Cooperative breeding beyond kinship: why else do helpers help?
Paul G. McDonaldBehavioural and Physiological Ecology Research Centre, Zoology, School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia. Email: paul.mcdonald@une.edu.au
Emu 114(2) 91-96 https://doi.org/10.1071/MUv114n2_ED
Submitted: 11 December 2012 Accepted: 13 December 2013 Published: 9 May 2014
Additional keywords: cooperation, group augmentation, inclusive fitness theory, kin selection theory, social prestige.
Introduction
One of the most widely studied forms of cooperative behaviour is the phenomenon of cooperative breeding, where ‘helpers’ care for young that are not their own (Cockburn 1998; Pacheco et al. 2008). Cooperative systems are present in taxa as diverse as slime mould, eusocial insects and a broad-range of vertebrates, including mammals, fish, reptiles and birds (Queller and Strassmann 1998; Clutton-Brock 2002; Mehdiabadi et al. 2006). Fittingly, a large body of research has been devoted to understanding the causes and consequences of cooperative interactions (e.g. Cockburn 1998; Lehmann and Keller 2006), stemming back to Darwin himself who pondered if eusociality might be a fatal flaw in his theory of natural selection (Darwin 1859). Interest in this research question has not been lost over time; a recent article (Nowak et al. 2010) prompted a joint reply from no less than 137 authors (Abbot et al. 2011)!
These issues are relevant to Emu: Austral Ornithology as this region is ideally placed to contribute to the field, because of a disproportionately high prevalence of cooperative avifauna, particularly in South Africa and Australia (Cockburn 1998; Jetz and Rubenstein 2011). Famous Australian examples include the diverse mating systems of Maluridae fairy-wrens (Margraf and Cockburn 2013), distinctive Corcoracidae societies built on long-term associations (Beck et al. 2008; Griesser et al. 2009), and the extraordinarily complex societies of Manorina honeyeaters (Dow and Whitmore 1990; Wright et al. 2010). In Africa, the Turdoides babblers have attracted considerable interest (Zahavi 1977; Ridley 2007) whereas Merops bee-eaters have long been a model system (Emlen and Wrege 1988). This extensive research effort has elucidated spectacular life histories, such as the identification of some of the world’s least faithful birds (Double et al. 1997; Durrant and Hughes 2005) and feats of extraordinary cooperative behaviour, such as broods of two to four Noisy Miner (Manorina melanocephala) nestlings being fed by as many as 20 helpers (Põldmaa et al. 1995)!
Despite this long history of research, few broad-scale factors that favour cooperative breeding have been uncovered, other than helpers tend to favour relatives. By contrast, there is comparatively little consensus on why cooperative breeding involving non-relatives has evolved (e.g. Clutton-Brock 2009). Although it is possible that there are no common drivers favouring cooperative breeding across taxa beyond kinship, I do not believe that present data allow us to definitively reach this conclusion. I therefore first investigate the evidence for any emerging consensus on cooperative breeding between non-relatives, before discussing suggestions as to how researchers might maximise the likelihood of uncovering, or at least refuting, non-kin based hypotheses for the evolution and maintenance of cooperative breeding.
Inclusive fitness is important, but not the only driver of cooperative breeding
How can selection favour the evolution and maintenance of such an apparently costly behaviour as feeding other’s offspring? Instead of incurring fitness costs, helpers might accrue a net benefit if they preferentially aid individuals with whom they share at least some genes, whether through random chance or kinship, termed ‘inclusive fitness theory’ (Hamilton 1964a, 1964b; Maynard Smith 1964; see Marshall 2011 for a useful review). This theory has been extraordinarily successful when applied to animal societies (Emlen 1995; Cockburn 1998; Clutton-Brock 2002), shaping patterns both across different species (Cornwallis et al. 2009) and also the cooperative efforts of individuals within species (Wright et al. 2010; Browning et al. 2012).
Despite this, inclusive fitness theory has not provided a standalone answer that explains cooperative breeding. Conservative estimates place cooperation between non-kin as regularly occurring in as many as 20% of avian families or 9% of cooperative species (Hatchwell 2009), with many systems displaying helping behaviour between both kin and non-kin (e.g. McDonald et al. 2009; West et al. 2011). Thus, whereas kin selection benefits are clearly a common driver for cooperative breeding in many systems, determining whether or not there are similar commonalities in non-kin based explanations for the evolution and maintenance of cooperative breeding remains a challenge for the field.
How can cooperative breeding involving non-relatives be explained?
