%3D&md5=2ee37be5a256a7a30ad021f77528b64eCAS |
Eyre, B. D. (2000). Regional evaluation of nutrient transformation and phytoplankton growth in nine river-dominated sub-tropical east Australian estuaries. Marine Ecology Progress Series 205, 61–83.
| Regional evaluation of nutrient transformation and phytoplankton growth in nine river-dominated sub-tropical east Australian estuaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhs1entg%3D%3D&md5=01136803ed825b48284434dd70422f35CAS |
Eyre, B., and Twigg, C. (1997). Nutrient behaviour during post-flood recovery of the Richmond River estuary northern NSW, Australia. Estuarine, Coastal and Shelf Science 44, 311–326.
| Nutrient behaviour during post-flood recovery of the Richmond River estuary northern NSW, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXivVyqs70%3D&md5=a401f9be0ae4653e22d1ea2a260849e8CAS |
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science 65, 414–432.
| Impacts of ocean acidification on marine fauna and ecosystem processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFegtL4%3D&md5=25f0681d20c358a38b757d741fc1cf94CAS |
Fairweather, P. G., and Lester, R. E. (2010). Predicting future ecological degradation based on modelled thresholds. Marine Ecology Progress Series 413, 291–304.
| Predicting future ecological degradation based on modelled thresholds.Crossref | GoogleScholarGoogle Scholar |
Feely, R. A., Alin, S. R., Newton, J., Sabine, C. L., Warner, M., Devol, A., Krembs, C., and Maloy, C. (2010). The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuarine, Coastal and Shelf Science 88, 442–449.
| The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVKmu7w%3D&md5=bdff2f5f0d4fccba15ea3f97eb2fed6aCAS |
Ferguson, G. J., Ward, T. M., and Geddes, M. C. (2008). Do recent age structures and historical catches of mulloway, Argyrosomus japonicus (Sciaenidae), reflect freshwater inflows in the remnant estuary of the Murray River, South Australia? Aquatic Living Resources 21, 145–152.
| Do recent age structures and historical catches of mulloway, Argyrosomus japonicus (Sciaenidae), reflect freshwater inflows in the remnant estuary of the Murray River, South Australia?Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M. (1995). Feeding ecology of the temperate marine fish Achoerodus viridis (Labridae): size, seasonal and site-specific differences. Marine and Freshwater Research 46, 1009–1020.
| Feeding ecology of the temperate marine fish Achoerodus viridis (Labridae): size, seasonal and site-specific differences.Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M., and Kingsford, M. J. (2002). Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanography and Marine Biology: An Annual Review 40, 233–309.
Gillanders, B. M., Elsdon, T. E., and Roughan, M. (2012). Connectivity of estuaries. In ‘Functioning of Estuaries and Coastal Ecosystems’. (Eds C. H. R. Heip, C. J. M. Philippart and J. J. Middelburg.) (Elsevier: Amsterdam.)
Gillson, J., Scandol, J., and Suthers, I. (2009). Estuarine gillnet fishery catch rates decline during drought in eastern Australia. Fisheries Research 99, 26–37.
| Estuarine gillnet fishery catch rates decline during drought in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Gräwe, U., Wolff, J. O., and Ribbe, J. (2010). Impact of climate variability on an east Australian bay. Estuarine, Coastal and Shelf Science 86, 247–257.
| Impact of climate variability on an east Australian bay.Crossref | GoogleScholarGoogle Scholar |
Griffiths, S. P. (2001). Factors influencing fish composition in an Australian intermittently open estuary. Is stability salinity-dependent? Estuarine, Coastal and Shelf Science 52, 739–751.
| Factors influencing fish composition in an Australian intermittently open estuary. Is stability salinity-dependent?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlGhsbw%3D&md5=07879547a3dafcd73935ddb3f0895ebfCAS |
Griffiths, S. P., and West, R. J. (1999). Preliminary assessment of shallow water fish in three small intermittently open estuaries in southeastern Australia. Fisheries Management and Ecology 6, 311–321.
| Preliminary assessment of shallow water fish in three small intermittently open estuaries in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Guisan, A., and Zimmermann, N. E. (2000). Predictive habitat distribution models in ecology. Ecological Modelling 135, 147–186.
| Predictive habitat distribution models in ecology.Crossref | GoogleScholarGoogle Scholar |
Halliday, I. A., Robins, J. B., Mayer, D. G., Staunton-Smith, J., and Sellin, M. J. (2008). Effects of freshwater flow on the year-class strength of a non-diadromous estuarine finfish, king threadfin (Polydactylus macrochir), in a dry-tropical estuary. Marine and Freshwater Research 59, 157–164.
