Climatic variation and breeding in the Australian Magpie (Gymnorhina tibicen): a case study using existing data
Heather GibbsSchool of Life and Environmental Sciences, Deakin University, Burwood, Vic. 3125, Australia. Present address: PO Box 2110, Lygon St North, East Brunswick, Vic. 3057, Australia. Email: hgi@deakin.edu.au
Emu 107(4) 284-293 https://doi.org/10.1071/MU07022
Submitted: 11 April 2007 Accepted: 3 October 2007 Published: 5 December 2007
Abstract
To anticipate the effects of climate change on Australia’s avifauna, it is first necessary to understand the current effects of climate (including climate variability) on life histories, and to examine the scope and nature of existing data that may provide the necessary historical context to anticipate the effects of climate change. This study examines naturally occurring geographical gradients (altitude, latitude) and the Southern Oscillation Index (SOI) as integrated measures of climate. These are then compared with the timing and ‘amount’ of breeding recorded for the Australian Magpie (Gymnorhina tibicen) using data from Birds Australia’s Nest Record Scheme and Atlas of Australian Birds, the NSW Bird Atlassers Inc.’s NSW Bird Atlas, and the Canberra Ornitholgists Group’s Garden Bird Survey. For this common, easily identified species, these data suggest links between Australian Magpie breeding and all three environmental variables. Breeding became later as altitude increased, the proportion of breeding records increased from north to south, and years of high SOI corresponded to more (and earlier) breeding in this species. That annual climatic fluctuations have a direct, immediate and substantial effect on breeding in the Australian Magpie, particularly on the amount of breeding that occurs, implies that longer term changes in climate will have substantial impacts on populations. Results were not solely temperature-driven, which makes predicting climate change impacts difficult. For rainfall, predictions are far less precise and regional variation is higher. The results also highlight the potential and limitations of current survey techniques for documenting the impacts of climate change on birds; in particular, the Nest Record Scheme does not measure the amount of breeding that occurs, but a useful index of this can be derived from bird atlassing data.
Additional keywords: altitude, Australia, birds, climate, ENSO, latitude, methodology, phenology, rainfall, SOI, survey, temperature.
Introduction
International studies indicate that climate change is influencing the timing of breeding in many species of birds. With the observed warmer conditions in recent years, birds are typically breeding earlier (Crick et al. 1997; Crick and Sparks 1999; Parmesan and Yohe 2003). However, in Australia, no similar patterns have been documented, except for one study in the Snowy Mountains, where the timing of breeding of Richard’s Pipit (Anthus novaeseelandiae) has advanced in line with an advance in the date of snow-melt (Norment and Green 2004). In general, there is relatively little published research on the biological effects of climate change on Australian species (Hughes 2003; Chambers et al. 2005), although there are recent studies on changes in the timing of migration in Australian birds (Green and Pickering 2002; Chambers 2005; Beaumont et al. 2006). The lack of published studies is partly because Australia lacks the diversity of long-term phenological datasets common in Europe and also because the biological effects of climate are not as well documented. This lack of data, lack of biological understanding and the challenges of a large, sparsely populated and climatically diverse continent, make it difficult to document such patterns in Australia. Further, the availability of water, rather than temperature, may be a critical factor influencing bird breeding over much of Australia (Barrett et al. 2002a).
Although less studied – particularly in relation to climate change – the ‘amount’ of breeding by birds also varies depending on the conditions they experience. Changes in the proportion of birds breeding may affect total reproductive output more than changes in the timing of breeding. For example, in Britain, a reduction in the average number of nesting attempts per pair of European Turtle-Doves (Streptopelia turtur) was sufficient to explain a population decrease of 17% per annum (Browne et al. 2005). Despite this, changes in the proportion of birds breeding has been relatively little studied. In top-order predators, changes between years can be very dramatic: Snowy Owls (Bubo scandiacus) and Pomarine Jaegers (Stercorarius pomarinus) are known to skip a breeding year or two when prey are scarce (Pitelka et al. 1955; Maher 1970; Hoffmann 1974), and in the Northern Spotted Owl (Strix occidentalis caurina) annual reproductive effort is varies greatly (McKelvey et al. 1993). In the Neotropic Cormorant (Phalacrocorax brasilianus) in central Chile, a decrease in breeding numbers linked to an El Niño event caused a considerable drop in production of offspring (Kalmbach et al. 2001). In Australia, a study on the Northern Tablelands of New South Wales (NSW) highlighted the importance of the ‘amount’ of breeding in terrestrial passerines: 18 of 46 species did not breed in the study area at all in a year when conditions were less suitable, while in some other species, including the Australian Magpie, substantially fewer pairs bred (McLean et al. 2005). The present study looks at links between climate and the amount and timing of breeding in the Australian Magpie.
Over the past 30 years, volunteers have collected large amounts of data on Australia’s birds and these provide a reservoir of information. For example, Birds Australia’s Nest Record Scheme (NRS) is similar to those elsewhere in the world that have provided useful evidence of the effects of climate change (Crick et al. 1997; Crick and Sparks 1999). The Atlas of Australian Birds (Barrett et al. 2002b), the NSW Bird Atlas (Cooper and McAllan 1995) and the Canberra Garden Bird Survey (Veerman 2003) also contain information on bird breeding, but only as part of more general bird surveys. This study uses data from these four sources to relate breeding in the Australian Magpie (hereafter, Magpie) to climatic variables.
