NSW Annual Immunisation Coverage Report, 2011
Brynley Hull A D , Aditi Dey A , Sue Campbell-Lloyd B , Robert I. Menzies A C and Peter B. McIntyre A CA National Centre for Immunisation Research and Surveillance, The Children’s Hospital at Westmead
B Centre for Health Protection, NSW Ministry of Health
C Sydney Medical School, The University of Sydney
D Corresponding author. Email: brynley.hull@health.nsw.gov.au
NSW Public Health Bulletin 23(10) 179-186 https://doi.org/10.1071/NB12084
Published: 12 December 2012
Abstract
Abstract: This annual report, the third in the series, documents trends in immunisation coverage in NSW for children, adolescents and the elderly, to the end of 2011. Methods: Data from the Australian Childhood Immunisation Register, the NSW School Immunisation Program and the NSW Population Health Survey were used to calculate various measures of population coverage. Results: During 2011, greater than 90% coverage was maintained for children at 12 and 24 months of age. For children at 5 years of age the improvement seen in 2010 was sustained, with coverage at or near 90%. For adolescents, there was improved coverage for all doses of human papillomavirus vaccine, both doses of hepatitis B vaccine, varicella vaccine and the dose of diphtheria, tetanus and acellular pertussis given to school attendees in Years 7 and 10. Pneumococcal vaccination coverage in the elderly has been steadily rising, although it has remained lower than the influenza coverage estimates. Conclusion: This report provides trends in immunisation coverage in NSW across the age spectrum. The inclusion of coverage estimates for the pneumococcal conjugate, varicella and meningococcal C vaccines in the official coverage assessments for ‘fully immunised’ in 2013 is a welcome initiative.
This is the third New South Wales (NSW) Annual Immunisation Coverage Report. This series of annual reports provides information on trends and issues in immunisation coverage in NSW to facilitate the monitoring of NSW immunisation programs. This report uses the longstanding international practice of reporting coverage at key milestone ages to measure coverage against national benchmarks and to track trends over time. It is adapted from annual national immunisation reports published since 2008.1
The Australian Childhood Immunisation Register was established on 1 January 1996 by incorporating demographic data from Medicare on all Medicare-registered children aged less than 7 years.2 The operations of the Australian Childhood Immunisation Register have been discussed in detail elsewhere.3
High levels of reporting to the Australian Childhood Immunisation Register are maintained by a system of incentive payments for immunisation providers and carers. These have been discussed in detail elsewhere.3 However, changes to immunisation policy, the incentive payment system and changes to the ‘fully immunised’ coverage algorithms may have an impact on reported vaccination coverage; some recent changes are highlighted in Box 1 and also referred to in this report.
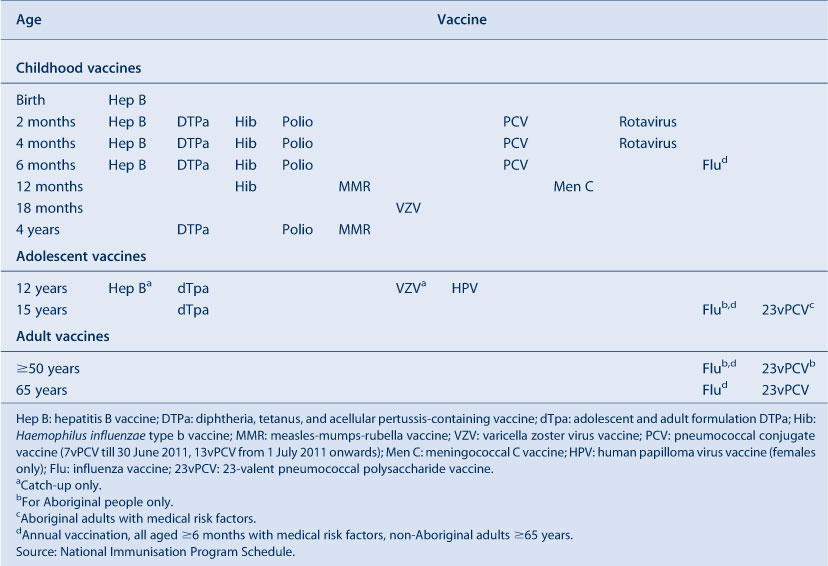
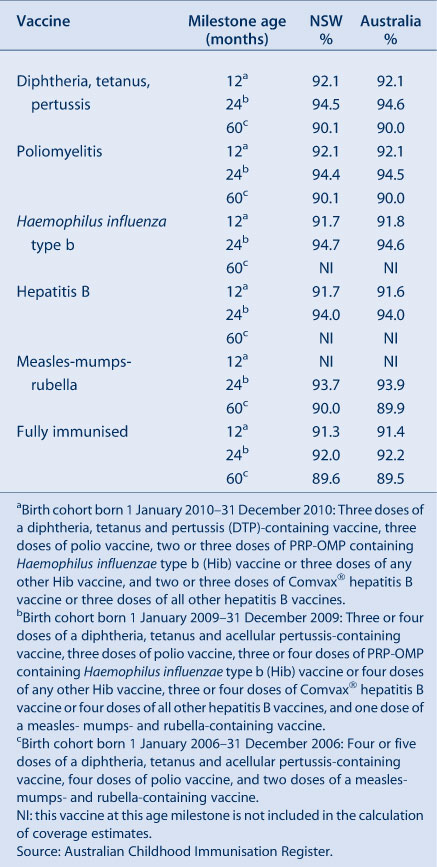
