115 SPERM CRYOPRESERVATION IN TRAGELAPHINE ANTELOPES
G. Wirtu A B , C. E. Pope A , A. Cole A , R. A. Godke B C , D. L. Paccamonti B and B. L. Dresser A DA Audubon Center for Research of Endangered Species, New Orleans, LA 70131, USA
B Veterinary Clinical Sciences, LSU School of Veterinary Medicine, Baton Rouge, LA 70803, USA
C Embryo Biotechnology Laboratory, Department of Animal Sciences, Louisiana State University Agricultural Center, Baton Rouge, LA 70803, USA
D Department of Biological Sciences, University of New Orleans, New Orleans, LA 70148, USA
Reproduction, Fertility and Development 18(2) 166-166 https://doi.org/10.1071/RDv18n2Ab115
Published: 14 December 2005
Abstract
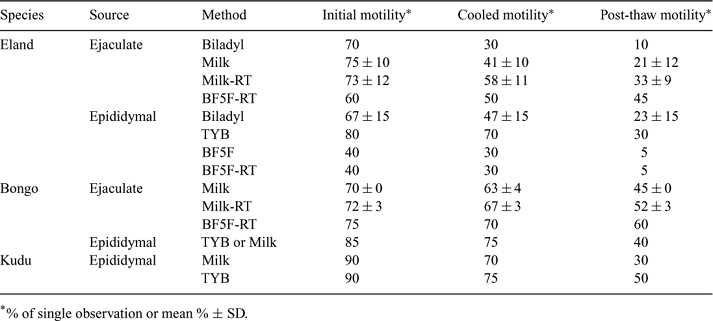
As an integral aspect of our program to develop assisted reproductive technologies in tragelaphine antelopes, we have been evaluating the effects of various extenders on cryosurvival of spermatozoa. Semen was collected by electroejaculation (EEJ) from an eland (Taurotragus oryx, n = 14 EEJs) and a bongo (Tragelaphus euryceros, n = 7 EEJs) male during sedation and standing restraint in a hydraulic chute. Epididymal spermatozoa were collected from the proximal vas deferens, and distal epididymides recovered from four common eland bulls after elective castration at the Hattiesburg Zoo, Mississippi (age = 46 months) and the San Diego Wild Animal Park, California (ages = 18, 20, and 23 months). Testes from a bongo (age = 6.5 yr) and a greater kudu bull (Tragelaphus strepsiceros, age = 7.5 yr) at the Audubon Nature Institute were recovered and processed immediately postmortem. Kudu, bongo, and the Hattiesburg eland testes were transported at ambient temperature whereas the testes from San Diego elands were shipped overnight in a cold-pack container. Sperm processing was done at room temperature (RT = 23°C) in HEPES-buffered Tyrode's solution. Four extenders containing 7% glycerol were evaluated: Biladyl®, TEST Yolk buffer (TYB), Beltsville extender (BF5F), and skim milk. For freezing, sperm samples were initially extended and gradually cooled to 4°C before sequential addition of extender containing glycerol at 4°C; the procedure was then modified by addition of the glycerol fraction at RT before the sample was cooled to 4°C. Spermatozoa were vapor-frozen in 0.5 mL straws placed 5 cm above liquid nitrogen before storage at −196°C. Straws were thawed in a water bath (38°C, 30 s) to evaluate cryosurvival. All eland and bongo electroejaculation procedures produced spermatozoa, although sperm quality varied. Ejaculate volume averaged 5.4 ± 1.2 mL and 3.7 ± 1.1 mL in the bongo and the eland, respectively. Sperm motility at collection, after cooling, and after freezing in the different extenders is presented in Table 1. No immediate decline in sperm motility was observed after adding glycerol to samples, whether at 23°C or 4°C. Bongo spermatozoa lost more motility during freezing than during cooling (15.0 ± 4.4 vs. 5.8 ± 2.0%; P = 0.006); whereas, in eland, motility loss during cooling and freezing was similar (25.3 ± 16.2 vs. 21.1 ± 7.7; P = 0.44). The present results indicate that cooling and freezing tolerance of spermatozoa in tragelaphine antelopes is influenced by species, extender type, and temperature at which a cryoprotectant is added.
|