| Effects of freshwater flow on the year-class strength of a non-diadromous estuarine finfish, king threadfin (Polydactylus macrochir), in a dry-tropical estuary.Crossref | GoogleScholarGoogle Scholar |
Hassell, K. L., Coutin, P. C., and Nugegoda, D. (2008a). Hypoxia impairs embryo development and survival in black bream (Acanthopagrus butcheri). Marine Pollution Bulletin 57, 302–306.
| Hypoxia impairs embryo development and survival in black bream (Acanthopagrus butcheri).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntlGms70%3D&md5=59462e37c0d6f2bdfda679ab15596894CAS |
Hassell, K. L., Coutin, P. C., and Nugegoda, D. (2008b). Hypoxia, low salinity and lowered temperature reduce embryo survival and hatch rates in black bream Acanthopagrus butcheri (Munro, 1949). Journal of Fish Biology 72, 1623–1636.
| Hypoxia, low salinity and lowered temperature reduce embryo survival and hatch rates in black bream Acanthopagrus butcheri (Munro, 1949).Crossref | GoogleScholarGoogle Scholar |
Heap, A. D., Bryce, S., and Ryan, D. A. (2004). Facies evolution of Holocene estuaries and deltas: a large-sample statistical study from Australia. Sedimentary Geology 168, 1–17.
| Facies evolution of Holocene estuaries and deltas: a large-sample statistical study from Australia.Crossref | GoogleScholarGoogle Scholar |
Hobday, A. J., Poloczanska, E. S., and Matear, R. J. (2008). Implications of climate change for Australian fisheries and aquaculture: a preliminary assessment. Report to the Department of Climate Change, Canberra.
Hoeksema, S. D., Chuwen, B. M., and Potter, I. C. (2006). Massive mortalities of the black bream Acanthopagrus butcheri (Sparidae) in two normally-closed estuaries, following extreme increases in salinity. Journal of the Marine Biological Association of the United Kingdom 86, 893–897.
| Massive mortalities of the black bream Acanthopagrus butcheri (Sparidae) in two normally-closed estuaries, following extreme increases in salinity.Crossref | GoogleScholarGoogle Scholar |
Hunt, C. W., Salisbury, J. E., Vandemark, D., and McGillis, W. (2011). Contrasting carbon dioxide inputs and exchange in three adjacent New England estuaries. Estuaries and Coasts 34, 68–77.
| Contrasting carbon dioxide inputs and exchange in three adjacent New England estuaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Sl&md5=201b6917b261ca970bab7f7891ced1bdCAS |
Hunter, K. A. (2007). XLCO2 – Seawater CO2 equilibrium calculations using Excel Version 2. University of Otago, New Zealand. Available at http://neon.otago.ac.nz/research/mfc/people/keith_hunter/software/swco2/ [Accessed 15 August 2011]
Jenkins, G. P., Conron, S. D., and Morison, A. K. (2010). Highly variable recruitment in an estuarine fish is determined by salinity stratification and freshwater flow: implications of a changing climate. Marine Ecology Progress Series 417, 249–261.
| Highly variable recruitment in an estuarine fish is determined by salinity stratification and freshwater flow: implications of a changing climate.Crossref | GoogleScholarGoogle Scholar |
Jiang, L. Q., Cai, W. J., and Wang, Y. C. (2008). A comparative study of carbon dioxide degassing in river- and marine-dominated estuaries. Limnology and Oceanography 53, 2603–2615.
| A comparative study of carbon dioxide degassing in river- and marine-dominated estuaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVOgu7nE&md5=e07852ffca329ce74109ac8977ac0658CAS |
Jones, M. V., and West, R. J. (2005). Spatial and temporal variability of seagrass fishes in intermittently closed and open coastal lakes in southeastern Australia. Estuarine, Coastal and Shelf Science 64, 277–288.
| Spatial and temporal variability of seagrass fishes in intermittently closed and open coastal lakes in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Jones, D. A., Wang, W., and Fawcett, R. (2009). High-quality spatial climate data-sets for Australia. Australian Meteorological and Oceanographic Journal 58, 233–248.