This is not a trivial problem, given that help is, in virtually all cases, energetically costly to provide. Simply invoking unselected care or misdirected care (Jamieson 1991) is neither satisfying nor a particularly likely explanation. Instead, multiple direct benefits of helping behaviour have been proposed, all of which identify pathways by which helpful donors could increase their ‘direct fitness’, that is, the number of offspring that they themselves produce (see West et al. 2011 for a readable summary). In a landmark review, in addition to inclusive fitness, Cockburn (1998) identified five other distinct categories of hypotheses proposed to account for cooperative breeding by some form of direct benefits (Table 1).
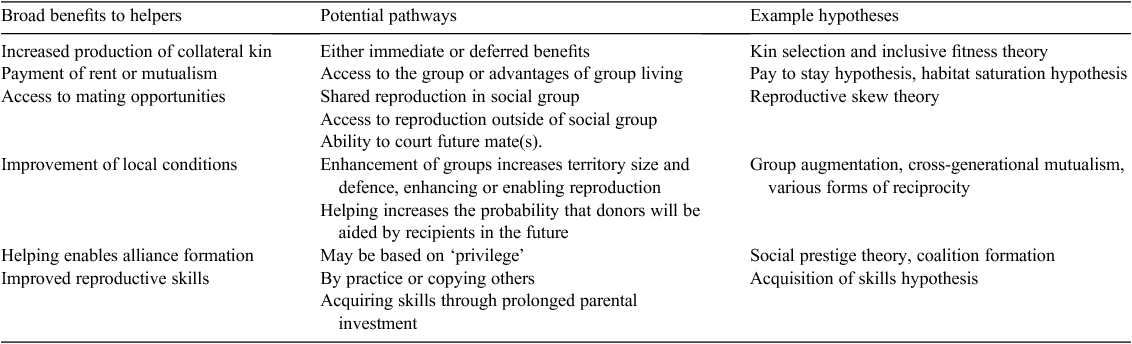
|
Even when collated into these groups, it is clear that there are many proposed pathways by which helpers might accrue direct benefits. Further, there is currently very little consensus on the broad applicability of these hypotheses, as Fig. 1 demonstrates. The data used to develop this figure are split into two time periods either side of Cockburn’s influential review: 1998 and earlier (136 papers), and 1999 through to the end of 2012 (424 papers). Contrary to my expectations, there is no significant change in the research effort devoted to each category of hypothesis across the two time frames (χ25 = 3.273, P = 0.658). If cooperative breeding research was converging on a consensus as to the most important direct benefit hypothesis or hypotheses as a field, we might expect increasing bias towards investigation of areas receiving frequent and broad support. Conversely, interest in a group of hypotheses should logically decline if negative evidence was continually reported. Neither process appears to be happening.
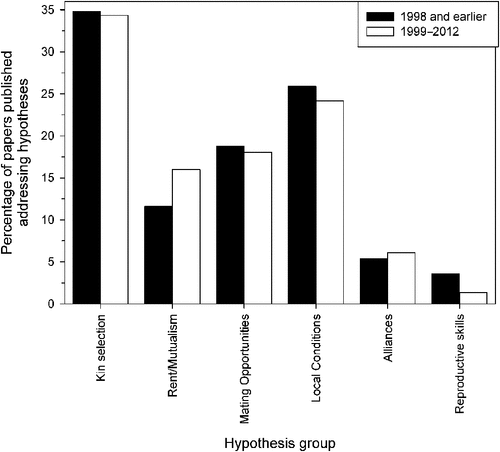
|
Two, non-mutually exclusive factors are likely to drive this result. First, there may well be no common explanation across species or at least groups of taxa that consistently underpins cooperation outside of kinship. Recent advances suggest that this might be a pessimistic outlook, as there appear to be at least some common factors (Jetz and Rubenstein 2011; but see Gonzalez et al. 2013). An alternative explanation may be that the types of studies and approaches being undertaken may be confounding attempts to effectively identify broad direct benefit-based hypotheses for cooperative breeding.
What factors might confound attempts to generate commonalities across taxa?
While acknowledging that direct benefits will be, by their very nature, somewhat specific to the ecological and behavioural traits of a given species, many direct benefit hypotheses have been developed without reference to more general cooperative theory (Bergmüller et al. 2007). Given this, formulating their precise empirical predictions, and thus points of difference, is not always straight forward. For example, empirically separating group augmentation v. reciprocal or mutualistic-based helping is perhaps unattainable (Wright 2007). Further, there is considerable debate about kin selection and how it may or may not overlap with the various forms of group selection, yet mathematically they are different ways of describing the same process (Marshall 2011; West et al. 2011).