Table 1 presents the vaccines delivered through the NSW Immunisation Program for children in 2011. The only new vaccine to be introduced into the NSW Immunisation Program in 2011 was the 13-valent pneumococcal conjugate vaccine, Prevenar 13®, which replaced Prevenar®, the 7-valent vaccine, on 1 July.

|
|
Methods
Measuring immunisation coverage using the Australian Childhood Immunisation Register
The cohort method has been used for calculating coverage at the population level (national and state/territory)4 since the inception of the Australian Childhood Immunisation Register. Cohort immunisation status is assessed at 12 months of age (for vaccines due at 6 months), 24 months of age (for vaccines due at 12 months), and 5 years of age (for vaccines due at 4 years). A 3-month lag period is allowed for the late notification of immunisations to the Australian Childhood Immunisation Register.4 If a child's records indicate receipt of the last dose of a vaccine that requires more than one dose to complete the series, it is assumed that earlier vaccinations in the sequence have been given. This assumption has been shown to be valid.5,6
The proportion of children designated as ‘fully immunised’ was calculated using the number of Medicare-registered children who were completely immunised with the vaccines of interest by the designated age as the numerator and the total number of Medicare-registered children in the age cohort as the denominator. Vaccines included were those used nationally for the purposes of incentive payments. ‘Fully immunised’ at 12 months of age was defined as a child having a record on the Australian Childhood Immunisation Register of three doses of a diphtheria, tetanus and pertussis (DTP)-containing vaccine, three doses of polio vaccine, two or three doses of PRP-OMP containing Haemophilus influenzae type b (Hib) vaccine or three doses of any other Hib vaccine, and two or three doses of Comvax® hepatitis B vaccine or three doses of all other hepatitis B vaccines. ‘Fully immunised’ at 24 months of age was defined as three or four doses of a DTP-containing vaccine, three doses of polio vaccine, three or four doses of PRP-OMP containing Hib vaccine or four doses of any other Hib vaccine, three or four doses of Comvax® hepatitis B vaccine or four doses of all other hepatitis B vaccines, and one dose of a measles- mumps- and rubella (MMR)-containing vaccine. ‘Fully immunised’ at 5 years of age was defined as four or five doses of a DTP-containing vaccine, four doses of polio vaccine, and two doses of an MMR-containing vaccine.
Previous reports included analysis by local health district (LHD), data on other National Immunisation Program vaccines not included in ‘fully immunised’ calculations (pneumococcal conjugate vaccine, rotavirus vaccine, varicella vaccine and meningococcal C vaccine), vaccination timeliness and data comparing Aboriginal and non-Aboriginal children. However, it was not possible to include these analyses in this report due to a review by the Department of Health and Ageing of processes for releasing data from the Australian Childhood Immunisation Register.