Justic, D., Rabalais, N. N., and Turner, R. E. (2005). Coupling between climate variability and coastal eutrophication: evidence and outlook for the northern Gulf of Mexico. Journal of Sea Research 54, 25–35.
| Coupling between climate variability and coastal eutrophication: evidence and outlook for the northern Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |
Justic, D., Bierman, V. J., Scavia, D., and Hetland, R. D. (2007). Forecasting Gulf’s hypoxia: the next 50 years? Estuaries and Coasts 30, 791–801.
| 1:CAS:528:DC%2BD1cXht12lsb4%3D&md5=b377e51a0b5dc48ce1ee6ef842f6fedeCAS |
Kench, P. S. (1999). Geomorphology of Australian estuaries: review and prospect. Australian Journal of Ecology 24, 367–380.
| Geomorphology of Australian estuaries: review and prospect.Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T., Walker, K. F., Lester, R. E., Fairweather, P. G., Sammut, J., and Geddes, M. C. (2011). A Ramsar wetland in crisis – the Coorong, Lower Lakes and Murray Mouth, Australia. Marine and Freshwater Research 62, 255–265.
| A Ramsar wetland in crisis – the Coorong, Lower Lakes and Murray Mouth, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsVKksbg%3D&md5=50a046216c7e92528af202f64520a64dCAS |
Klausmeyer, K. R., and Shaw, M. R. (2009). Climate change, habitat loss, protected areas and the climate adaptation potential of species in Mediterranean ecosystems worldwide. Plos One 4, e6392.
| Climate change, habitat loss, protected areas and the climate adaptation potential of species in Mediterranean ecosystems worldwide.Crossref | GoogleScholarGoogle Scholar |
Koch, M. S., Schopmeyer, S. A., Kyhn-Hansen, C., Madden, C. J., and Peters, J. S. (2007). Tropical seagrass species tolerance to hypersalinity stress. Aquatic Botany 86, 14–24.
| Tropical seagrass species tolerance to hypersalinity stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1agtrrF&md5=e4722105bca51ccc95aea5508ee88c82CAS |
Kurihara, H. (2008). Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Marine Ecology Progress Series 373, 275–284.
| Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVCrsL4%3D&md5=f92507ddda12dadb6109a2a2c60093beCAS |
Last, P. R., White, W. T., Gledhill, D. C., Hobday, A. J., Brown, R., Edgar, G. J., and Pecl, G. (2011). Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Global Ecology and Biogeography 20, 58–72.
| Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices.Crossref | GoogleScholarGoogle Scholar |
Lester, R. E., and Fairweather, P. G. (2009). Modelling future conditions in the degraded semi-arid estuary of Australia’s largest river using ecosystem states. Estuarine Coastal and Shelf Science 85, 1–11.
| Modelling future conditions in the degraded semi-arid estuary of Australia’s largest river using ecosystem states.Crossref | GoogleScholarGoogle Scholar |
Lester, R. E., Webster, I. T., Fairweather, P. G., and Young, W. J. (2011). Linking water-resource models to ecosystem-response models to guide water-resource planning – an example from the Murray–Darling Basin, Australia. Marine and Freshwater Research 62, 279–289.
| Linking water-resource models to ecosystem-response models to guide water-resource planning – an example from the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsVKktr8%3D&md5=ba2a4dd45b5b5d1472192f2cb3d399bdCAS |
Levin, L. A., Ekau, W., Gooday, A. J., Jorissen, F., Middelburg, J. J., Naqvi, S. W. A., Neira, C., Rabalais, N. N., and Zhang, J. (2009). Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 6, 2063–2098.
| Effects of natural and human-induced hypoxia on coastal benthos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltVCkug%3D%3D&md5=5abe57bd0a71118d345ad76b448af120CAS |
Loneragan, N. R., and Bunn, S. E. (1999). River flows and estuarine ecosystems: implications for coastal fisheries from a review and a case study of the Logan River, southeast Queensland. Australian Journal of Ecology 24, 431–440.
| River flows and estuarine ecosystems: implications for coastal fisheries from a review and a case study of the Logan River, southeast Queensland.Crossref | GoogleScholarGoogle Scholar |
Lough, J. M., Hobday, A. J., and Jones, D. (2011). Observed climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 984–999.
| Observed climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Lui, L. C. (1969). Salinity tolerance and osmoregulation of Taeniomembras microstomus (Gunther, 1861) (Pisces–Mugiliformes–Atherinidae) from Australian salt lakes. Australian Journal of Marine and Freshwater Research 20, 157–162.
| Salinity tolerance and osmoregulation of Taeniomembras microstomus (Gunther, 1861) (Pisces–Mugiliformes–Atherinidae) from Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Mallin, M. A., Paerl, H. W., Rudek, J., and Bates, P. W. (1993). Regulation of estuarine primary production by watershed rainfall and river flow. Marine Ecology Progress Series 93, 199–203.
| Regulation of estuarine primary production by watershed rainfall and river flow.Crossref | GoogleScholarGoogle Scholar |
Malone, T. C., Crocker, L. H., Pike, S. E., and Wendler, B. W. (1988). Influences of river flow on the dynamics of phytoplankton production in a partially stratified estuary. Marine Ecology Progress Series 48, 235–249.
| Influences of river flow on the dynamics of phytoplankton production in a partially stratified estuary.Crossref | GoogleScholarGoogle Scholar |
McDowall, R. M. (1988). ‘Diadromy in Fishes. Migrations Between Freshwater and Marine Environments.’ (Croom Helm: London.)
