
Australian Journal of Chemistry
Volume 67 Numbers 8 & 9 2014
Dedication to Roger F. C. Brown

The foreword to this special issue dedicated to Professor Roger F. C. Brown provides a biographical introduction, an account of his scientific education in the 1950s, and a selection of research contributions from 1962. It highlights his work on flash vacuum pyrolysis, the study of reactive intermediates, and some natural product syntheses.
Ian Rae, Roger Brown’s first Ph.D. student, explored dithiole chemistry but failed to synthesise thiolutin. Now a historian of chemistry, he recounts the story of J. F. G. Fletcher, who returned to Australia in 1919 after munitions work in Britain, dreaming of the synthesis of indigo from naphthalene. He succeeded, but was unable to commercialise the process.
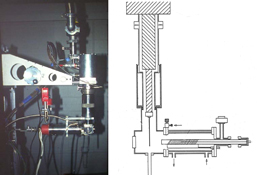
Types of pyrolysis equipment, methods, and reactions are narrated including flash vacuum pyrolysis (FVP) for preparative or spectroscopic use, very low pressure pyrolysis, laser pyrolysis, pulsed pyrolysis, solvent spray pyrolysis, pipto-pyrolysis (‘falling solid pyrolysis’), vacuum gas–solid reactions, microwave and flash-flow pyrolysis..
CH14105Recent Studies on Flash Vacuum Thermolysis in Tandem with UV-Photoelectron Spectroscopy and Quantum Calculations
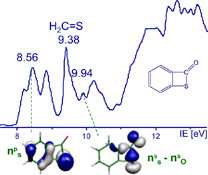
Flash vacuum thermolysis, in tandem with UV-photoelectron spectroscopy and quantum calculations, is a very useful tool for mechanistic investigations and electronic structure studies.
CH13670C8H6 Thermal Chemistry. 7-Methylenecyclohepta-1,3,5-dienyne (Heptafulvyne) by Flash Vacuum Thermolysis–Matrix Isolation. Chemical Activation in the Rearrangements of Phenylenedicarbenes and of Benzocyclobutadiene to Phenylacetylene
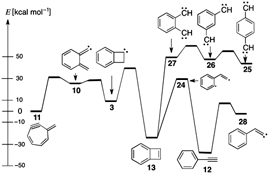
Methylenecycloheptadienyne 11 (heptafulvyne) is obtained very cleanly by flash vacuum thermolysis (FVT), but its facile thermal rearrangement to phenylacetylene 12 and benzocyclobutadiene 13 is ascribed to chemical activation, which is also invoked in the rearrangement of p-, m-, and o-phenylenebiscarbenes 25–27 to phenylacetylene 12.
CH14071A Critical Investigation on the Existence of Selective Microwave Absorption in the Synthesis of CdSe Quantum Dots
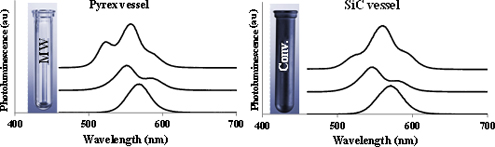
In recent years, selective microwave heating of specific reagents has been reported several times in the literature for microwave-assisted synthesis of different nanoparticles. In the current study, the existence of selective microwave absorption in the synthesis of CdSe quantum dots using silicon carbide reactor technology as a control experiment was investigated and no evidence for this phenomenon was observed.
CH14030Development of the Claisen Rearrangement/Organocatalytic Diels-Alder Approach for the Synthesis of Eunicellins
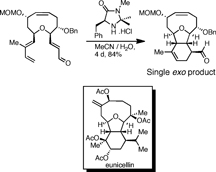
The tricyclic core of eunicellin natural products was synthesised, employing an exo-selective Diels-Alder cycloaddition catalysed by the MacMillan imidazolidinone catalyst. This approach was further developed to address the issue of hydroxylation at the C8 position, and a novel scalable method for the extension of the medium-ring lactone to a cyclic ether was developed.
CH14098The Elusive Ethenediselone, Se=C=C=Se
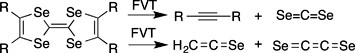
Ethenediselone, Se=C=C=Se, is characterised by neutralisation–reionisation mass spectrometry as a product of flash vacuum thermolysis (FVT) of tetraselenafulvalenes. Alkynes, CSe2, and selenoketene, H2C=C=Se were identified by matrix infrared (IR) spectroscopy. Se=C=C=Se is predicted to be a ground state triplet molecule with a very small singlet-triplet gap.
CH14095Unexpected Products from Mesoionic 1,3-Thiazinium and Oxazinium Olates: A Novel Access to 3,5-Diaryl-1,3-thiazine-2,4,6-trione and Alkoxy-3,5-diphenyl-3H-1,3-oxazine-2,6-dione Derivatives
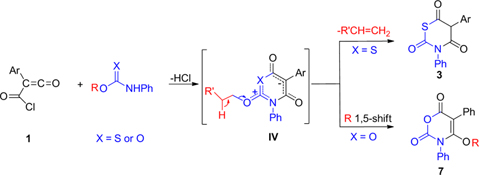
The condensation of (chlorocarbonyl)ketenes 1 with N-phenylthiocarbamates 2 and N-phenylcarbamates 6 is postulated to lead to the formation of unstable mesoionic 1,3-thiazinium 4-olates I or 1,3-oxazinium 4-olates II, respectively.