This overlap in predictions and thus expected outcomes between hypotheses is relevant, as it can result in studies claiming support for one hypothesis, when another or others may have also been relevant but not considered. Inclusive fitness theory provides a good example, as frequently direct pathways are subsequently ignored once kin-directed aid has been identified in at least part of a population. Previous research into Manorina honeyeaters illustrates this, concluding the group was a largely kin-based helping system, whereas significant levels of help actually occurs between non-relatives (Wright et al. 2010; McDonald and Wright 2011). This issue is also prevalent if one of the hypotheses favoured is considered more ‘exciting’ or novel. For example, social prestige suggests that helpers may be showing off their prowess within a group to gain prestige and later benefits based on their status (Zahavi 1977). Although intuitive to humans, the process is likely highly cognitively demanding and requires significant information that might be difficult for most species to obtain (Wright 2007; McDonald et al. 2008). Evidence supporting the hypothesis has been claimed in only two avian studies (Carlisle and Zahavi 1986; Doutrelant and Covas 2007), yet both are likely more parsimoniously explained by either observer-induced disturbance (McDonald et al. 2007) or responses to potential predators (as noted by the authors: Doutrelant and Covas 2007). When viewed with other negative experimental results (e.g. McDonald et al. 2008; Nomano et al. 2013), support for social prestige has thus far been very limited at best, yet research in this area continues (Fig. 1).
Suggestions on the way forward
Given that many direct benefit based hypotheses are very difficult to separate empirically, and the considerable debate over even the terms of reference in this field (West et al. 2007), how can ornithology move forward to definitively define, or exclude, common traits underpinning cooperative breeding involving non-relatives? I recommend three strategies that researchers should consider before embarking on a study investigating cooperative breeding in birds, or indeed other taxa to ensure maximum explanatory power when testing hypotheses in this field, and thus contribute effectively to the search for commonalities in direct-benefit based cooperative breeding.
1. The assessment of underlying mechanisms that shape cooperative interactions
Science routinely has areas that fall in and out of favour in terms of popularity and, in avian cooperative research, an area that has been under utilised recently is the elucidation of the mechanisms behind cooperative interactions. This is unfortunate, as understanding mechanism remains as important now as it was when flagged by Tinbergen as one of the four key questions in behavioural research (Tinbergen 1963). In cooperative breeding research, elucidating mechanisms shaping cooperative decisions offer a way forward in differentiating between hypotheses with similar empirical predictions, but different mechanistic pathways. For example, if kin selection is driving helping behaviour, some form of kin discrimination, be that a simple rule-of-thumb such as ‘help familiar or nearby individuals’ in viscous populations (Hatchwell 2009), through to complex, fine-scale kin differentiation capabilities in more dispersed systems is required (McDonald and Wright 2011). Further, the degree of kin recognition in systems appears to be modulated by the costs of helping, as kin recognition capabilities are greater in species where the costs of incorrectly aiding non-kin are highly detrimental (Cornwallis et al. 2009). Thus, by extension, identifying the kin recognition capabilities in a system likely identifies the relative importance of inclusive fitness theory in the focal society, yet this method has not been routinely utilised by researchers.
The same logic applies to direct benefit hypotheses as well. Many require a potential donor to differentiate between two groups of recipients, thus if test subjects cannot achieve this differentiation, the theory is extremely unlikely to be relevant. For example, under direct reciprocity, an individual must discern between those that have helped it previously and those that have not. If individuals in such a system cannot differentiate between past helpers and past cheaters, than this direct benefit hypothesis would seem to be on very shaky ground as an explanation for the maintenance of help. The modality of interest will differ between taxa, but in birds, acoustic discrimination (Boeckle et al. 2012; McDonald 2012) seems the most likely pathway to investigate; however, visual and even olfactory cues are increasingly being recognised as important (Bonadonna and Sanz-Aguilar 2012).
If understanding mechanism is given priority in studies, it simplifies the task of testing potentially relevant hypotheses, as many can be ruled out before devoting limited resources to their study. Second, it enables subsequent experiments and correlational work to target the key variables of interest, something that has become increasingly important in academia. Finally, if researchers are aware of an animal’s capabilities and discriminatory powers, even null results in an experiment become highly informative and enable not only support for, but crucially definitive evidence against, particular hypotheses. Take a hypothetical acoustic playback experiment where receivers are exposed to two types of stimuli as an example. Null results in this context might be due to either poor equipment being unable to replicate appropriate signal details, the subjects being unable to differentiate between the stimuli (i.e. both groups sound functionally identical to receivers) or the subjects might identify differences in the stimuli, but the observable response to both in the experimental context is identical. These possibilities lead to very different conclusions, but can be effectively resolved by better identifying the abilities of the focal animal, and thus the mechanism(s) behind cooperative behaviour that are likely important. By doing so, all results, not just significant differences, become helpful in understanding the social system.