Meynecke, J. O., Lee, S. Y., Duke, N. C., and Warnken, J. (2006). Effect of rainfall as a component of climate change on estuarine fish production in Queensland, Australia. Estuarine, Coastal and Shelf Science 69, 491–504.
| Effect of rainfall as a component of climate change on estuarine fish production in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Miller, A. W., Reynolds, A. C., Sobrino, C., and Riedel, G. F. (2009). Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries. Plos One 4, e5661.
| Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries.Crossref | GoogleScholarGoogle Scholar |
Milton, D., Halliday, I., Sellin, M., Marsh, R., Staunton-Smith, J., and Woodhead, J. (2008). The effect of habitat and environmental history on otolith chemistry of barramundi Lates calcarifer in estuarine populations of a regulated tropical river. Estuarine Coastal and Shelf Science 78, 301–315.
| The effect of habitat and environmental history on otolith chemistry of barramundi Lates calcarifer in estuarine populations of a regulated tropical river.Crossref | GoogleScholarGoogle Scholar |
Morrongiello, J. R., Beatty, S. J., Bennett, J., Crook, D. A., Ikedife, D. N. E. N., Kennard, M. J., Kerezsy, A., Lintermans, M., McNeil, D. G., Pusey, B. J., and Rayner, T. (2011a). Climate change and its implications for Australia's freshwater fish. Marine and Freshwater Research 62, 1082–1098.
| Climate change and its implications for Australia's freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Morrongiello, J. R., Crook, D. A., King, A. J., Ramsey, D. S. L., and Brown, P. (2011b). Impacts of drought and predicted effects of climate change on fish growth in temperate Australian lakes. Global Change Biology 17, 745–755.
| Impacts of drought and predicted effects of climate change on fish growth in temperate Australian lakes.Crossref | GoogleScholarGoogle Scholar |
Mosley, L. M., Peake, B. M., and Hunter, K. A. (2010). Modelling of pH and inorganic carbon speciation in estuaries using the composition of the river and seawater end members. Environmental Modelling & Software 25, 1658–1663.
| Modelling of pH and inorganic carbon speciation in estuaries using the composition of the river and seawater end members.Crossref | GoogleScholarGoogle Scholar |
Munday, P. L., Crawley, N. E., and Nilsson, G. E. (2009a). Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Marine Ecology Progress Series 388, 235–242.
| Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1SrtrnM&md5=d4ff6d30a9d56ac2d9fc60193d0456efCAS |
Munday, P. L., Dixson, D. L., Donelson, J. M., Jones, G. P., Pratchett, M. S., Devitsina, G. V., and Doving, K. B. (2009b). Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proceedings of the National Academy of Sciences, USA 106, 1848–1852.
| Ocean acidification impairs olfactory discrimination and homing ability of a marine fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitV2isrg%3D&md5=b393a4ea34b06c3afc4a75c959e7efeeCAS |
Munday, P. L., Donelson, J. M., Dixson, D. L., and Endo, G. G. K. (2009c). Effects of ocean acidification on the early life history of a tropical marine fish. Proceedings of the Royal Society, B – Biological Sciences 276, 3275–3283.
| Effects of ocean acidification on the early life history of a tropical marine fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFCgsLrJ&md5=39f3ac5437e108caa78e0f735a3df105CAS |
Munday, P. L., Dixson, D. L., McCormick, M. I., Meekan, M., Ferrari, M. C. O., and Chivers, D. P. (2010). Replenishment of fish populations is threatened by ocean acidification. Proceedings of the National Academy of Sciences, USA 107, 12930–12934.
| Replenishment of fish populations is threatened by ocean acidification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsVagur4%3D&md5=29915a1998e7190bf98e919f51aaec55CAS |
Najjar, R. G., Pyke, C. R., Adams, M. B., Breitburg, D., Hershner, C., Kemp, M., Howarth, R., Mulholland, M. R., Paolisso, M., Secor, D., Sellner, K., Wardrop, D., and Wood, R. (2010). Potential climate-change impacts on the Chesapeake Bay. Estuarine Coastal and Shelf Science 86, 1–20.