This study uses large-scale climate indices derived from monthly values of the Southern Oscillation Index (SOI). The SOI has been related to enhanced success or catastrophic failure in breeding seabirds (Bunce et al. 2002; Smithers et al. 2003; Chambers 2004) and to abundance of waterbirds (Norman and Nicholls 1991; Chambers and Loyn 2006) but no studies have investigated the relationship between the SOI and the biology of terrestrial passerines in Australia. This is despite substantial effects being documented overseas using similar indices: for example, in the Pacific north-west of North America, the North Atlantic Oscillation (NAO) and the El Niño–Southern Oscillation (ENSO) Precipitation Index (ESPI; closely related to the SOI) explained 50–90% of the annual variation in avian productivity for 10 landbird species (Nott et al. 2002). In the southern hemisphere, a study on Burrowing Parrots (Cyanoliseus patagonus) in Patagonia documented an apparently SOI-related effect on breeding success between two seasons (Masello and Quillfeldt 2003), and in New Zealand, Common Starlings (Sturnus vulgaris) laid earlier at both extremes of an ENSO index, presumably as food availability fluctuated according to climatic events (Tryjanowski et al. 2006).
As well as variation in climate between years, which for much of eastern Australia is reasonably well represented by the SOI (Pittock 2003), altitude and latitude also have marked effects on local climate and can be useful as proxies in studies of climate change (Fielding et al. 1999; Dingle et al. 2000). High altitudes are typically much colder than the surrounding lowlands, with the decrease in mean temperature with altitude known as the lapse rate. For the Snowy Mountains, the lapse rate is ~7.3°C per 1000 m (Galloway 1988). Similarly, there is a general cooling with distance from equatorial latitudes. In Australia the main influence of latitude on some species could relate more to seasonal changes in daylength; these are minimal in the tropics, but very significant further south. For example, in Darwin the longest and shortest days differ by only 1.5 h, whereas in Hobart the difference is 6.3 h (http://www.ga.gov.au/geodesy/astro/sunrise.jsp, accessed 26 June 2006). Longer summer days would give largely visual foragers such as the Magpie substantially longer to obtain enough food to provision hungry nestlings or fledglings and would also affect biological productivity such as plant growth and attendant flow-on effects to higher trophic levels.
The objective of this study was to use existing Australian data on Magpies to document relationships between breeding and climate, using natural geographical gradients in climate (altitude, latitude) and the SOI as integrated measures of climate. The Magpie was chosen because it is common, widespread and easy to identify, resulting in fairly accurate data and good sample sizes. It is also resident, generally single-brooded (Carrick 1972; Higgins et al. 2006) and breeds seasonally, simplifying the analysis of the timing of breeding.
Methods
Magpie variables
Magpie data were sourced from:
-
The Atlas of Australian Birds database of Birds Australia (hereafter Atlas 2), available at http://www.birdsaustralia.com.au/atlas/index.html accessed 13 November 2007 (Barrett et al. 2003).
-
The Birds Australia Nest Record Scheme database (hereafter NRS), available at http://www.birdsaustralia.com.au/projects/nrs.html, accessed 13 November 2007.
-
The NSW Bird Atlas, of the NSW Bird Atlassers Inc. (hereafter NSW Atlas), available at http://www.nswbirdatlassers.com/, accessed 13 November 2007.
-
The Garden Bird Survey of the Canberra Ornithologists Group (hereafter GBS), Canberra Ornithologists Group Inc., available at http://garden.canberrabirds.org.au/linkPages/theSurveyLink.htm, accessed 13 November 2007 (Veerman 2003).
The Atlas 2 data that were supplied included details for the location, duration and date of all surveys, not just those where Magpies were recorded. In contrast, for the NSW Atlas data, only records of Magpies were provided.
The survey techniques for each data source differ in some important respects. Both atlases aim to record the distribution of bird species, and include breeding information only incidentally. In Atlas 2, short local surveys (2 ha, 20 min) predominate, whereas the NSW Atlas largely consists of longer surveys (days, weeks or months) covering larger areas (usually blocks of 10′ latitude × 10′ longitude). The NRS aims to record the details of individual breeding attempts: observers are encouraged to make repeat visits to nests that they have found. Whereas for the atlas data each survey records many different species at the one location, in the NRS every breeding attempt is recorded independently. There is thus no obvious measure of ‘survey effort’ in the NRS. The GBS is different again: it involves filling in a chart that divides the year into weeks (columns) and species (rows). For each week, observers record the species present as well as the stage of breeding (if any). This has some advantages: first, breeding information is deliberately sought and is recorded in the context of all the other species seen; second, survey effort is relatively stable throughout the year. The disadvantage is the amount of recording effort that it takes, which may partly explain why this type of data is only available for suburban gardens in the Canberra region.
These data sources differ not only in their methodology, but also in the quantity of data and in the periods over which they were collected. The NRS database contained ~1250 Magpie nest records generated over more than 30 years (mainly 1967–97); the Atlas 2 data contained ~4000 observations of Magpie breeding over 5 years (1998–2002); the NSW Atlas contained ~1500 observations of Magpie breeding over more than 30 years (mainly 1977–99); and the GBS held ~3000 observations of Magpie breeding (representing at least 200 breeding events) over 21 years (1981–2002).
