Invasive pneumococcal disease in western Sydney, 2002–2010
Kristina L. Flego A C , George Truman A , Vicky Sheppeard A and Robin E. Gilmour BA Centre for Population Health, Clinical Support Division Western
B Communicable Diseases Branch, NSW Department of Health
C Corresponding author. Email: Kristina.Flego@swahs.health.nsw.gov.au
NSW Public Health Bulletin 22(12) 219-221 https://doi.org/10.1071/NB11012
Published: 22 December 2011
In January 2005 the 7-valent pneumococcal conjugate vaccine (7vPCV) was funded on the National Immunisation Program for all children as a three-dose regimen given at 2, 4 and 6 months of age with a catch-up program for children up to 3 years of age. In the same year, the 23-valent pneumococcal polysaccharide vaccine (23vPPV) was funded for all Australians aged 65 years and over. A 23vPPV nationally funded program for Indigenous Australians aged 50 years and over, as well as those aged 15–50 years with specified underlying medical risk factors, has been in place since 1999.1
We review the burden of invasive pneumococcal disease (IPD) in the former Sydney West Area Health Service (SWAHS) during the period 2002–2010 with particular attention to the proportion of IPD due to serotypes covered or not covered by the vaccines (Box 1).
Methods
IPD (defined by the isolation of Streptococcus pneumoniae from a normally sterile site such as blood or cerebrospinal fluid) has been a notifiable condition under the NSW Public Health Act 1991 since January 2001. All New South Wales (NSW) laboratories are required to report positive culture results to their local public health unit (PHU). All serotyping was performed at the Institute for Clinical Pathology and Medical Research, Westmead, one of three reference laboratories for this purpose in Australia. PHU staff enter this information, including serotyping results if available, into a statewide database.2
The population investigated was that of the former SWAHS (estimated 2010 population of 1 168 076). To demonstrate vaccine impact on disease burden the population was divided into three age categories for analysis: 0–4 years, 5–64 years and 65 years and over. Non-Aboriginal people aged 5–64 years are not routinely vaccinated. Annual age-specific rates and Poisson confidence intervals were calculated from January 2002 to December 2010 in Microsoft Excel® using notification data and Australian Bureau of Statistics population estimates from a population health database held by the NSW Department of Health (Health Outcomes and Information Statistical Toolkit). The average annual age-adjusted rates for each of the three age categories for the period 2002–2004 (baseline) were compared to the 2006–2010 post-vaccination implementation period. The serotype incidence percentage was calculated by summing the total cases caused by that serotype divided by the total notified cases for that time period.
Results
Changing incidence of IPD over time by age category
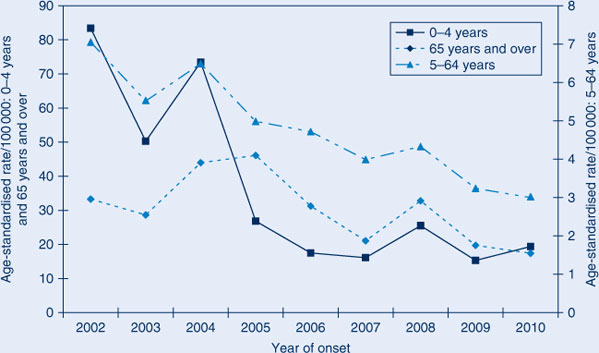
All three age categories showed a reduction in the age-specific IPD incidence over time with the greatest reduction in the 0–4-year age group (Figure 1).
|
Comparison of average annual IPD notification rates for the two time periods also shows that the greatest reduction in IPD incidence has been in the 0–4-year age group (72.8% reduction) (Table 1). The 5–64-year age group had a 39.4% decrease which is greater than that seen in the 65 years and over age group (31.4%), despite this population being targeted for 23vPPV.

|
Comparison of serotype incidence
The proportion of IPD cases due to serotypes contained in the 7vPCV was reduced in 2006–2010 compared with 2002–2004. Serotype 19A demonstrated the biggest increase, followed by serotype 3. Twelve of the 15 non-vaccine serotypes found in this population were relatively more common during 2006–2010. Non-vaccine serotypes caused 6% of all notified cases in 2002–2004 and 16% of all notified cases in 2006–2010.