| Potential climate-change impacts on the Chesapeake Bay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1WisrvF&md5=ae8094dd4067a6b7dfeb34441b37b79fCAS |
Nicholson, G., Jenkins, G. P., Sherwood, J., and Longmore, A. (2008). Physical environmental conditions, spawning and early-life stages of an estuarine fish: climate change implications for recruitment in intermittently open estuaries. Marine and Freshwater Research 59, 735–749.
| Physical environmental conditions, spawning and early-life stages of an estuarine fish: climate change implications for recruitment in intermittently open estuaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVaisrvK&md5=cb0a33c233eaae3d2506d58b67a49195CAS |
Nicolas, D., Lobry, J., Lepage, M., Sautour, B., Le Pape, O., Cabral, H., Uriarte, A., and Boet, P. (2010). Fish under influence: a macroecological analysis of relations between fish species richness and environmental gradients among European tidal estuaries. Estuarine Coastal and Shelf Science 86, 137–147.
| Fish under influence: a macroecological analysis of relations between fish species richness and environmental gradients among European tidal estuaries.Crossref | GoogleScholarGoogle Scholar |
Nix, H. A. (1981). The environment of Terra Australis. In ‘Ecological Biogeography of Australia’. (Ed. A. Keast.) pp. 103–133. (W. Junk: The Hague.)
Noell, C. J., Ye, Q., Short, D. A., Bucater, L. B., and Wellman, N. R. (2009). Fish assemblages of the Murray Mouth and Coorong region, South Australia, during an extended drought period. CSIRO, Water for a Healthy Country National Research Flagship, Canberra.
Nordlie, F. G. (2009). Environmental influences on regulation of blood plasma/serum components in teleost fishes: a review. Reviews in Fish Biology and Fisheries 19, 481–564.
| Environmental influences on regulation of blood plasma/serum components in teleost fishes: a review.Crossref | GoogleScholarGoogle Scholar |
Nunes, R. A., and Lennon, G. W. (1986). Physical property distributions and seasonal trends in Spencer Gulf, South Australia – an inverse estuary. Australian Journal of Marine and Freshwater Research 37, 39–53.
| Physical property distributions and seasonal trends in Spencer Gulf, South Australia – an inverse estuary.Crossref | GoogleScholarGoogle Scholar |
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R. M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R. G., Plattner, G. K., Rodgers, K. B., Sabine, C. L., Sarmiento, J. L., Schlitzer, R., Slater, R. D., Totterdell, I. J., Weirig, M. F., Yamanaka, Y., and Yool, A. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686.
| Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVCjsL%2FE&md5=9390bc0fada8743ec20f80a7ffe47e99CAS |
Ortega, T., Ponce, R., Forja, J., and Gomez-Parra, A. (2005). Fluxes of dissolved inorganic carbon in three estuarine systems of the Cantabrian Sea (north of Spain). Journal of Marine Systems 53, 125–142.
| Fluxes of dissolved inorganic carbon in three estuarine systems of the Cantabrian Sea (north of Spain).Crossref | GoogleScholarGoogle Scholar |
Parker, L. M., Ross, P. M., and O’Connor, W. A. (2009). The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Global Change Biology 15, 2123–2136.
| The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850).Crossref | GoogleScholarGoogle Scholar |
Parker, L. M., Ross, P. M., and O’Connor, W. A. (2010). Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters. Marine Biology 157, 2435–2452.
| Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters.Crossref | GoogleScholarGoogle Scholar |
Perkins, S. E., and Pitman, A. J. (2009). Do weak AR4 models bias projections of future climate changes over Australia? Climatic Change 93, 527–558.
| Do weak AR4 models bias projections of future climate changes over Australia?Crossref | GoogleScholarGoogle Scholar |
Pollard, D. A. (1994). A comparison of fish assemblages and fisheries in intermittently open and permanently open coastal lagoons on the south coast of New South Wales, south eastern Australia. Estuaries 17, 631–646.
| A comparison of fish assemblages and fisheries in intermittently open and permanently open coastal lagoons on the south coast of New South Wales, south eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Pörtner, H. O., and Farrell, A. P. (2008). Physiology and climate change. Science 322, 690–692.
| Physiology and climate change.Crossref | GoogleScholarGoogle Scholar |
Pörtner, H. O., and Peck, M. A. (2010). Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. Journal of Fish Biology 77, 1745–1779.