Coverage in adolescents and the elderly
Information describing coverage for vaccines given to adolescents was collected from the NSW School Immunisation Program. Vaccination status is recorded by school immunisation teams and counts are collated by LHDs and NSW Health. The denominator is the number of school enrolments at the start of the year. The coverage rates may underestimate the true vaccination coverage as they represent only those vaccinations received through the school program and do not include doses received from general practitioners or other immunisation providers. Methods are presented in more detail elsewhere.7 For varicella and hepatitis B vaccines, students who were not vaccinated due to previous vaccination or varicella infection are included in the denominator. The proportion of these students is unknown.
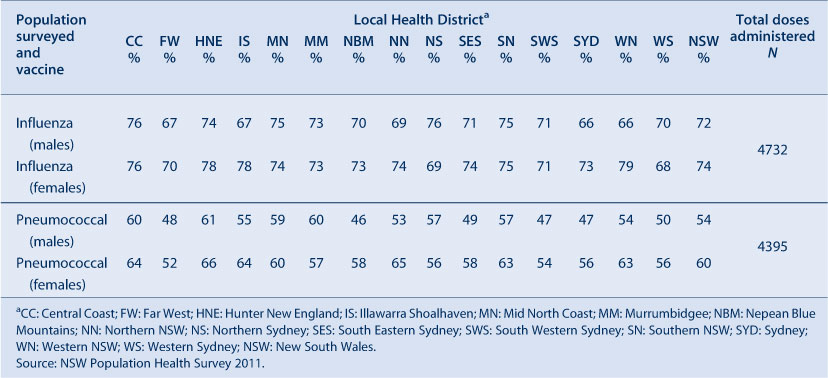
Influenza and pneumococcal vaccination coverage estimates in the elderly were obtained from the NSW Population Health Survey. This is a rolling random digit-dialled telephone survey, with vaccination status determined from patient recall at the time of the interview. Influenza and pneumococcal vaccination coverage estimates are based on 4732 and 4395 respondents in NSW, respectively. Methods and results are presented in more detail elsewhere.8
Results
Overall coverage estimates
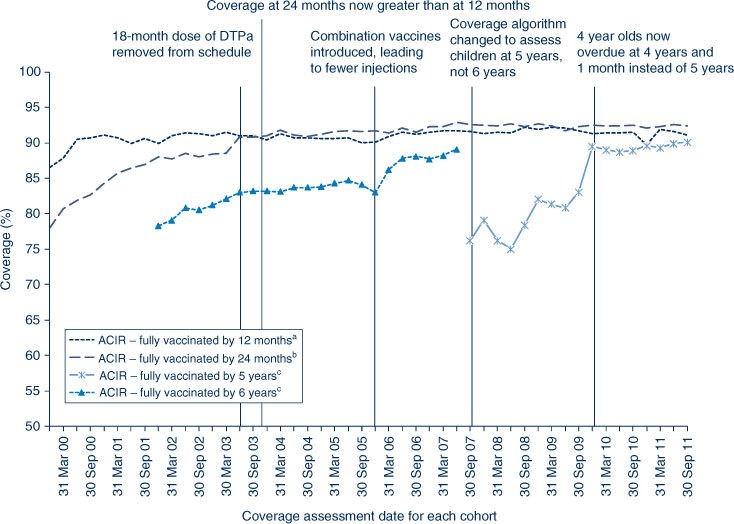
In NSW, coverage for all individual vaccines for the 12-month age group is greater than 91%. Similarly, for the 24-month age group, coverage for all individual vaccines is also higher than 90%. Recorded coverage for the 5-year age group is higher than 90% for all vaccines (Table 2). Figure 1 shows time trends in ‘fully immunised’ childhood vaccination coverage at three milestone ages in NSW. The proportion ‘fully immunised’ at 1 and 2 years of age has been at high levels since 2003 whereas coverage at 5 years of age increased markedly during 2010 and remained at that level during 2011.
