108 THE ROLE OF NITRIC OXIDE SYNTHASE IN IN VITRO DEVELOPMENT OF BOVINE OOCYTES AND PRE-IMPLANTATION EMBRYOS
A.K. Kadanga A , D. Tesfaye A , S. Ponsuksili B , K. Wimmers B , M. Gilles A and K. Schellander AA Institute of Animal Breeding and Genetics, University of Bonn, Bonn, Germany
B Research Institute for Biology of Farm Animals, Dummerstorf, Germany. Email: akad@itz.uni-bonn.de
Reproduction, Fertility and Development 17(2) 204-205 https://doi.org/10.1071/RDv17n2Ab108
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
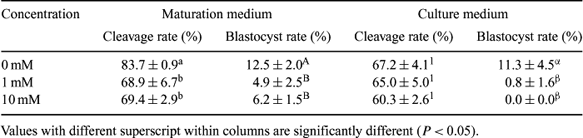
Nitric oxide (NO) is a free radical that serves as a key-signal molecule in various physiological processes including reproduction. Four isoforms of nitric oxide synthase (NOS) have been characterized: endothelial (eNOS), inducible (iNOS), neuronal (nNOS), and mitochondrial (mtNOS). The first two isoforms are reported to be expressed in mouse follicles, oocytes, and pre-implantation embryos (Nishikimi A et al. 2001 Reproduction 122, 957–963). However, the role of any of these isoforms have not yet been investigated in bovine embryos. Here we aimed to examine the role of NOS in in vitro development of bovine embryos by treating embryos with NOS inhibitor, N-omega-L-nitro-arginine methyl esther (L-NAME), and examining the localization of the protein in pre-implantation embryos. Oocytes and embryos were grown in the media with NOS inhibitor added at a level of 0 mM (control), 1 mM, and 10 mM to either maturation or culture medium. Each experiment was conducted in four replicates each containing 100 oocytes for IVP. Cleavage and blastocyst rate were recorded at Days 2 and 7, respectively. Data were analyzed using the General Linear Model in SAS version 8.02 (SAS Institute, Inc., Cary, NC, USA) with the main factors being the level of L-NAME and the point of application. Pairwise comparisons were done using the Tukey test. Protein localization in bovine oocytes and embryos was performed by immunocytochemistry using eNOS- and iNOS-specific antibodies. Embryos were fixed in 3.7% paraformaldehyde, permeabilized in 0.1% Triton-X100, and washed three times in PBS supplemented with BSA. They were incubated with eNOS and iNOS primary antibody (1:200 dilutions) and washed before incubation with secondary antibody conjugated to FITC. After washing they were mounted on glass slides and examined under a confocal laser scanning microscope (Carl Zeiss Jena, Carl Zeiss AG, Oberkochen, Germany). In the controls the primary antibodies were omitted. As shown in the table below, the presence of L-NAME in the maturation medium significantly reduced the cleavage and blastocyst rate independent of the dosage applied. However the presence of L-NAME in the culture medium had an influence only on the blastocyst rate. The immunocytochemical staining results showed that both eNOS and iNOS are expressed in the cytoplasm of the MII oocytes, and during the pre-implantation stage the fluorescence signal was observed in nuclei and cytoplasm. However, the nuclear signal was much weaker. In conclusion, the present study is the first to determine the role of NO and to detect NOS protein in bovine oocytes and pre-implantation embryos. These results indicate that nitric oxide may play an important role as diffusible regulator of bovine oocyte maturation and preimplantation embryo development.
|


