Preparation and Characterization of Copper(ii) and Nickel(ii) Complexes with N-Benzyliminodiacetamide Derivatives
Neven Smrečki A B , Vladimir Stilinović A , Maja Merkaš A , Andrea Lučić A , Boris-Marko Kukovec A and Zora Popović AA Laboratory of General and Inorganic Chemistry, Department of Chemistry, Faculty of Science, University of Zagreb, Horvatovac 102a, 10000 Zagreb, Croatia.
B Corresponding author. Email: nsmrecki@chem.pmf.hr
Australian Journal of Chemistry 69(8) 896-904 https://doi.org/10.1071/CH16046
Submitted: 26 January 2016 Accepted: 25 February 2016 Published: 12 April 2016
Abstract
The reactions of substituted N-benzyliminodiacetamides (o-CH3Bnimda, m-CH3Bnimda, p-CH3Bnimda, o-ClBnimda, m-ClBnimda, p-ClBnimda, p-FBnimda, and p-BrBnimda; o-CH3Bn = ortho-methylbenzyl, m-CH3Bn = meta-methylbenzyl, p-CH3Bn = para-methylbenzyl, o-ClBn = ortho-chlorobenzyl, m-ClBn = meta-chlorobenzyl, p-ClBn = para-chlorobenzyl, p-FBn = para-fluorobenzyl, and p-BrBn = para-bromobenzyl) with copper(ii) and nickel(ii) nitrate in aqueous solutions were investigated. Sixteen new complexes [Cu(o-CH3Bnimda)2](NO3)2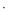 0.5H2O (1), [Cu(m-CH3Bnimda)2](NO3)2 (2), [Cu(p-CH3Bnimda)2](NO3)2
0.5H2O (1), [Cu(m-CH3Bnimda)2](NO3)2 (2), [Cu(p-CH3Bnimda)2](NO3)2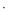 2H2O (3), [Cu(o-ClBnimda)2](NO3)2
2H2O (3), [Cu(o-ClBnimda)2](NO3)2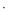 H2O (4), [Cu(m-ClBnimda)2](NO3)2 (5), [Cu(p-ClBnimda)2](NO3)2
H2O (4), [Cu(m-ClBnimda)2](NO3)2 (5), [Cu(p-ClBnimda)2](NO3)2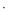 2H2O (6), [Cu(p-FBnimda)2](NO3)2
2H2O (6), [Cu(p-FBnimda)2](NO3)2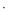 2H2O (7), [Cu(p-BrBnimda)2](NO3)2
2H2O (7), [Cu(p-BrBnimda)2](NO3)2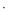 2H2O (8), [Ni(o-CH3Bnimda)2](NO3)2
2H2O (8), [Ni(o-CH3Bnimda)2](NO3)2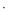 H2O (9), [Ni(m-CH3Bnimda)2](NO3)2
H2O (9), [Ni(m-CH3Bnimda)2](NO3)2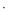 2H2O (10), [Ni(p-CH3Bnimda)2](NO3)2
2H2O (10), [Ni(p-CH3Bnimda)2](NO3)2 H2O (11), [Ni(o-ClBnimda)2](NO3)2 (12), [Ni(m-ClBnimda)2](NO3)2
H2O (11), [Ni(o-ClBnimda)2](NO3)2 (12), [Ni(m-ClBnimda)2](NO3)2 2DMF (13) (DMF = N,N-dimethylformamide), [Ni(p-ClBnimda)2](NO3)2
2DMF (13) (DMF = N,N-dimethylformamide), [Ni(p-ClBnimda)2](NO3)2 2H2O (14), [Ni(p-FBnimda)2](NO3)2
2H2O (14), [Ni(p-FBnimda)2](NO3)2 H2O (15), and [Ni(p-BrBnimda)2](NO3)2
H2O (15), and [Ni(p-BrBnimda)2](NO3)2 2H2O (16) were prepared and characterized by infrared spectroscopy and thermal analysis (thermogravimetric and differential thermal analyses). The molecular and crystal structures of three complexes (2, 5, and 13) were determined by X-ray crystal structure analysis. The octahedral coordination environments around the copper(ii) and nickel(ii) ions in complexes 2, 5, and 13 consist of two O,N,O'-tridentate iminodiacetamide ligands. The complexes are trans-isomers with fac-configuration of the chelators.
2H2O (16) were prepared and characterized by infrared spectroscopy and thermal analysis (thermogravimetric and differential thermal analyses). The molecular and crystal structures of three complexes (2, 5, and 13) were determined by X-ray crystal structure analysis. The octahedral coordination environments around the copper(ii) and nickel(ii) ions in complexes 2, 5, and 13 consist of two O,N,O'-tridentate iminodiacetamide ligands. The complexes are trans-isomers with fac-configuration of the chelators.