As the NSW Atlas data are generally recorded monthly and without details on the stage of breeding, data on the timing of breeding have relatively low precision. However, the quantity, consistency and geographical extent of the data make them extremely valuable. It is also relatively independent of, yet comparable with, the GBS, since it covers a similar location and period. Thus, it provides an opportunity to compare results between these quite different survey techniques.
All data were checked for obvious errors (for example, months greater than 12), which were either corrected or excluded. Altitudes were estimated for each survey location, using the nearest grid-point in the GEODATA 9 Second Digital Elevation Model (DEM) Version 2 (CD-ROM, see http://www.ga.gov.au/meta/ANZCW0703005624.html, accessed 13 November 2007). Where this grid-point fell over sea but near land, an altitude of sea level was assumed. This approximation was used because subtle climatic differences between altitudes close to sea level were not of interest in this study.
The data were processed to produce simple indices of the amount and timing of breeding, as follows.
Timing of breeding
This is taken here to mean an estimate of the average date for any point in the breeding cycle. For the NRS data, this was calculated by taking the midpoint between the last record of eggs and the first record of nestlings for each recorded breeding attempt, or where this was not available, the last record of eggs (Griffioen 2001). This provides an estimate of timing that is near the hatching date. For Magpies, this method excluded about one-third of all NRS breeding records (e.g. those where only fledglings were recorded). However, the increase in precision made the reduced sample size worthwhile (for this species). This method was modified to calculate breeding dates from GBS data. In the GBS, participants are asked to record the stage of breeding each week throughout the breeding season. During the first 12 years (1981–93) of the 21 years of data obtained, only two breeding categories were used: nests with eggs or nestlings (N) and those with fledglings (F) (Veerman 2003). Therefore, the most accurate estimate of breeding date is an estimate of the fledging date, that is, the midpoint between the last record of ‘N’ and the first record of ‘F’ (hereafter MidWk). Survey weeks were numbered 1–52 (beginning the first week of July) and only records where the difference between the earliest ‘F’ and the last ‘N’ was 1–5 weeks were used. Thus a maximum of one breeding date per site-year was included in the analysis. This was considered reasonable as Magpies are usually single-brooded, and including records where re-nesting occurred would complicate the analysis. The data were averaged for each year, rather than using all data points, as this provides a more conservative result: it reduces the possibility of results for a few years dominating the analysis owing to their having a higher number of data points. Also, all years were included (even those with few values), because the interaction between the SOI and amount of breeding (see separate analyses below) meant that excluding years with minimal breeding would have biased the results.
For both types of atlas data, only a relatively poor estimate of breeding date was available – the day of year (DOY) for surveys where breeding was recorded. The DOY value (originally 1–365, ignoring 29 February) was adjusted such that breeding records later than 31 December but which were still part of the same breeding season were increased by 365, to represent the fact that late December and early January are close together. This simplification, in lieu of using circular statistics, was possible because there is a period (late June) where almost no Magpie breeding is recorded. The surveys selected for this analysis were those completed within a single day (Atlas 2) and within 1 week (NSW Atlas), and the midpoint between survey start-date and end-date was used to calculate the DOY value.
Percentage of breeding records (hereafter Br%) – an index of the ‘amount’ of breeding
This was not calculated for NRS data, because there is no adequate way to quantify survey effort. For Atlas 2 data, the total number of Magpie breeding records in a latitudinal range (e.g. 30–35°S) was divided by the total number of Magpie records (both breeding and non-breeding) in that range. When comparing different breeding years using NSW Atlas data, the total number of breeding records in a breeding year (July–June) was divided by the total number of sighting records in that year. For Atlas 2 data, which consisted of mostly short surveys, only single-day surveys were used; this minimised the effects of different survey lengths. However, for the NSW Atlas, monthly data predominated, and thus all surveys under 33 days in length were used. This was temporally accurate enough to generate monthly averages, but the effects of survey length did have to be included in the binary logistic regression. For the GBS data, Br% was the number of sites where breeding Magpies were recorded divided by the number of sites where any Magpies were recorded.
Reporting rate and breeding reporting rate
For Atlas 2 data, the reporting rate (number of surveys where Magpies were recorded / total number of surveys) and breeding reporting rate (number of surveys where Magpies were recorded breeding / total number of surveys) were also calculated.
Breeding year (hereafter Year)
For correlation analysis and correspondingly in the figures, the breeding year is denoted by the first year of the two calendar years spanned by the breeding season (for example, 1981 denotes the 1981–82 breeding season).