| Climate change effects on fishes and fisheries: towards a cause-and-effect understanding.Crossref | GoogleScholarGoogle Scholar |
Potter, I. C., Chuwen, B. M., Hoeksema, S. D., and Elliott, M. (2010). The concept of an estuary: a definition that incorporates systems which can become closed to the ocean and hypersaline. Estuarine Coastal and Shelf Science 87, 497–500.
| The concept of an estuary: a definition that incorporates systems which can become closed to the ocean and hypersaline.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVemsrk%3D&md5=5fd28a3027d0d3e0c8be8f93d57e5f3aCAS |
Rand, P. S., Hinch, S. G., Morrison, J., Foreman, M. G. G., MacNutt, M. J., Macdonald, J. S., Healey, M. C., Farrell, A. P., and Higgs, D. A. (2006). Effects of river discharge, temperature, and future climates on energetics and mortality of adult migrating Fraser River sockeye salmon. Transactions of the American Fisheries Society 135, 655–667.
| Effects of river discharge, temperature, and future climates on energetics and mortality of adult migrating Fraser River sockeye salmon.Crossref | GoogleScholarGoogle Scholar |
Randall, D. A., Wood, R. A., Bony, S., Colman, R., Fichefet, T., Fyfe, J., Kattsov, V., Pitman, A., Shukla, J., Srinivasan, J., Stouffer, R. J., Sumi, A., and Taylor, K. E. (2007). Climate models and their evaluation. In ‘Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor and H. L. Miller.). pp. 589–662. (Cambridge University Press: Cambridge, UK)
Ribbe, J. (2006). A study into the export of saline water from Hervey Bay, Australia. Estuarine, Coastal and Shelf Science 66, 550–558.
| A study into the export of saline water from Hervey Bay, Australia.Crossref | GoogleScholarGoogle Scholar |
Richardson, A. J., and Schoeman, D. S. (2004). Climate impact on plankton ecosystems in the northeast Atlantic. Science 305, 1609–1612.
| Climate impact on plankton ecosystems in the northeast Atlantic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntlGrsr4%3D&md5=27ee860356df147bed5c8e466cdca001CAS |
Robins, J. B., Halliday, I. A., Staunton-Smith, J., Mayer, D. G., and Sellin, M. J. (2005). Freshwater-flow requirements of estuarine fisheries in tropical Australia: a review of the state of knowledge and application of a suggested approach. Marine and Freshwater Research 56, 343–360.
| Freshwater-flow requirements of estuarine fisheries in tropical Australia: a review of the state of knowledge and application of a suggested approach.Crossref | GoogleScholarGoogle Scholar |
Robins, J., Mayer, D., Staunton-Smith, J., Halliday, I., Sawynok, B., and Sellin, M. (2006). Variable growth rates of the tropical estuarine fish barramundi Lates calcarifer (Bloch) under different freshwater flow conditions. Journal of Fish Biology 69, 379–391.
| Variable growth rates of the tropical estuarine fish barramundi Lates calcarifer (Bloch) under different freshwater flow conditions.Crossref | GoogleScholarGoogle Scholar |
Rose, K. A., Adamack, A. T., Murphy, C. A., Sable, S. E., Kolesar, S. E., Craig, J. K., Breitburg, D. L., Thomas, P., Brouwer, M. H., Cerco, C. F., and Diamond, S. (2009). Does hypoxia have population-level effects on coastal fish? Musings from the virtual world. Journal of Experimental Marine Biology and Ecology 381, S188–S203.
| Does hypoxia have population-level effects on coastal fish? Musings from the virtual world.Crossref | GoogleScholarGoogle Scholar |
Roughan, M., Macdonald, H. S., Baird, M. E., and Glasby, T. M. (2011). Modelling coastal connectivity in a western boundary current: seasonal and inter-annual variability. Deep-sea Research. Part II. Topical Studies in Oceanography 58, 628–644.
| Modelling coastal connectivity in a western boundary current: seasonal and inter-annual variability.Crossref | GoogleScholarGoogle Scholar |
Roy, P. S., Williams, R. J., Jones, A. R., Yassini, I., Gibbs, P. J., Coates, B., West, R. J., Scanes, P. R., Hudson, J. P., and Nichol, S. (2001). Structure and function of south-east Australian estuaries. Estuarine, Coastal and Shelf Science 53, 351–384.
| Structure and function of south-east Australian estuaries.Crossref | GoogleScholarGoogle Scholar |
Rustomji, P. (2007). Flood and drought impacts on the opening regime of a wave-dominated estuary. Marine and Freshwater Research 58, 1108–1119.
