135 REGULATION OF GLUCOSE METABOLISM TO DECREASE LIPID CONTENT OF IN VIRTO-PRODUCED BOVINE EMBRYOS
J. De La Torre-Sanchez A , D. Gardner B , K. Preis B and G. Seidel Jr AA Animal Reproduction and Biotechnology Laboratory, Colorado State University, Fort Collins, CO 80521, USA
B Colorado Center for Reproductive Medicine, Englewood, CO 80110, USA. Email: gseidel@colostate.edu
Reproduction, Fertility and Development 17(2) 218-218 https://doi.org/10.1071/RDv17n2Ab135
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
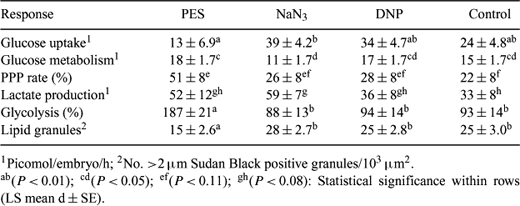
Our objective was to improve normality of embryos produced in vitro with regulators of carbohydrate metabolism at doses optimized in earlier experiments. Eight- to 16-cell embryos were produced in vitro in the G1/G2 system (chemically defined sequential medium with recombinant human serum albumin), and then cultured 3 days in G2 containing metabolic regulators as follows: phenazine ethosulfate (PES), 0.3 μM; NaN3, 27 μM; 2,4-dinitrophenol (DNP), 30 μM; and control. The following responses were analyzed by ANOVA in 2 to 4 replicates of 8–12 embryos each: glucose uptake and metabolism (uptake measured by microfluorometry of medium after incubating an embryo 3 h; metabolism measured as 3H2O released after incubating an embryo 3 h in medium containing 5-3H glucose), % of glucose metabolized via the pentose phosphate pathway (PPP rate), lactate production, glycolysis (% of lactate produced from glucose taken up on a molar basis), lipid accumulation (number of >2 μM Sudan Black B positive granules/103 μm2), % live Day 14 embryos recovered from embryos transferred to recipients at Day 7, and average surface area of embryos collected. In vivo-derived embryos were included as a second control for lipid evaluation. PES-treated embryos had higher glucose metabolism (P < 0.05) and lower glucose uptake (P < 0.01) than embryos in NaN3 and tended to have a higher PPP rate (P < 0.11) than controls; however, glycolysis was higher for PES than other treatments (P < 0.01) (Table 1). Lipid accumulation of embryos from PES was markedly lower than any other in vitro treatments (P < 0.01), but higher than in vivo embryos (3.31 ± 2.78 lipid granules) (P < 0.01). NaN3- and DNP-treated embryos both accumulated lipid similar to in vitro controls. No treatment differences were found in developmental competence when Day 7 embryos were transferred to recipients and recovered 1 week later (43 to 54% live embryos recovered), nor were there any significant differences (P > 0.1) in surface area. Embryos exposed to PES at the compaction and post-compaction stages accumulated much less lipid than controls or embryos exposed to other metabolic regulators, making this a very promising treatment. PES oxidizes NADPH; the molecular mechanism of PES appears to involve increased flux of glucose through the PPP while decreasing availability of NADPH for fatty acid synthesis.
|