The importance of elucidating mechanisms applies beyond acoustic examples, such as understanding the feeding effort of unrelated helpers in a system. Social prestige-based helping is predicted to occur independently of brood demand, as helpers gain benefits through showing off to others in the group, not through nourishing offspring (McDonald et al. 2009). In contrast, group augmentation theory (Woolfenden and Fitzpatrick 1978) predicts helpers should aid all nestlings that will improve group size or composition, and should therefore feed hungry chicks more to improve their condition. Both scenarios predict that unrelated helpers should provide significant amounts of help to offspring, but crucially by very different mechanistic pathways. Only by monitoring help and a likely mechanism (in this scenario brood demand changes) can the two be separated and the correct conclusion reached.
2. Examine the society as a whole
Regardless of the species involved, understanding cooperative breeding necessarily involves understanding the interactions that occur in an entire society. Although these are often modelled and discussed as a two-party system for convenience, cooperation typically involves groups of many more individuals (Cockburn 2007). Ignoring this might result in over-simplification and risks throwing out critical information fundamental to understanding cooperative breeding. Instead, adopting frameworks such as multi-level selection that can account for selection pressures across all relevant levels in a society offers a more informative approach (Okasha 2006).
Further, cooperative breeding behaviour is typically just one facet of interaction in highly social systems. Typically individuals frequently cooperate in contexts away from the nest, such as predator mobbing or social foraging (Kennedy et al. 2009; Sorato et al. 2012). Indeed, many austral species live in year-round groups that cooperate outside of the breeding season (Cockburn 1998; Arnold et al. 2005). Understanding these cooperative events is critical to understanding a species social behaviour and thus any broad-scale patterns shaping cooperative breeding, as evidence exists that not all members of a population contribute equally to each cooperative modality (Arnold et al. 2005).
Fortunately, powerful tools for analysing these types of relationships do exist and have been developed in other fields. For example, social network analysis (Croft et al. 2008) or tools focussing on information use (Dall et al. 2005) offer a way of assessing multiple players simultaneously, and technological advances such as automatic monitoring systems provide researchers with ever more affordable and powerful research tools (Blumstein et al. 2011). Deploying these approaches in future research will likely unravel previously cryptic facets of societies, and thus provide a new understanding of cooperative breeding behaviour that may assist in reaching broad conclusions.
3. Detailed methodology is crucial
Researchers should also learn from the mistakes of the past, and directly test for important, but cryptic factors that have been shown to shape behaviour. The effect of disturbance is a key example, setting equipment too close to a sensitive area such as a nest site (McDonald et al. 2007) can be easily ruled out, yet few papers explicitly test for these effects. This can lead to difficulty interpreting unusual behaviours that may simply be a by-product of displacement behaviour. Likewise, there is considerable misunderstanding and misapplication of molecular tools, and researchers should be aware of the limitations and resolution of the molecular assessments that they use to determine kinship. For example, raw estimates of relatedness are often fitted as a linear covariate in analyses, when either threshold assessments or likelihood modelling is typically more appropriate (Blouin 2003; Rollins et al. 2012). Methodological issues such as these are simple to ameliorate, yet critical to generating datasets that allow unambiguous cross-species comparisons.
Concluding remarks
Some of these recommendations are challenging to undertake – elucidating the mechanisms behind cooperative breeding is not a trivial task. However, failure to at least begin to do so risks overlooking and misinterpreting the key factors driving cooperative breeding. This also decreases the likelihood of partitioning out common direct benefits important in avian societies, if they truly exist. Calls for an integration of mechanism and function are neither unique to cooperative breeding research nor new (McNamara and Houston 2009); however, this approach is critical if researchers are able to both falsify or find support for direct benefit hypotheses broadly, without violating assumptions associated with an over-extension of the ‘behavioural gambit’ (Fawcett et al. 2013). There is hope, as holistic and detailed approaches can elucidate detailed pictures of societal structure in even reasonably short time frames (e.g. Russell et al. 2007; Kingma et al. 2011). By clarifying mechanistic pathways, researchers can also simplify the equation by identifying the key variables that need to be monitored to ensure that experimental procedures are fully interpretable. By coupling this approach with analysis of all relevant cooperative behaviours for a given species, the appropriate molecular tools and choosing suitable model systems that abound in our region, austral ornithologists are well placed to contribute to the next major breakthroughs in the study of cooperative breeding and cooperation more generally. Whether consensus on the important direct benefits will ever reached is debatable, but I argue that these approaches should make the search more definitive and efficient.
Acknowledgements
Thanks to Kate Buchanan for encouraging this editorial, and to Simon Griffith and anonymous reviewers for providing helpful comments on earlier drafts.
References
Abbot, P., Abe, J., Alcock, J., Alizon, S., Alpedrinha, J. A. C., Andersson, M., Andre, J.-B., van Baalen, M., Balloux, F., Balshine, S., Barton, N., Beukeboom, L. W., et al (2011). Inclusive fitness theory and eusociality. Nature 471, E1–E4.| Inclusive fitness theory and eusociality.Crossref | GoogleScholarGoogle Scholar |
Arnold, K. E., Owens, I. P. F., and Goldizen, A. W. (2005). Division of labour within cooperatively breeding groups. Behaviour 142, 1577–1590.
| Division of labour within cooperatively breeding groups.Crossref | GoogleScholarGoogle Scholar |
Beck, N. R., Peakall, R., and Heinsohn, R. G. (2008). Social constraint and an absence of sex-biased dispersal drive fine-scale genetic structure in white-winged choughs. Molecular Ecology 17, 4346–4358.
| Social constraint and an absence of sex-biased dispersal drive fine-scale genetic structure in white-winged choughs.Crossref | GoogleScholarGoogle Scholar |
Bergmüller, R., Johnstone, R. A., Russell, A. F., and Bshary, R. (2007). Integrating cooperative breeding into theoretical concepts of cooperation. Behavioural Processes 76, 61–72.
| Integrating cooperative breeding into theoretical concepts of cooperation.Crossref | GoogleScholarGoogle Scholar |
Blouin, M. S. (2003). DNA-based methods for pedigree reconstruction and kinship analysis in natural populations. Trends in Ecology & Evolution 18, 503–511.
| DNA-based methods for pedigree reconstruction and kinship analysis in natural populations.Crossref | GoogleScholarGoogle Scholar |
Blumstein, D. T., Mennill, D. J., Clemins, P., Girod, L., Yao, K., Patricelli, G., Deppe, J. L., Krakauer, A. H., Clark, C., Cortopassi, K. A., Hanser, S. F., McCowan, B., Ali, A. M., and Kirschel, A. N. G. (2011). Acoustic monitoring in terrestrial environments using microphone arrays: applications, technological considerations and prospectus. Journal of Applied Ecology 48, 758–767.
| Acoustic monitoring in terrestrial environments using microphone arrays: applications, technological considerations and prospectus.Crossref | GoogleScholarGoogle Scholar |
Boeckle, M., Szipl, G., and Bugnyar, T. (2012). Who wants food? Individual characteristics in raven yells. Animal Behaviour 84, 1123–1130.
| Who wants food? Individual characteristics in raven yells.Crossref | GoogleScholarGoogle Scholar |
Bonadonna, F., and Sanz-Aguilar, A. (2012). Kin recognition and inbreeding avoidance in wild birds: the first evidence for individual kin-related odour recognition. Animal Behaviour 84, 509–513.
| Kin recognition and inbreeding avoidance in wild birds: the first evidence for individual kin-related odour recognition.Crossref | GoogleScholarGoogle Scholar |
Browning, L. E., Patrick, S. C., Rollins, L. A., Griffith, S. C., and Russell, A. F. (2012). Kin selection, not group augmentation, predicts helping in an obligate cooperatively breeding bird. Proceedings of the Royal Society of London – B. Biological Sciences 279, 3861–3869.
| Kin selection, not group augmentation, predicts helping in an obligate cooperatively breeding bird.Crossref | GoogleScholarGoogle Scholar |
Carlisle, T. R., and Zahavi, A. (1986). Helping at the nest, allofeeding and social status in immature Arabian Babblers. Behavioral Ecology and Sociobiology 18, 339–351.
| Helping at the nest, allofeeding and social status in immature Arabian Babblers.Crossref | GoogleScholarGoogle Scholar |
Clutton-Brock, T. H. (2002). Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72.
| Breeding together: kin selection and mutualism in cooperative vertebrates.Crossref | GoogleScholarGoogle Scholar |
Clutton-Brock, T. (2009). Cooperation between non-kin in animal societies. Nature 462, 51–57.
| Cooperation between non-kin in animal societies.Crossref | GoogleScholarGoogle Scholar |
Cockburn, A. (1998). Evolution of helping behavior in cooperatively breeding birds. Annual Review of Ecology and Systematics 29, 141–177.
| Evolution of helping behavior in cooperatively breeding birds.Crossref | GoogleScholarGoogle Scholar |
Cockburn, A. (2007). Solitary obsession or menage a trois, but cooperative breeding is never a two-party game. Behavioural Processes 76, 106–108.
| Solitary obsession or menage a trois, but cooperative breeding is never a two-party game.Crossref | GoogleScholarGoogle Scholar |
Cornwallis, C. K., West, S., and Griffin, A. S. (2009). Routes to indirect fitness in cooperatively breeding vertebrates: kin discrimination and limited dispersal. Journal of Evolutionary Biology 22, 2445–2457.
| Routes to indirect fitness in cooperatively breeding vertebrates: kin discrimination and limited dispersal.Crossref | GoogleScholarGoogle Scholar |
Croft, D. P., James, R., and Krause, J. (2008). ‘Exploring Animal Social Networks.’ (Princeton University Press: Princeton, NJ.)