Southern Oscillation Index variables
The SOI values were obtained from the Bureau of Meteorology (http://www.bom.gov.au/climate/current/soihtm1.shtml/, accessed 1 August 2005). An average of the winter monthly SOI values (June–August) was calculated for each year (hereafter SOI-W). These months were chosen as they are just before the peak of the Magpie breeding season and although there are more sophisticated climate prediction tools now available (especially useful for studies at a regional level), simple 3-month averages of SOI can be used with some success to predict conditions in the following season (Drosdowsky and Chambers 1998). An annual average of SOI (hereafter SOI-Y) was also calculated, based on values of SOI covering the entire breeding year (July–June). This index seemed to summarise the overall seasonal conditions better, but had the disadvantage of not being ‘predictive’, because much of the data used to calculate it would have been generated after breeding began. Generally, negative values of the SOI correspond to warm conditions and, in eastern Australia, usually to dry conditions. Similarly, positive SOI values correspond to cold conditions and, in eastern Australia, usually to wet conditions (see http://www.bom.gov.au/climate/ahead/ENSO_background.html, accessed 12 November 2007).
Temperature and rainfall
For the GBS data, it was also possible to calculate simple indices of temperature and rainfall for each year, based on average monthly values for maximum and minimum temperatures and rainfall recorded at Canberra Airport (for more details about this weather station, please refer to http://www.bom.gov.au/weather/nsw/canberra/climate.shtml/, accessed 1 June 2006). Monthly values were averaged for the first three months of the Magpie breeding season (July, August and September) in each year and denoted as JASmax, JASmin and JASrain, respectively.
Analyses
Relationships between the geographical and climatic variables and the Magpie breeding variables were investigated using visual inspection (graphs by month and year), correlation and regression. Most analyses were performed in SPSS version 12.0.1 (SPSS Inc., Chicago, IL, USA). Binary logistic regression was used for all regression analyses involving binary data (breeding = 1, not breeding = 0; in summary form, Br%). Ordinary least-squares regression was used for analyses involving only continuous variables (e.g. DOY v. altitude, JASmax, etc.). Locally weighted regression (Systat 5.02, SYSTAT Inc., Evanston, IL, USA) was used to display changes in the timing of breeding with altitude, because it did this effectively without presupposing a linear relationship between the two variables. Br% was plotted for different latitudes using Microsoft Excel 2002 and the binomial standard error (√Br% × (1 – Br%) / n), where n is the sample size, was calculated and used to display error bars. These are indicative of the amount of error owing to sample size variation, but because no corrections were made to account for the actual distribution of the data (in which sampling is non-random), the true standard errors may be substantially greater.
Results
Altitude and breeding
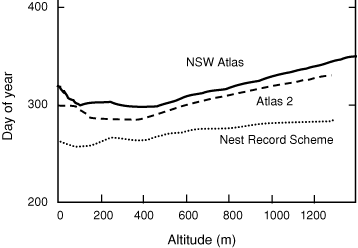
Altitude appeared to influence the timing of breeding in Magpies. Locally weighted regression lines for all three datasets for which altitude was available suggested that Magpies bred later at high altitudes (Fig. 1). The results from fitting linear regressions, using data above 250 m only, were highly significant (P < 0.001) and suggest that breeding is some 2.7–3.9 days later per 100 m of altitude increase, depending on the dataset. There appeared to be no relationship with altitude below 250 m, probably because at low altitudes other factors (such as distance from the coast) were more important determinants of local climate.
|
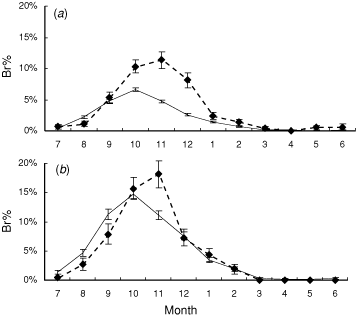
An analysis using the amount of breeding instead of the timing of breeding indicates the potential for these two measures to interact. Using the percentage of breeding records (Br%) calculated from Atlas 2 data and comparing values at weekly intervals, both above and below 600 m, shows that the peak in Br% is greater in magnitude (approximately double) at higher altitudes (Fig. 2a). It is also slightly later in the year. However, this pattern was apparently due, at least in part, to geographical confounding of the data. When only data from latitude–longitude grid-squares with at least five surveys for both high and low altitudes were used, the difference in peak height was markedly reduced, though the change in timing remained (Fig. 2b). The results for Br% are quite robust to the seasonal differences in survey effort, which can influence simple averages such as those in Fig. 1 since the survey effort is incorporated into these calculations.
|
Latitude and breeding
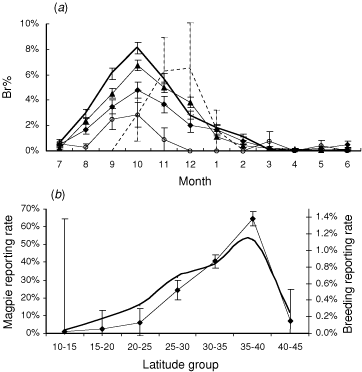
Only Atlas 2 data had a sufficient geographical range for latitudinal analysis. For mainland Australia, the proportion of Magpie records that were breeding records (Br%) was much higher in southern Australia than in northern Australia (Fig. 3a). There were also latitudinal changes in reporting rate and breeding reporting rate (Fig. 3b). These trends were concurrent and could not be clearly separated from each other.
|
Splitting the data east–west at 136°E (not shown) showed that the pattern in Western Australia (WA) was similar to that in eastern Australia: the proportion of Magpies breeding south of 20°S (the only part of WA where there were sufficient data) was not noticeably different to that observed in the east.