Discussion
A significant reduction in the overall incidence of IPD occurred in the SWAHS population since the National Immunisation Program pneumococcal vaccines were introduced. The greatest reduction was in children aged less than 5 years, although significant reductions were noted across all age groups. These results are similar to those from other settings where unvaccinated cohorts enjoyed a reduced incidence of IPD due to a herd immunity effect.3–5
Serotype epidemiology has also changed since the pre-vaccination period. All 7vPCV-containing serotypes have reduced in relative frequency across the entire population. Serotype 19A, a component of the 23vPPV, is now the dominant serotype. Factors in the emergence of 19A may include: poor 23vPPV coverage rates in the 65 years and over age group (54%);6 inferior efficacy of the polysaccharide vaccine in elderly persons compared to that of 7vPCV in infants; and pressure from antibiotic use, as 19A is frequently resistant to penicillin and erythromycin.7–10
| Box 1. Serotypes covered by the three pneumococcal vaccines provided in NSW, 2004–2011 |
| 7-valent pneumococcal conjugate vaccine (7vPCV): 4, 6B, 9V, 14, 18C, 19F, 23F |
| 13-valent pneumococcal conjugate vaccine (13vPCV): all 7vPCV serotypes plus 1, 3, 5, 6A, 7F, 19A |
| 23-valent pneumococcal conjugate vaccine (23vPPV): all 7vPCV serotypes plus 1, 2, 3, 5, 7F, 8, 9N, 10A, 11A, 12F, 15B, 17F, 19A, 20, 22F, 33F |
Conclusion
The introduction of the 7vPCV into the National Immunisation Program has significantly reduced the incidence of IPD across all ages but particularly in 0–4-year olds. Non-7vPCV serotypes now predominate, particularly serotype 19A. A 13-valent pneumococcal conjugate vaccine 13vPCV (which includes serotypes 19A and 3) will replace 7vPCV from July 2011 (Box 1). This is expected to reduce the incidence of IPD in the 0–4-year age group and potentially the remainder of the population through a herd immunity effect.
References
[1] Department of Health and Ageing Vaccine Preventable Diseases in Australia, 2005–2007. Commun Dis Intell 2010; 34[2] Health NSW. Response Protocol for NSW Public Health Units. Pneumococcal Disease (Invasive). September 2004. Available from: http://www.health.nsw.gov.au/factsheets/guideline/pneumo.html (Cited 27 May 2011.)
[3] Isaacman DJ, Fletcher MA, Fritzell B, Ciuryla V, Shranz J. Indirect effects associated with widespread vaccination of infants with heptavalent pneumococcal conjugate vaccine (PCV7;Prevenar). Vaccine 2007; 25 2420–7..
| Indirect effects associated with widespread vaccination of infants with heptavalent pneumococcal conjugate vaccine (PCV7;Prevenar).Crossref | GoogleScholarGoogle Scholar |
[4] Metlay JP, Fishman NO, Joffe M, Edelstein PH. Impact of pediatric vaccination with pneumococcal conjugate vaccine on the risk of bacteremic pneumococcal pneumonia in adults. Vaccine 2006; 24 468–75.
| Impact of pediatric vaccination with pneumococcal conjugate vaccine on the risk of bacteremic pneumococcal pneumonia in adults.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XkvVak&md5=88d61d190c794e565492df7a01eecaecCAS |
[5] Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin Infect Dis 2009; 49 205–12.
| Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study.Crossref | GoogleScholarGoogle Scholar |
[6] Sydney West Immunisation Strategy 2010–13. Parramatta: Centre for Population Health; 2010.
[7] Dias R, Caniça M. Invasive pneumococcal disease in Portugal prior to and after the introduction of pneumococcal heptavalent conjugate vaccine. FEMS Immunol Med Microbiol 2007; 51 35–42.
| Invasive pneumococcal disease in Portugal prior to and after the introduction of pneumococcal heptavalent conjugate vaccine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFeksrrI&md5=2df51e6ce87474820d4f239e1c47ba23CAS |
[8] Lynch JP, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med 2010; 16 217–25.
[9] Harboe ZB, Benfield TL, Valentiner-Branth P, Hjuler T, Lambersten L, Kaltoft M., et al. Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades. Clin Infect Dis 2010; 50 329–37.
| Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades.Crossref | GoogleScholarGoogle Scholar |
[10] Liñares J, Ardanuy C, Pallares R, Fenoll A. Changes in anitmicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect 2010; 16 402–10.
| Changes in anitmicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period.Crossref | GoogleScholarGoogle Scholar |