References
[1] N. Smrečki, B.-M. Kukovec, M. Đaković, Z. Popović, Polyhedron 2015, 93, 106.| Crossref | GoogleScholarGoogle Scholar |
[2] J.-W. Ran, J. Pei, Acta Crystallogr. 2013, E69, m325.
| Crossref | GoogleScholarGoogle Scholar |
[3] X.-H. Deng, Q.-J. Nie, F.-J. Zhu, Acta Crystallogr. 2013, E69, m89.
| Crossref | GoogleScholarGoogle Scholar |
[4] M. Sekizaki, Bull. Chem. Soc. Jpn. 1974, 47, 1447.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXks12ltb8%3D&md5=9db68bdf402970014f67bb9fc5af2801CAS |
[5] M. Sekizaki, Acta Crystallogr. 1976, B32, 1568.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XksFCqtr4%3D&md5=9a1620f602675a78a92900767bcb2013CAS |
[6] M. Sekizaki, Bull. Chem. Soc. Jpn. 1981, 54, 3861.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xnsl2jug%3D%3D&md5=45e03644831711411a7fa4439ff3e1f2CAS |
[7] L. A. Clapp, C. J. Siddons, D. G. Van Deerver, J. H. Reibenspies, S. B. Jones, R. D. Hancock, Dalton Trans. 2006, 2001.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjtlCisrg%3D&md5=1c3a726271e85d16613d34bd52ea0b22CAS | 16609771PubMed |
[8] D. A. Smith, S. Sucheck, A. A. Pinkerton, J. Chem. Soc., Chem. Commun. 1992, 367.
| Crossref | GoogleScholarGoogle Scholar |
[9] E. S. Claudio, M. A. Horst, C. E. Forde, C. L. Stern, M. K. Zart, H. A. Godwin, Inorg. Chem. 2000, 39, 1391.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhsV2qur0%3D&md5=1df00eb379764baed84787ed801f190bCAS | 12526441PubMed |
[10] L. A. Clapp, C. J. Siddons, J. R. Whitehead, D. G. Van Derveer, R. D. Rogers, S. T. Griffin, S. B. Jones, R. D. Hancock, Inorg. Chem. 2005, 44, 8495.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFSjt7zE&md5=27b224307345225e06aad2561f456eb7CAS | 16270989PubMed |
[11] D. Burdinski, J. A. Pikkemaat, J. Lub, P. de Peinder, L. N. Garrido, T. Weyhemuller, Inorg. Chem. 2009, 48, 6692.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvFOju7k%3D&md5=eb7260b2f96d9300131f2fc84587339cCAS | 19507818PubMed |
[12] K. Krot, A. L. Liamas-Saiz, N. Vembu, K. B. Nollan, Z. Anorg. Allg. Chem. 2007, 633, 1900.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVynu7nP&md5=f7f459d266dbb346314bae78bdae989cCAS |
[13] E. Skrzypczak-Jankun, D. A. Smith, Acta Crystallogr. 1994, C50, 1585.
| 1:CAS:528:DyaK2MXhvVCitLk%3D&md5=bb16ad1b82b99ed9735dac249817c473CAS |
[14] M. C. Etter, J. C. MacDonald, J. Bernstein, Acta Crystallogr. 1990, B46, 256.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXisVentL0%3D&md5=9e4a667e8636f1d9e72a1d74c384f612CAS |
[15] R. M. Silverstein, F. X. Webster, D. Kiemle, Spectrometric Identification of Organic Compounds, seventh edition 2005 (Wiley: New York, NY).
[16] K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, sixth edition 2009 (Wiley: Hoboken).
[17] E. Müller, O. Bayer, H. Meerwein, K. Ziegler, Methoden der Organischen Chemie (Houben-Weyl), Band XI/1 – Stickstoffverbindungen II 1957 (Georg Thieme Verlag: Stuttgart).
[18] W. A. Jacobs, M. Heidelberger, J. Biol. Chem. 1915, 20, 659.
| 1:CAS:528:DyaC2MXhtVertQ%3D%3D&md5=9dcc68d59f01128aeb35077c7758a25eCAS |
[19] Oxford Diffraction CrysAlis CCD and CrysAlis RED version 1.171.32.29 2008 (Oxford Diffraction Ltd: Abingdon, Oxfordshire, England).
[20] Oxford Diffraction CrysAlisPro Version 1.171.33.55 2010 (Oxford Diffraction Ltd: Abingdon, Oxfordshire, England).
[21] G. M. Sheldrick, Acta Crystallogr. 2008, A64, 112.
| Crossref | GoogleScholarGoogle Scholar |
[22] A. L. Spek, J. Appl. Crystallogr. 2003, 36, 7.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltlChtw%3D%3D&md5=fef1b94db322ce3c12ee7ccdd0c49499CAS |