There was no obvious latitudinal pattern north of 20°S, but this may have been a result of a lack of sufficient data (results not shown). Br% data were also calculated for 5 × 5° grid-blocks, and visual inspection suggested that central Australian inland areas, between latitudes 10° and 20°S, had fewer breeding Magpies than coastal regions at corresponding latitudes, in addition to the more general latitudinal trends that were still evident.
A time-slice through the data on the proportion of Magpies breeding for the month of October showed a strong linear relationship (P < 0.001, R2 = 0.99) between Br% and latitude across mainland Australia, but data for Tasmania (i.e. all records south of 40°S) did not fit this latitudinal pattern. Although there were insufficient data to form firm conclusions, the breeding season in Tasmania appeared to be shorter and to start and peak later in the year (see Fig. 3a).
Southern Oscillation Index, annual climatic variation and breeding
Timing of breeding – GBS
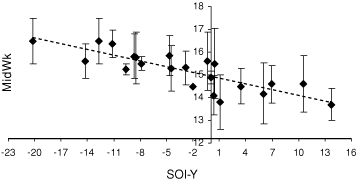
Twenty-one years of GBS data (1981–2001) were analysed, using an average MidWk value for each year (an estimate of fledging date) and the two SOI variables, SOI-Y and SOI-W (Fig. 4).
|
For timing of breeding, SOI-Y showed a consistently stronger relationship with the Magpie data than SOI-W. In order to assess why this might be, correlations were performed against monthly SOI variables and local climate parameters (Table 1). This showed that monthly SOI values for August, September and October were also highly correlated with the timing of Magpie breeding and with SOI-Y. These SOI variables were also highly correlated with minimum temperatures and rainfall at Canberra Airport (JASmin and JASrain) over this period, but the correlation between timing of Magpie breeding and SOI-Y remained the strongest of any of the variables tested. This was unsurprising, as it is difficult to identify the specific seasonal combination(s) of rainfall and temperature that indicate a ‘good’ or ‘bad’ year, whereas SOI reflects different climatic patterns, allowing years to be sorted into a rough, overall gradient (Stenseth et al. 2003).
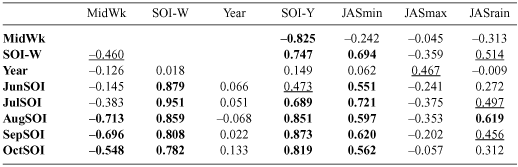
|
For the GBS data, a change in the estimated fledging date of around 3 weeks occurred over the range of SOI-Y values observed in this study, giving approximate fledging dates of 1 October to 21 October. The estimated fledging dates became later as SOI-Y became more negative (in part representing warmer, drier conditions).
Because the GBS involves observers monitoring the same site over many years, a preliminary analysis of differences between years at the same sites was also performed. However, few sites had breeding data for more than 3 years. Interestingly, all trendlines for individual sites (where breeding was recorded in at least four years; n = 5 sites) were in the same direction as that of the overall regression with respect to SOI-Y.
Timing of breeding – NSW Atlas
Using the average breeding date for each year, for years with at least 20 breeding records (i.e. 1984, 1986–98), a significant negative relationship with SOI-Y was found (Fig. 5). This result corresponds with the results from the GBS data, a pattern of earlier breeding at higher SOI-Y (cooler, wetter years), with a predicted range of ‘breeding dates’ over the range of SOI-Y values observed in this study of around 3 weeks (c. 25 October to 15 November).
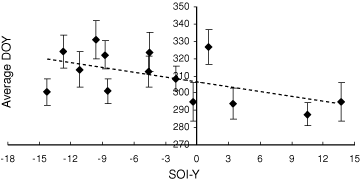
|
The exact point of the breeding cycle represented by the average breeding date (DOY) in the NSW Atlas is unknown, as it depends on the ‘average’ stage of breeding recorded by the observers. Therefore, a direct comparison of the timing of breeding between the NSW Atlas and the GBS data is not possible.
Not surprisingly, given that the years included in this analysis overlapped with those of the GBS, correlations between the SOI variables were similar, i.e. SOI-Y was highly correlated with monthly SOI values for July to September (Table 2). Again, the correlation between timing of Magpie breeding and SOI-Y was strongest.
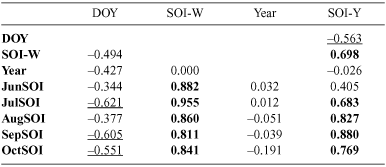
|
Amount of breeding – GBS
The proportion of Magpies recorded breeding (Br%) using Canberra GBS data appeared to be related to SOI-Y. However, this result was entirely from one outlier, the 1982 drought year, in which SOI-Y was extremely negative and Br% extremely low. Excluding this year, there was no relationship between either SOI-W or SOI-Y and Br%. However, there was a strong trend over time in Br% and a concurrent trend in averaged July–September maximum temperatures (JASmax), with both increasing over time. Logistic regression of Br% against JASmax, excluding 1982 gave B = 0.293, P < 0.000, Nagelkerke R Square 0.019), but using year alone actually explained more variation (B = 0.044, P < 0.000, Nagelkerke R Square 0.023). Although temperature probably influences Magpie breeding, in this analysis its effects could not be separated from other changes over time (see Discussion). The temporal trend in Br% was investigated further to try and determine if it was caused by autocorrelation (r = 0.53). However, there was minimal autocorrelation in these data after the trend was removed (r = –0.09), suggesting that any apparent serial correlation may have been a result of the trend itself (Yue et al. 2002).