|
Coverage estimates for children aged less than 3 years
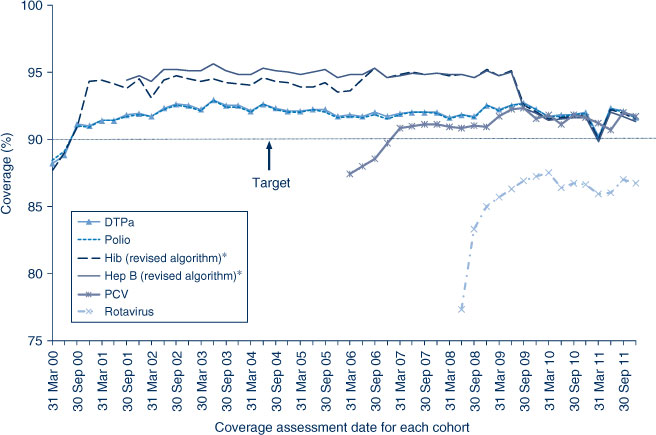
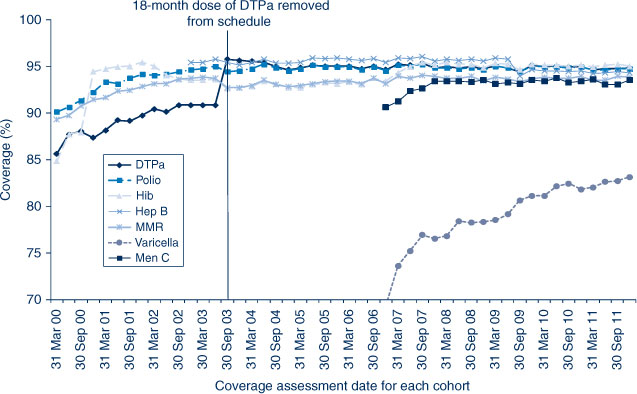
In NSW before 2009, coverage for the 12-month and 24-month age groups for Hib and hepatitis B vaccines was greater than for DTPa and polio, due to a less stringent algorithm for calculating coverage. Since the change in algorithm in the latter half of 2009, coverage estimates for Hib and hepatitis B have lowered and become similar to those of DTPa and polio at just under 92% (Figure 2) for the 12-month age group and approximately 95% (Figure 3) for the 24-month age group. These newer estimates more accurately reflect the true proportion of children fully vaccinated for these vaccines. Also in 2011, coverage for the MMR vaccine for the 24-month age group was around 94% (Table 2).
|
Coverage estimates for children aged 5–6 years
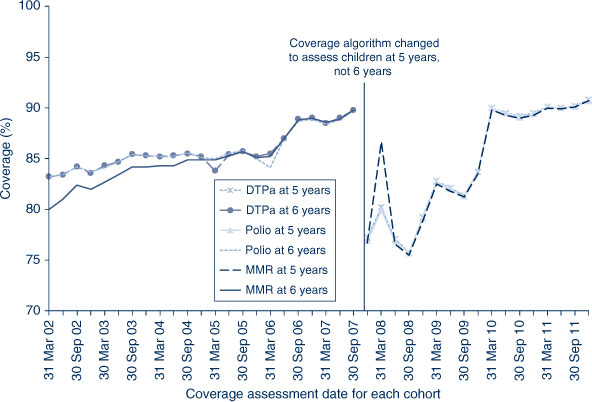
The trends in childhood vaccination coverage in NSW for individual vaccines (DTPa, polio and MMR) at 5 years of age (6 years of age prior to December 2007) are shown in Figure 4. Coverage for all three vaccines was almost identical and remained steady across the whole period until mid-2006 when a sharp increase of almost 5% was recorded, likely due to the introduction of combination vaccines. Coverage at 5 years of age was substantially lower than at 6 years of age due to the shorter time for the recording of delayed vaccinations. However, in 2011, the 5-year coverage for DTPa, polio and MMR increased markedly to be slightly above 90% following a change to due and overdue rules. The overall ‘fully immunised’ rate for 5-year coverage was 89.6% in NSW, which was similar to the national 5-year coverage rate of 89.5% (Table 2). However, at 5 years of age, the proportion recorded as being ‘fully immunised’ was lower than that at earlier age milestones.