| Flood and drought impacts on the opening regime of a wave-dominated estuary.Crossref | GoogleScholarGoogle Scholar |
Sakabe, R., and Lyle, J. M. (2010). The influence of tidal cycles and freshwater inflow on the distribution and movement of an estuarine resident fish Acanthopagrus butcheri. Journal of Fish Biology 77, 643–660.
| The influence of tidal cycles and freshwater inflow on the distribution and movement of an estuarine resident fish Acanthopagrus butcheri.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cjktFOnsQ%3D%3D&md5=487fd4e86f68a983d8d95a7b4fa6fd8cCAS |
Samarasinghe, J. R. D., and Lennon, G. W. (1987). Hypersalinity, flushing and transient salt-wedges in a tidal gulf – an inverse estuary. Estuarine, Coastal and Shelf Science 24, 483–498.
| Hypersalinity, flushing and transient salt-wedges in a tidal gulf – an inverse estuary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXkvVent7s%3D&md5=bfb3b7c8c8a319153b3297655510b573CAS |
Samarasinghe, J. R. D., Bode, L., and Mason, L. B. (2003). Modelled response of Gulf St Vincent (South Australia) to evaporation, heating and winds. Continental Shelf Research 23, 1285–1313.
| Modelled response of Gulf St Vincent (South Australia) to evaporation, heating and winds.Crossref | GoogleScholarGoogle Scholar |
Sammut, J., White, I., and Melville, M. D. (1996). Acidification of an estuarine tributary in eastern Australia due to drainage of acid sulfate soils. Marine and Freshwater Research 47, 669–684.
| Acidification of an estuarine tributary in eastern Australia due to drainage of acid sulfate soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmvF2jtLk%3D&md5=bde60ed63e472e2b8a62c4c054aafdd2CAS |
Sanchez-Jerez, P., Gillanders, B. M., and Kingsford, M. J. (2002). Spatial variability of trace elements in fish otoliths: comparison with dietary items and habitat constituents in seagrass meadows. Journal of Fish Biology 61, 801–821.
| Spatial variability of trace elements in fish otoliths: comparison with dietary items and habitat constituents in seagrass meadows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XotFGgsrg%3D&md5=420e2b4c39ccd39c06a05f602daf5398CAS |
Schlacher, T. A., Skillington, A. J., Connolly, R. M., Robinson, W., and Gaston, T. F. (2008). Coupling between marine plankton and freshwater flow in the plumes off a small estuary. International Review of Hydrobiology 93, 641–658.
| Coupling between marine plankton and freshwater flow in the plumes off a small estuary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFyitbg%3D&md5=0bc39a89f79eb871445f3d39a0c5aa81CAS |
Sheaves, M., and Johnston, R. (2008). Influence of marine and freshwater connectivity on the dynamics of subtropical estuarine wetland fish metapopulations. Marine Ecology Progress Series 357, 225–243.
| Influence of marine and freshwater connectivity on the dynamics of subtropical estuarine wetland fish metapopulations.Crossref | GoogleScholarGoogle Scholar |
Sheaves, M., and Johnston, R. (2009). Ecological drivers of spatial variability among fish fauna of 21 tropical Australian estuaries. Marine Ecology Progress Series 385, 245–260.
| Ecological drivers of spatial variability among fish fauna of 21 tropical Australian estuaries.Crossref | GoogleScholarGoogle Scholar |
Sheaves, M., Johnston, R., and Abrantes, K. (2007). Fish fauna of dry tropical and subtropical estuarine floodplain wetlands. Marine and Freshwater Research 58, 931–943.
| Fish fauna of dry tropical and subtropical estuarine floodplain wetlands.Crossref | GoogleScholarGoogle Scholar |
Snow, G. C., Adams, J. B., and Bate, G. C. (2000). Effect of river flow on estuarine microalgal biomass and distribution. Estuarine, Coastal and Shelf Science 51, 255–266.
| Effect of river flow on estuarine microalgal biomass and distribution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmsFemtrY%3D&md5=df97bd9270dd94dd48b6467a658fb915CAS |
Staunton-Smith, J., Robins, J. B., Mayer, D. G., Sellin, M. J., and Halliday, I. A. (2004). Does the quantity and timing of fresh water flowing into a dry tropical estuary affect year-class strength of barramundi (Lates calcarifer)? Marine and Freshwater Research 55, 787–797.