Amount of breeding – NSW Atlas
The relationship between SOI-W and the proportion of Magpies breeding based on the NSW Atlas data was striking (Fig. 6) and highly statistically significant (P < 0.000). Over the range of SOI values observed, the predicted Br% values were 1.7–1.8 times greater at the highest SOI (+20) compared with the lowest SOI value (–21), depending on survey length (for 3-day surveys, 2.2–3.9%, and for 31 day surveys, 7.7–13.2%). This provides strong evidence that annual fluctuations in climate affect the proportion of Magpies breeding.
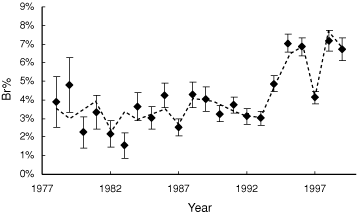
|
Discussion
In general, timing of breeding in Magpies is remarkably constant throughout Australia, despite dramatic differences in climate and seasonality (Higgins et al. 2006). However, the timing, amount or success of breeding does vary depending on seasonal conditions (Robinson 1956; Bedggood 1973; Rollinson 2004; McLean et al. 2005). This study provides quantitative evidence for the first time of widespread and systematic differences in Magpie breeding patterns over geographical and temporal climatic gradients. Of particular interest is that the timing and amount of Magpie breeding varied annually in response to climatic conditions, demonstrating that: (1) Magpies have relatively flexible breeding behaviour and (2) climate-change impacts will be both substantial and immediate. While the SOI seemed to reflect annual climatic variation better than local temperature and rainfall data, the latter were also useful, especially where a temperature trend over time was not reflected in the SOI variables.
The response of species’ life cycles to present temperature gradients – such as those represented by altitude and latitude – can be used to predict the likely effects of future climatic warming (Fielding et al. 1999; Mazerolle et al. 2005). Although differences between high and low altitudes were as expected from temperature considerations alone, the results for the SOI were not: earlier breeding (and more breeding) occurred when SOI values indicated colder, wetter conditions. This suggests that in many regions rainfall, or a combination of temperature and rainfall, is more important than temperature alone.
Effect of altitude
In several Australian species, laying is thought to occur later at high altitudes (e.g. Magpie-lark (Grallina cyanoleuca), Frith 1984; Welcome Swallow (Hirundo neoxena), Marchant and Fullager 1983; and Willie Wagtail (Rhipidura leucophrys), Marchant 1974). Magpies follow a similar pattern (Fig. 2), though not as obviously as some other species (Frith 1984). In this they are similar to their northern hemisphere counterparts, presumably because extremes of cold (and resultant biological productivity and food availability) are usually the limiting factors at high altitudes. High-elevation populations may shift investment from quantity of offspring towards offspring quality (Badyaev and Ghalambor 2001; Lu 2005). Results from this study are broadly consistent with this theory if Br% is considered to represent a measure of reproductive ‘effort’, since it peaked higher and later and dropped more sharply at high altitude (Fig. 2).
Effect of latitude
The Australian Magpie shows little variation in the timing of its breeding season across its range. Although timing was not analysed separately (for example, using DOY), this pattern is evident in the similar timing of peaks in breeding across a range of latitudes (Fig. 3a). Magpie breeding in southern Australia was only slightly later than in northern Australia, peaking around October in all regions. In more southerly regions, increased seasonality (e.g. change in daylength) could allow greater foraging time, or contribute to greater prey availability, or both.
The concurrent changes in reporting rate with latitude (Fig. 3b) make it unclear if the change in Br% reflects a change in the proportion of Magpies choosing to breed, or alternatively, reflect differences in population density between regions. Br% needs to be used (and interpreted) cautiously in these circumstances. Nonetheless, the results raise some interesting questions and possibilities. There are reasons to suspect that Br% does vary with reporting rate in Magpies. If the reporting rate does not accurately reflect population density (a highly mobile, vocal and visible species such as the Magpie could be recorded relatively often even if extremely rare), then the breeding reporting rate may give an alternative indication of relative population densities (Fig. 3b). The downside of using breeding reporting rates is that there is only about one-fiftieth of the amount of data, and even that is strongly seasonal. Also, Br% in Magpies may be influenced by social factors, as either groups or pairs may defend a territory, and territories themselves vary dramatically in size, depending on the spatial distribution and quality of foraging habitat (Higgins et al. 2006). But as Fig. 3b shows, use of breeding reporting rates changes our understanding of how populations vary spatially. The sharp decline in breeding reporting rate while reporting rate decreases more gradually (Fig. 3b) suggests that most of northern Australia is quite marginal for Magpies, with low densities and hardly any breeding. Many of these northerly regions showed decreases in Magpie abundance between 1977–81 and 1998–2002, while predominantly no changes in abundance were observed in south-eastern Australia (Barrett et al. 2002a). So, while Magpie populations in Australia have generally increased – probably because farmland is a preferred habitat (Higgins et al. 2006) – climate change does have the potential to reduce the range of the species. If northern, inland and desert areas become hotter and drier, many could quite quickly become unable to sustain resident populations of Magpies.