Dall, S. R. X., Giraldeau, L. A., Olsson, O., McNamara, J. M., and Stephens, D. W. (2005). Information and its use by animals in evolutionary ecology. Trends in Ecology & Evolution 20, 187–193.
| Information and its use by animals in evolutionary ecology.Crossref | GoogleScholarGoogle Scholar |
Darwin, C. (1859). ‘On the Origin of Species.’ (John Murray: London.)
Double, M. C., Dawson, D., Burke, T., and Cockburn, A. (1997). Finding the fathers in the least faithful bird: a microsatellite-based genotyping system for the Superb Fairy-wren Malurus cyaneus. Molecular Ecology 6, 691–693.
| Finding the fathers in the least faithful bird: a microsatellite-based genotyping system for the Superb Fairy-wren Malurus cyaneus.Crossref | GoogleScholarGoogle Scholar |
Doutrelant, C., and Covas, R. (2007). Helping has signalling characteristics in a cooperatively breeding bird. Animal Behaviour 74, 739–747.
| Helping has signalling characteristics in a cooperatively breeding bird.Crossref | GoogleScholarGoogle Scholar |
Dow, D. D., and Whitmore, M. J. (1990). Noisy Miners: variations on the theme of communality. In ‘Cooperative Breeding in Birds: Long Term Studies of Ecology and Behaviour’. (Eds P. B. Stacey, W. D. Koenig.) pp. 559–592. (Cambridge University Press: Cambridge, UK.)
Durrant, K. L., and Hughes, J. M. (2005). Differing rates of extra-group paternity between two populations of the Australian Magpie (Gymnorhina tibicen). Behavioral Ecology and Sociobiology 57, 536–545.
| Differing rates of extra-group paternity between two populations of the Australian Magpie (Gymnorhina tibicen).Crossref | GoogleScholarGoogle Scholar |
Emlen, S. T. (1995). An evolutionary theory of family. Proceedings of the National Academy of Sciences of the United States of America 92, 8092–8099.
| An evolutionary theory of family.Crossref | GoogleScholarGoogle Scholar |
Emlen, S. T., and Wrege, P. H. (1988). The role of kinship in helping decisions among White-fronted Bee-Eaters. Behavioral Ecology and Sociobiology 23, 305–315.
| The role of kinship in helping decisions among White-fronted Bee-Eaters.Crossref | GoogleScholarGoogle Scholar |
Fawcett, T. W., Hamblin, S., and Giraldeau, L.-A. (2013). Exposing the behavioral gambit: the evolution of learning and decision rules. Behavioral Ecology 24, 2–11.
| Exposing the behavioral gambit: the evolution of learning and decision rules.Crossref | GoogleScholarGoogle Scholar |
Gonzalez, J.-C. T., Sheldon, B. C., and Tobias, J. A. (2013). Environmental stability and the evolution of cooperative breeding in hornbills. Proceedings of the Royal Society of London – B. Biological Sciences 280, 20131297.
| Environmental stability and the evolution of cooperative breeding in hornbills.Crossref | GoogleScholarGoogle Scholar |
Griesser, M., Barnaby, J., Schneider, N. A., Figenschau, N., Wright, J., Griffith, S. C., Kazem, A., and Russell, A. F. (2009). Influence of winter ranging behaviour on the social organization of a cooperatively breeding bird species, the Apostlebird. Ethology 115, 888–896.
| Influence of winter ranging behaviour on the social organization of a cooperatively breeding bird species, the Apostlebird.Crossref | GoogleScholarGoogle Scholar |
Hamilton, W. D. (1964a). The genetical evolution of social behaviour. I. Journal of Theoretical Biology 7, 1–16.
| The genetical evolution of social behaviour. I.Crossref | GoogleScholarGoogle Scholar |
Hamilton, W. D. (1964b). The genetical evolution of social behaviour. II. Journal of Theoretical Biology 7, 17–52.
| The genetical evolution of social behaviour. II.Crossref | GoogleScholarGoogle Scholar |
Hatchwell, B. J. (2009). The evolution of cooperative breeding in birds: kinship, dispersal and life history. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364, 3217–3227.
| The evolution of cooperative breeding in birds: kinship, dispersal and life history.Crossref | GoogleScholarGoogle Scholar |
Jamieson, I. G. (1991). The unselected hypothesis for the evolution of helping behavior: too much or too little emphasis on natural selection? American Naturalist 138, 271–282.
| The unselected hypothesis for the evolution of helping behavior: too much or too little emphasis on natural selection?Crossref | GoogleScholarGoogle Scholar |
Jetz, W., and Rubenstein, D. R. (2011). Environmental uncertainty and the global biogeography of cooperative breeding in birds. Current Biology 21, 72–78.