|
Coverage in adolescents
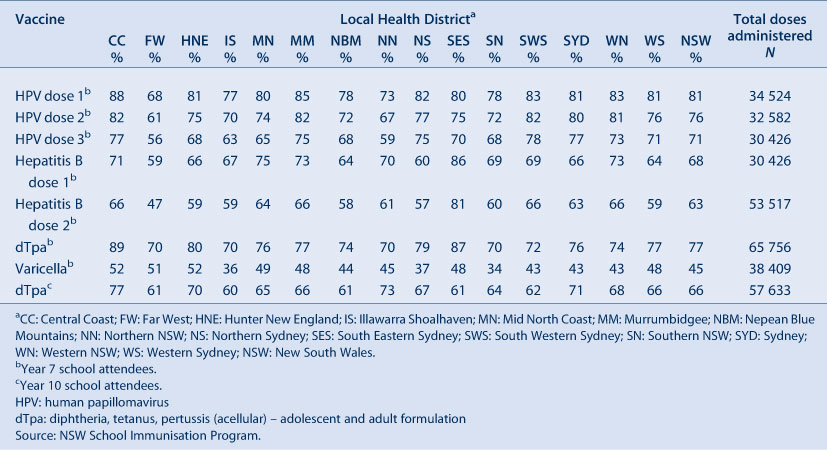
NSW Adolescent Vaccination Program coverage data for high school students for 2011 by LHD are shown in Table 3. For NSW, coverage varied by vaccine and dose with better coverage for the first and second doses of human papillomavirus vaccine (HPV) and the dose of dTpa in Year 7 attendees. In 2011, coverage in adolescents increased for all vaccine types and doses compared to the previous year.9 The greatest improvement was seen with varicella vaccine, with coverage increasing 13 percentage points from 32% to 45%. Vaccine coverage in adolescents varied by LHD (Table 3).
|
Vaccines for the elderly (pneumococcal and influenza)
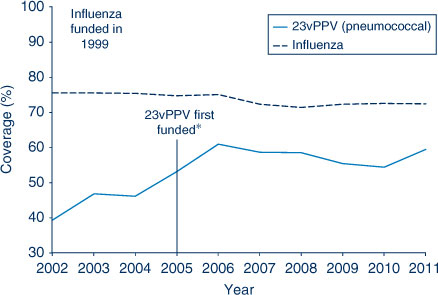
The proportion of people aged 65 years and over who were vaccinated for influenza in the past 12 months has remained relatively stable at over 70% in NSW during the period 2002–2011. However, pneumococcal vaccination (23-valent pneumococcal polysaccharide vaccine, 23vPPV) in the previous 5 years remained lower than the influenza coverage estimates. The highest coverage estimate for pneumococcal vaccination in the elderly was observed in 2006 at 61%, the year after its inclusion on the National Immunisation Program (Figure 5). Coverage in 2011 was 60%. Vaccine coverage in the elderly varied by LHD (Table 4).
|
Discussion
These data reveal that 90% coverage benchmarks have been reached for children at both 12 and 24 months of age for NSW. During 2011, there was an increase in coverage at the 5-year milestone, to approximately 90%.
Coverage at 24 months of age exceeds that at 12 months in NSW. This is likely related to the greater period of time between due date and assessment time (12 and 6 months respectively), and potentially the maternity incentive payment assessed at 18–24 months. The continued improvement in coverage at 5 years of age is due to further improved timeliness of vaccination, and is probably related to the change to the overdue rules in January 2009, where children became overdue for their pre-school boosters at 4 years and 1 month of age instead of the previous 5 years (Box 1). This change had an impact on eligibility for child care benefits for parents and outcome payments for providers. It is also possible that the splitting of the Maternity Immunisation Allowance in 2011 could have affected these data, as it applied to children turning 4 years from 2011 onwards.
It should be noted that at present several vaccines on the National Immunisation Program schedule are not included in the assessment of ‘fully immunised’ (i.e. PCV, meningococcal C, rotavirus and varicella). This annual report does not provide coverage data on these vaccines. Only data for the more longstanding and established vaccines are provided to Medicare Locals, public health units and immunisation providers. As these non-assessable vaccines have been routinely incorporated into the childhood immunisation schedule for some time, their inclusion in the official coverage assessments for ‘fully immunised’ (except for rotavirus vaccine) has been made policy by the Department of Health and Ageing and is effective from 1 July 2013. This will facilitate more complete monitoring of program delivery, potentially boost coverage by allowing existing incentive payments to apply to them, and provide a more realistic assessment of ‘fully immunised’.