Effect of the Southern Oscillation Index
Annual fluctuations in climate have a dramatic and immediate effect on breeding in Magpies. As there were no significant trends over time in the SOI-related variables used (Tables 1 and 2), the relationships found between SOI-Y and timing of breeding in Magpies (Figs 4 and 5) are strong evidence of a direct and immediate link to climate. Further, the amount of breeding in Magpies from NSW Atlas data corresponds very strongly with SOI-W (Fig. 6), a particularly dramatic and interesting result. In Canberra gardens, the 1982 drought saw a sharp dip in the amount of breeding reported across many species, including Magpies (Veerman 2003). However for the remaining years, the trend over time was stronger than any detected link to climatic variables. Although trends over time may relate to co-temporal warming trends (Beaumont et al. 2006), any analysis of this is best suited to multiple-species assessments. For an individual species, relationships with annual fluctuations in climate provide stronger evidence of direct links with climate. There was a concurrent trend in July–September maximum temperatures (JASmax), but there were also other relevant trends in the Canberra GBS data. Magpies showed a clear, gradual increase in abundance over the period of the GBS (Veerman 2003), which could be either a cause or an effect of the increase in Br% (or be unrelated). Thus it is difficult to ascribe long-term changes in Br% to climate trends in this instance. While the population changes could themselves be a result of climate warming, they might also derive from urbanisation and related factors, such as the availability of supplemental food (Higgins et al. 2006).
There are strong correlations between large-scale climate indices and breeding in birds (Grant and Grant 1987; Nott et al. 2002; Chambers 2004; Mazerolle et al. 2005; Sandvik et al. 2005) and other biological parameters (Grant and Grant 1987; Przybylo et al. 2000; Stenseth et al. 2003; Sandvik et al. 2005). Large-scale climatic indices may better represent climatic effects than any single local weather variable (Stenseth et al. 2003) and in many cases explain as much or more variation in biological parameters as do local climatic variables (Post and Stenseth 1999). In terrestrial species, correlations with such indices probably reflect changes in local seasonal weather conditions, which in turn affect plant productivity and insect abundance (Nott et al. 2002). Despite the number of such papers, few are directly comparable to this study. One that is involved Yellow Warblers (Dendroica petechia) in a riparian forest in southern Manitoba, Canada. Estimates of adult survival and the production of young were positively correlated with the SOI, and the authors concluded that climate change could negatively affect Yellow Warbler populations and those of other Neotropical migrants (Mazerolle et al. 2005). Magpies may also be disadvantaged by climate change, although community dynamics and other factors such as habitat modification would need to be factored in before the net effect on Magpie populations could be predicted. It is also possible that widespread, common and adaptable species such as the Magpie would be less negatively affected by climate changes than other more specialised species, leading to a competitive advantage over the medium to long-term that may to some degree compensate for any direct disruption to their breeding cycle. However, there is also good evidence (Veerman 2003; Higgins et al. 2006; Robinson et al. 2007) that more frequent and severe droughts would negatively affect Magpie populations.
The analyses presented here suggest that a major cause of reduced breeding output in ‘bad’ years is birds ‘choosing’ not to breed at all. This has significant implications for monitoring. Breeding success alone will not be adequate to predict population trends. Further, surveys such as the NRS provide little indication of observer effort and therefore cannot easily be used to obtain an estimate of the amount of breeding. However, it is possible to get a satisfactory yearly index of breeding activity (Br%) from Atlas data, even though it cannot be assumed that survey technique and observer behaviour remains constant over time. For instance, in the case of the NSW Atlas, it was not surprising that Br% was higher in longer surveys, but what was surprising was that this mattered to the analysis. Survey length had to be included as a factor because there was a step change in the proportion of longer surveys between 1993 and 1994. Ironically, this was probably caused by a request that participants limit surveys to a maximum of 1 month. So, while the longest surveys were excluded from earlier years by the ‘less than 33 day survey length’ criterion, more long surveys were included when participants shortened their longest survey periods to 1 month! This highlights the difficulties of maintaining strictly comparable survey techniques over the long-term. Other analyses using Br% (e.g. Fig. 3) suggest it can be influenced by changes in population density, and therefore that it must be used cautiously where populations themselves change over time or space – fortunately this is much less of a problem, at least in resident species, when considering annual fluctuations in Br% (e.g. Fig. 6).
So, what do the results mean in terms of the biology of the species and proximal causes? It is thought that birds generally try to optimise food supply and availability for their offspring at fledging (Thompson 1950) – critical to fledgling survival and recruitment into the breeding population. However, the availability of adequate food for breeding adults before breeding may also be an important influence (Davies 1979). Therefore, it seems likely that the climate influences documented in this study affect Magpie breeding via their impacts on food supply (i.e. invertebrate abundance – both directly through the creation of suitable moisture and temperature conditions and indirectly via its influence on plant biological productivity). Understanding both ultimate and proximate causes is important, as a mismatch between the cue a species uses to determine timing of breeding and the requirements of its fledglings for food could be catastrophic. Unfortunately, this was beyond the scope of this study, but by showing that Magpies alter their breeding patterns in line with annual climatic fluctuations, this study has provided a base to which further details can be added. It also documents and explores techniques which can be generalised to other species in time.
This study adds further weight to existing evidence that there are relatively complex relationships between species’ biology and climate in Australia (Chambers 2005; Beaumont et al. 2006). In contrast to some results for the northern hemisphere, temperature change alone is not adequate to predict species response to climate change in Australia. This makes predicting the biological effects of climate change in Australia much more difficult, as climate models predict varying changes in rainfall across Australia (some areas will have increased rainfall while others will have less) in contrast to temperature (nearly all areas will become warmer). Predictions for rainfall are also not as precise – for many regions there is uncertainty even whether rainfall will increase or decrease (CSIRO 2001).
Further research
It would be useful to extend these analyses to other species to see whether relationships between breeding and climate parameters are generally consistent, and to further describe the effects of specific extreme events, such as the 1982 drought. Field studies could usefully extend the biological interpretation of these results, e.g. to determine how the SOI affects the timing and amount of breeding in Magpies (and other species). However, the value of the expected results would have to be weighed against the large investment of time and effort involved. It may be more feasible to integrate some of the sorts of analyses presented here into existing long-term studies, as has for example been done successfully in the case of the Little Penguin (Eudyptula minor) (Chambers 2004).
Acknowledgements
Many thanks are due to my supervisors at Deakin University, Ashley Bunce and Andrew Bennett, and to Lynda Chambers (Bureau of Meteorology), who also provided climate data; also to the Stuart Leslie Bird Research Award (Birds Australia) and the Norman Wettenhall Foundation, for their financial support of this project. The following organisations and individuals helped with access to or interpretation of data, or both: Geoff Barrett, Henry Nix, Rory Poulter, Andrew Silcocks and Mike Weston (Birds Australia – Nest Record Scheme and Atlas 2 databases), Philip Veerman, Martin Butterfield and David Rosalky (Canberra Ornithologists Group – Garden Bird Survey data) and Ian McAllan and Dick Cooper (NSW Atlas data). The New South Wales Bird Atlas database is a highly valued resource and the generous access arrangements afforded by the New South Wales Bird Atlassers Inc. are greatly appreciated, as are those provided by Birds Australia and the Canberra Ornithologists Group. Graeme Newell, Peter Griffioen, Mike Clarke and Leslie Hughes also provided invaluable help, and finally, extra special thanks go to all the volunteers whose bird observations made this study possible.
Badyaev, A. V. , and Ghalambor, C. K. (2001). Evolution of life histories along elevational gradients: trade-off between parental care and fecundity. Ecology 82, 2948–2960.
Beaumont, L. J. , McAllan, I. A. W. , and Hughes, L. (2006). A matter of timing: changes in the first date of arrival and last date of departure of Australian migratory birds. Global Change Biology 12, 1339–1354.
| Crossref | GoogleScholarGoogle Scholar |
Chambers, L. E. (2005). Migration dates at Eyre Bird Observatory: links with climate change? Climate Research 29, 157–165.
| Crossref | GoogleScholarGoogle Scholar |
Crick, H. Q. P. , and Sparks, T. H. (1999). Climate change related to egg-laying trends. Nature 399, 423–424.
| Crossref | GoogleScholarGoogle Scholar |
Davies, S. J. J. F. (1979). The breeding seasons of birds in south-western Australia. Journal of the Royal Society of Western Australia 62, 53–64.
Fielding, C. A. , Whittaker, J. B. , Butterfield, J. E. L. , and Coulson, J. C. (1999). Predicting responses to climate change: the effect of altitude and latitude on the phenology of the Spittlebug Neophilaenus lineatus. Functional Ecology 13, 65–73.
| Crossref | GoogleScholarGoogle Scholar |
Grant, P. R. , and Grant, B. R. (1987). The extraordinary El Niño event of 1982–1983 effects on Darwin’s Finches on Isla Genovesa Galapagos Ecuador. Oikos 49, 55–66.
| Crossref | GoogleScholarGoogle Scholar |
Hughes, L. (2003). Climate change and Australia: trends, projections and impacts. Austral Ecology 28, 423–443.
| Crossref | GoogleScholarGoogle Scholar |
McLean, I. G. , Recher, H. F. , Studholme, B. J. S. , Given, A. , and Duncan, C. (2005). Breeding success and timing of nesting by forest birds on northern tablelands of New South Wales, Australia. Corella 29, 53–62.
Post, E. , and Stenseth, N. C. (1999). Climatic variability, plant phenology, and northern ungulates. Ecology 80, 1322–1329.
Sandvik, H. , Erikstad, K. E. , Barrett, R. T. , and Yoccoz, N. G. (2005). The effect of climate on adult survival in five species of North Atlantic seabirds. Journal of Animal Ecology 74, 817–831.
| Crossref | GoogleScholarGoogle Scholar |
Yue, S. , Pilon, P. , Phinney, B. , and Cavadias, G. (2002). The influence of autocorrelation on the ability to detect trend in hydrological series. Hydrological Processes 16, 1807–1829.
| Crossref | GoogleScholarGoogle Scholar |