| Environmental uncertainty and the global biogeography of cooperative breeding in birds.Crossref | GoogleScholarGoogle Scholar |
Kennedy, R. A. W., Evans, C. S., and McDonald, P. G. (2009). Individual distinctiveness in the mobbing call of a cooperative bird, the noisy miner Manorina melanocephala. Journal of Avian Biology 40, 481–490.
| Individual distinctiveness in the mobbing call of a cooperative bird, the noisy miner Manorina melanocephala.Crossref | GoogleScholarGoogle Scholar |
Kingma, S. A., Hall, M. L., and Peters, A. (2011). Multiple benefits drive helping behavior in a cooperatively breeding bird: an integrated analysis. American Naturalist 177, 486–495.
| Multiple benefits drive helping behavior in a cooperatively breeding bird: an integrated analysis.Crossref | GoogleScholarGoogle Scholar |
Lehmann, L., and Keller, L. (2006). The evolution of cooperation and altruism – a general framework and a classification of models. Journal of Evolutionary Biology 19, 1365–1376.
| The evolution of cooperation and altruism – a general framework and a classification of models.Crossref | GoogleScholarGoogle Scholar |
Margraf, N., and Cockburn, A. (2013). Helping behaviour and parental care in fairy-wrens (Malurus). Emu 113, 294–301.
| Helping behaviour and parental care in fairy-wrens (Malurus).Crossref | GoogleScholarGoogle Scholar |
Marshall, J. A. R. (2011). Group selection and kin selection: formally equivalent approaches. Trends in Ecology & Evolution 26, 325–332.
| Group selection and kin selection: formally equivalent approaches.Crossref | GoogleScholarGoogle Scholar |
Maynard Smith, J. (1964). Group selection and kin selection. Nature 201, 1145–1147.
| Group selection and kin selection.Crossref | GoogleScholarGoogle Scholar |
McDonald, P. G. (2012). Cooperative bird differentiates between the calls of different individuals, even when vocalizations were from completely unfamiliar individuals. Biology Letters 8, 365–368.
| Cooperative bird differentiates between the calls of different individuals, even when vocalizations were from completely unfamiliar individuals.Crossref | GoogleScholarGoogle Scholar |
McDonald, P. G., and Wright, J. (2011). Bell miner provisioning calls are more similar among relatives and are used by helpers at the nest to bias their effort towards kin. Proceedings of the Royal Society of London – B. Biological Sciences 278, 3403–3411.
| Bell miner provisioning calls are more similar among relatives and are used by helpers at the nest to bias their effort towards kin.Crossref | GoogleScholarGoogle Scholar |
McDonald, P. G., Kazem, A. J. N., and Wright, J. (2007). A critical analysis of ‘false-feeding’ behaviour in a cooperatively breeding bird: disturbance effects, satiated nestlings or deception? Behavioral Ecology and Sociobiology 61, 1623–1635.
| A critical analysis of ‘false-feeding’ behaviour in a cooperatively breeding bird: disturbance effects, satiated nestlings or deception?Crossref | GoogleScholarGoogle Scholar |
McDonald, P. G., Kazem, A. J. N., Clarke, M. F., and Wright, J. (2008). Helping as a signal: does removal of potential audiences alter helper behavior in the bell miner? Behavioral Ecology 19, 1047–1055.
| Helping as a signal: does removal of potential audiences alter helper behavior in the bell miner?Crossref | GoogleScholarGoogle Scholar |
McDonald, P. G., Kazem, A. J. N., and Wright, J. (2009). Cooperative provisioning dynamics: fathers and unrelated helpers show similar responses to manipulations of begging. Animal Behaviour 77, 369–376.
| Cooperative provisioning dynamics: fathers and unrelated helpers show similar responses to manipulations of begging.Crossref | GoogleScholarGoogle Scholar |
McNamara, J. M., and Houston, A. I. (2009). Integrating function and mechanism. Trends in Ecology & Evolution 24, 670–675.
| Integrating function and mechanism.Crossref | GoogleScholarGoogle Scholar |
Mehdiabadi, N. J., Jack, C. N., Farnham, T. T., Platt, T. G., Kalla, S. E., Shaulsky, G., Queller, D. C., and Strassmann, J. E. (2006). Kin preference in a social microbe. Nature 442, 881–882.
| Kin preference in a social microbe.Crossref | GoogleScholarGoogle Scholar |
Nomano, F. Y., Browning, L. E., Rollins, L. A., Nakagawa, S., Griffith, S. C., and Russell, A. F. (2013). Feeding nestlings does not function as a signal of social prestige in cooperatively breeding chestnut-crowned babblers. Animal Behaviour 86, 277–289.
| Feeding nestlings does not function as a signal of social prestige in cooperatively breeding chestnut-crowned babblers.Crossref | GoogleScholarGoogle Scholar |
Nowak, M. A., Tarnita, C. E., and Wilson, E. O. (2010). The evolution of euscociality. Nature 466, 1057–1062.
| The evolution of euscociality.Crossref | GoogleScholarGoogle Scholar |
Okasha, S. (2006). ‘Evolution and the Levels of Selection.’ (Oxford University Press: New York.)