School-based vaccination in NSW has achieved relatively high coverage for most vaccines, which is similar to or better than that achieved in other Australian jurisdictions and higher than in settings where adolescent vaccines are implemented through primary care.7 Coverage for varicella is lower than other vaccines in all other jurisdictions as well as NSW. This is likely due to a combination of students not vaccinated due to previous clinical history of chickenpox, students with previous receipt of varicella vaccine, and perceived less serious clinical outcomes. The change in advice given to parents about the need for a dose at 12 years of age that occurred in 2011 may also have had an impact. Coverage for all adolescent vaccines increased from 2010 to 2011.
Coverage for the elderly has been consistently high for influenza vaccine, but less so for pneumococcal vaccine, perhaps due to greater awareness of the yearly influenza vaccination programs. Uptake of pneumococcal vaccine in 2011 is likely to have been negatively affected by reported adverse events in that year and the associated temporary suspension of any doses of pneumococcal vaccine in NSW. These results may also be partly explained by an Australian study in the elderly that found, in comparison to written records held by immunisation providers, a telephone survey over-estimated influenza coverage in the elderly by 3%, and under-estimated pneumococcal coverage by 8%.10
Conclusion
Data provided in this report by the Australian Childhood Immunisation Register reflect the successful delivery of the National Immunisation Program in NSW, while identifying some areas for improvement. The Australian Childhood Immunisation Register, the NSW Population Health Survey and monitoring through the NSW School Vaccination Program continue to be very useful tools for administering the National Immunisation Program and monitoring its implementation in NSW.
References
[1] Hull B, Deeks S, Menzies R, McIntyre P. Immunisation coverage annual report, 2007. Commun Dis Intell 2009; 33 170–87.[2] Hull BP, McIntyre PB, Heath TC, Sayer GP. Measuring immunisation coverage in Australia. A review of the Australian Childhood Immunisation Register. Aust Fam Physician 1999; 28 55–60.
| 1:STN:280:DyaK1M7ktlClsw%3D%3D&md5=fbcf77a4c001124cc2aa56701fe36479CAS |
[3] Hull BP, Deeks SL, McIntyre PB. The Australian Childhood Immunisation Register – a model for universal immunisation registers? Vaccine 2009; 27 5054–60.
| The Australian Childhood Immunisation Register – a model for universal immunisation registers?Crossref | GoogleScholarGoogle Scholar |
[4] O'Brien ED, Sam GA, Mead C. Methodology for measuring Australia's childhood immunisation coverage. Commun Dis Intell 1998; 22 36–7.
| 1:STN:280:DyaK1c3hvVWntA%3D%3D&md5=bf6a3b2035fa7583d72c3867ec0a4402CAS |
[5] Hull BP, McIntyre PB. Immunisation coverage reporting through the Australian Childhood Immunisation Register – an evaluation of the third-dose assumption. Aust N Z J Public Health 2000; 24 17–21.
| Immunisation coverage reporting through the Australian Childhood Immunisation Register – an evaluation of the third-dose assumption.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c3ktVCnsw%3D%3D&md5=63b9a830900f117c0c5c720e2b4e0a76CAS |
[6] Hull BP, Lawrence GL, MacIntyre CR, McIntyre PB. Estimating immunisation coverage: is the ‘third dose assumption’ still valid? Commun Dis Intell 2003; 27 357–61.
[7] Ward KF, Menzies RI, Quinn HE, Campbell-Lloyd S. School-based vaccination in NSW. N S W Public Health Bull 2010; 21 237–42.
| School-based vaccination in NSW.Crossref | GoogleScholarGoogle Scholar |
[8] Centre for Epidemiology and Evidence. Health Statistics New South Wales. Sydney: NSW Ministry of Health. Available at: http://www.healthstats.nsw.gov.au/Indicator/com_flupneumoimmu_age/com_flupneumoimmu_age_trend (Cited 17 October 2012).
[9] Hull BP, Dey A, Campbell-Lloyd S, Menzies RI, McIntyre PB. NSW Annual Immunisation Coverage Report, 2011. N S W Public Health Bull 2010; 22 179–95.
[10] Andrews RM. Assessment of vaccine coverage following the introduction of a publicly funded pneumococcal vaccine program for the elderly in Victoria, Australia. Vaccine 2005; 23 2756–61.
| Assessment of vaccine coverage following the introduction of a publicly funded pneumococcal vaccine program for the elderly in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |