31 EFFECTS OF CYCLOHEXIMIDE ON CAPRINE SOMATIC CELL NUCLEAR TRANSFER EMBRYO AND FETAL DEVELOPMENT
R.E Butler A , D. Melican A , N. Hawkins A , T. Jellerette A , S. Nims A , K. Graslie A and W. Gavin AGTC Biotherapeutics 175 Crossing Boulevard, Framingham, MA, USA. email: Robin.Butler@GTC-Bio.com
Reproduction, Fertility and Development 16(2) 138-138 https://doi.org/10.1071/RDv16n1Ab31
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
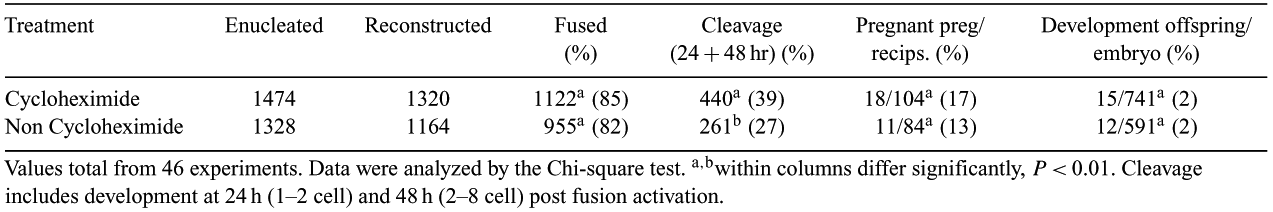
The use of protein synthesis inhibitors to down regulate the levels of Maturation Promoting Factor (MPF) following fusion and activation are widely used in the field of Nuclear Transfer (NT). Cycloheximide is a protein synthesis inhibitor that blocks the levels of cyclin B, a component of MPF which is required to maintain MII stage arrest in oocytes. However, it is unclear what, if any, effects these broadbased inhibitors may have on nonspecific protein expression in the oocytes or karyoplasts. The purpose of this study was to examine the effects of treatment with and without cycloheximide on embryo and fetal development to term. Ovulated in vivo MII oocytes from superovulated does during the traditional breeding season were surgically collected and then enucleated and reconstructed with either transfected fetal or adult skin cell karyoplasts or transgenic primary somatic skin cells. Couplets were simultaneously fused and activated with a single electrical pulse between 2.6 and 3.0 kV cm−1 for 20 μs. An additional electrical pulse was given to fused couplets and non-fused couplets were re-fused. Reconstructed embryos were either treated with 5 μg mL−1 cyclohexamide (Sigma, St. Louis, MO, USA) for a minimum of 3 h or cultured directly post-fusion and activation. The embryos were cultured in SOF + BSA at 38°C in a humidified modular incubation chamber (Billrups-Rothenberg, USA) containing 6% O2, 5% CO2, and 89% N2 for 24–48 h. Cleavage was assessed, and nuclear transfer embryos, with age appropriate development (up to 2 cells at 24 h or 2 to 8 cells by 48 h), were surgically transferred to the oviduct of surrogate recipient does. There were 18 and 11 confirmed pregnancies by Day 50 post-fusion and activation from the cycloheximide and non cycloheximide groups, respectively. A total of 12 recipients that received cycloheximide treated embryos produced term pregnancies and yielded 15 offspring. Alternatively, 9 recipients that received untreated embryos produced term pregnancies which yielded 12 offspring. A total of 27 NT offspring were produced and one offspring from a set of quadruplets died several days post-natally. While significantly more cycloheximide treated NT embryos cleaved, there was no significant difference in pregnancy rate or offspring born between treatment groups. These results suggest that the use of cycloheximide for embryo development in somatic cell nuclear transfer may not be necessary for establishing and maintaining caprine NT pregnancies.
|