| Does the quantity and timing of fresh water flowing into a dry tropical estuary affect year-class strength of barramundi (Lates calcarifer)?Crossref | GoogleScholarGoogle Scholar |
Thresher, R. E., Koslow, J. A., Morison, A. K., and Smith, D. C. (2007). Depth-mediated reversal of the effects of climate change on long-term growth rates of exploited marine fish. Proceedings of the National Academy of Sciences, USA 104, 7461–7465.
| Depth-mediated reversal of the effects of climate change on long-term growth rates of exploited marine fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlslGhtr0%3D&md5=f38b039a9f90cac9a957c29aee59a223CAS |
Timbal, B., Fernandez, E., and Li, Z. (2009). Generalization of a statistical downscaling model to provide local climate change projections for Australia. Environmental Modelling & Software 24, 341–358.
| Generalization of a statistical downscaling model to provide local climate change projections for Australia.Crossref | GoogleScholarGoogle Scholar |
Turner, L., Tracey, D., Tilden, J., and Dennison, W. C. (2004). ‘Where River Meets Sea: Exploring Australia’s Estuaries.’ (Cooperative Research Centre for Coastal Zone Estuary and Waterway Management: Brisbane.)
Vörösmarty, C. J., Green, P., Salisbury, J., and Lammers, R. B. (2000). Global water resources: vulnerability from climate change and population growth. Science 289, 284–288.
| Global water resources: vulnerability from climate change and population growth.Crossref | GoogleScholarGoogle Scholar |
Warfe, D. M., Pettit, N. E., Davies, P. M., Pusey, B. J., Hamilton, S. K., Kennard, M. J., Townsend, S. A., Bayliss, P., Ward, D. P., Douglas, M. M., Burford, M. A., Finn, M., Bunn, S. E., and Halliday, I. A. (2011). The ‘wet–dry’ in the wet-dry tropics drives ecosystem structure and processes in northern Australian rivers. Freshwater Biology , in press..
| The ‘wet–dry’ in the wet-dry tropics drives ecosystem structure and processes in northern Australian rivers.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S. D., Walker, K. F., and Zampatti, B. P. (2008). Salinity may cause fragmentation of hardyhead (Teleostei: Atherinidae) populations in the River Murray, Australia. Marine and Freshwater Research 59, 254–258.
| Salinity may cause fragmentation of hardyhead (Teleostei: Atherinidae) populations in the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltl2jtLY%3D&md5=f511ed38653e6b944500b993af28ed8dCAS |
Wetz, M. S., Hutchinson, E. A., Lunetta, R. S., Paerl, H. W., and Taylor, J. C. (2011). Severe droughts reduce estuarine primary productivity with cascading effects on higher trophic levels. Limnology and Oceanography 56, 627–638.
| Severe droughts reduce estuarine primary productivity with cascading effects on higher trophic levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltVGktLw%3D&md5=45d34d7c4ff3f51ff964f8e17c3b1023CAS |
Yates, C. J., Elith, J., Latimer, A. M., Le Maitre, D., Midgley, G. F., Schurr, F. M., and West, A. G. (2010). Projecting climate change impacts on species distributions in megadiverse South African Cape and southwest Australian floristic regions: opportunities and challenges. Austral Ecology 35, 374–391.
| Projecting climate change impacts on species distributions in megadiverse South African Cape and southwest Australian floristic regions: opportunities and challenges.Crossref | GoogleScholarGoogle Scholar |
Young, G. C., and Potter, I. C. (2002). Influence of exceptionally high salinities, marked variations in freshwater discharge and opening of estuary mouth on the characteristics of the ichthyofauna of a normally-closed estuary. Estuarine, Coastal and Shelf Science 55, 223–246.
| Influence of exceptionally high salinities, marked variations in freshwater discharge and opening of estuary mouth on the characteristics of the ichthyofauna of a normally-closed estuary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmsF2msb4%3D&md5=d1642eeab63caf4b6281d67c9d314418CAS |
Zampatti, B. P., Bice, C. M., and Jennings, P. R. (2010). Temporal variability in fish assemblage structure and recruitment in a freshwater-deprived estuary: the Coorong, Australia. Marine and Freshwater Research 61, 1298–1312.
| Temporal variability in fish assemblage structure and recruitment in a freshwater-deprived estuary: the Coorong, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVagt7vP&md5=9ae849eb259972ed63a5f2ac54ffb300CAS |
Zhong, L. J., Li, M., and Foreman, M. G. G. (2008). Resonance and sea level variability in Chesapeake Bay. Continental Shelf Research 28, 2565–2573.
| Resonance and sea level variability in Chesapeake Bay.Crossref | GoogleScholarGoogle Scholar |