Pacheco, M. L., McDonald, P. G., Wright, J., Kazem, A. J. N., and Clarke, M. F. (2008). Helper contributions to antiparasite behavior in the cooperatively breeding Bell Miner. Behavioral Ecology 19, 558–566.
| Helper contributions to antiparasite behavior in the cooperatively breeding Bell Miner.Crossref | GoogleScholarGoogle Scholar |
Põldmaa, T., Montgomerie, R., and Boag, P. (1995). Mating system of the cooperatively breeding Noisy Miner Manorina melanocephala, as revealed by DNA profiling. Behavioral Ecology and Sociobiology 37, 137–143.
| Mating system of the cooperatively breeding Noisy Miner Manorina melanocephala, as revealed by DNA profiling.Crossref | GoogleScholarGoogle Scholar |
Queller, D. C., and Strassmann, J. E. (1998). Kin selection and social insects. Bioscience 48, 165–175.
| Kin selection and social insects.Crossref | GoogleScholarGoogle Scholar |
Ridley, A. R. (2007). Factors affecting offspring survival and development in a cooperative bird: social, maternal and environmental effects. Journal of Animal Ecology 76, 750–760.
| Factors affecting offspring survival and development in a cooperative bird: social, maternal and environmental effects.Crossref | GoogleScholarGoogle Scholar |
Rollins, L.-A., Browning, L. E., Holleley, C. E., Savage, J. L., Russell, A. F., and Griffith, S. C. (2012). Building genetic networks using relatedness information: a novel approach for the estimation of dispersal and characterization of group structure in social animals. Molecular Ecology 21, 1727–1740.
| Building genetic networks using relatedness information: a novel approach for the estimation of dispersal and characterization of group structure in social animals.Crossref | GoogleScholarGoogle Scholar |
Russell, A. F., Langmore, N. E., Cockburn, A., Astheimer, L. B., and Kilner, R. M. (2007). Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science 317, 941–944.
| Reduced egg investment can conceal helper effects in cooperatively breeding birds.Crossref | GoogleScholarGoogle Scholar |
Sorato, E., Gullett, P. R., Griffith, S. C., and Russell, A. F. (2012). Effects of predation risk on foraging behaviour and group size: adaptations in a social cooperative species. Animal Behaviour 84, 823–834.
| Effects of predation risk on foraging behaviour and group size: adaptations in a social cooperative species.Crossref | GoogleScholarGoogle Scholar |
Tinbergen, N. (1963). On aims and methods of Ethology. Zeitschrift für Tierpsychologie 20, 410–433.
| On aims and methods of Ethology.Crossref | GoogleScholarGoogle Scholar |
West, S. A., Griffin, A. S., and Gardner, A. (2007). Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. Journal of Evolutionary Biology 20, 415–432.
| Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection.Crossref | GoogleScholarGoogle Scholar |
West, S. A., El Mouden, C., and Gardner, A. (2011). Sixteen common misconceptions about the evolution of cooperation in humans. Evolution and Human Behavior 32, 231–262.
| Sixteen common misconceptions about the evolution of cooperation in humans.Crossref | GoogleScholarGoogle Scholar |
Woolfenden, G. E., and Fitzpatrick, J. W. (1978). The inheritance of territory in group-breeding birds. Bioscience 28, 104–108.
| The inheritance of territory in group-breeding birds.Crossref | GoogleScholarGoogle Scholar |
Wright, J. (2007). Cooperation theory meets cooperative breeding: exposing some ugly truths about social prestige, reciprocity and group augmentation. Behavioural Processes 76, 142–148.
| Cooperation theory meets cooperative breeding: exposing some ugly truths about social prestige, reciprocity and group augmentation.Crossref | GoogleScholarGoogle Scholar |
Wright, J., McDonald, P. G., te Marvelde, L., Kazem, A. J. N., and Bishop, C. (2010). Helping effort increases with relatedness in bell miners, but ‘unrelated’ helpers of both sexes still provide substantial care. Proceedings of the Royal Society of London – B. Biological Sciences 277, 437–445.
| Helping effort increases with relatedness in bell miners, but ‘unrelated’ helpers of both sexes still provide substantial care.Crossref | GoogleScholarGoogle Scholar |
Zahavi, A. (1977). Reliability in communication systems and the evolution of altruism. In ‘Evolutionary Ecology’. (Eds B. Stonehouse, C. Perrins.) pp. 253–259. (University Park Press: Baltimore, MD.)