CH14107Acanthocyclamine A From the Indonesian Marine Sponge Acanthostrongylophora ingens
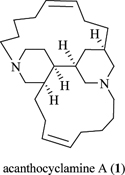
A new 3-alkylpiperidine compound (-) acanthocyclamine A (1) has been obtained from the methanolic extract of Acanthostrongylophora ingens (order Haplosclerida, family Petrosiidae). The structure of 1 was investigated by extensive 1D- and 2D-NMR experiments. The absolute configuration of 1 has been established by X-ray crystallography from anomalous dispersion effects using Cu radiation as C2 (R), C3 (R), C7 (R), and C9 (R). A plausible biosynthetic scheme leading to 1 is presented.
CH14116Synthesis of Indoxylic Acid Esters by Rhodium-catalyzed Carbene N–H Insertion and Thermal Cyclization
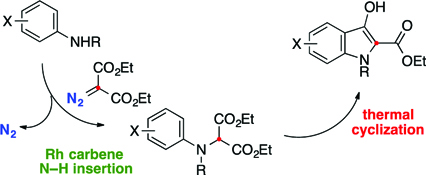
A two-step conversion of anilines into indoxylic acid esters by carbene N–H insertion and thermal cyclization is described.
CH14055Annulation of Eight- to Ten-Membered Oxaza Rings to the Benzo[b]thiophene System by Intramolecular Nucleophilic Displacement
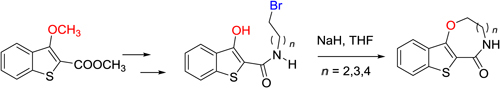
Fused N,O-containing medium-sized ring systems provide significant opportunities as novel scaffolds for pharmaceutical development. Further synthetic methodologies are required to access these systems. Intramolecular nucleophilic displacement is one such methodology which now provides a concise entry to benzo[b]thieno-annulated [1,5]-oxazocinone, oxazoninone, and oxazecinone derivatives.
CH14085Use of Ethyl (Benzothiazol-2-ylsulfonyl)acetate for Malonic Ester-type Syntheses of Carboxylic Acids and Esters
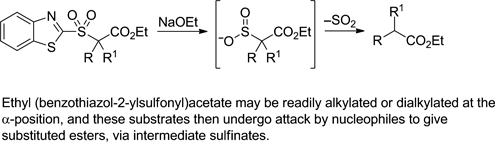
Ethyl (benzothiazol-2-ylsulfonyl)acetate can be readily alkylated or dialkylated at the α-position. The resulting substrates then undergo attack by nucleophiles to give substituted esters via intermediate sulfinates.
CH14119Synthesis of Heterocyclic-fused Imidazoles by Pyrolysis of N-Heterocyclic Isoxazol-5(2H)-ones
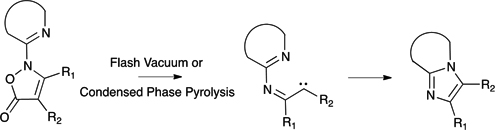
Heterocyclic-fused imidazoles were synthesised by flash vacuum pyrolysis (FVP) and condensed phase pyrolysis of N-heterocyclic isoxazol-5(2H)-ones in good-to-excellent yields.
CH14093Use of Flash Vacuum Thermolysis in a Stereocontrolled Synthesis of Optically Active Alkyl-substituted Cyclopentenones with Fragrant Properties
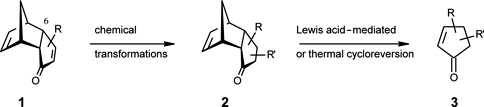
Enantiopure precursors 2 were obtained using enzymatic resolution of 1 with R = 6-CO2Et. The 6-CO2Et was replaced with Br, which upon treatment with Me2CuLi followed by an appropriate electrophile gave a variety of precursors 2. Subsequent FVT gave enantiopure cyclopentenones 3 in high optical and chemical yields.
CH14094Facial Selectivity in the Addition of Lithium Dimethylcuprate to 6-Substituted Tricyclo[5.2.1.02,6]deca-4,8-dienones. Synthesis of β-Substituted Cyclopentenones Using Flash Vacuum Thermolysis
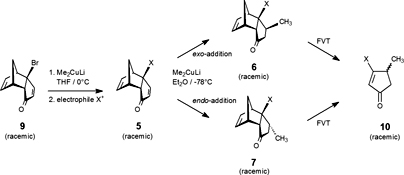
The stereoselectivity of the nucleophilic addition of lithium dimethylcuprate and lithium di-n-pentylcuprate to 6-substituted tricyclo[5.2.1.02,6]deca-4,8-dienones was investigated. Depending on the nature of the substituent at C-6, in some cases complete endo selectivity was observed, whilst in other cases the exo–endo ratio varied. The results were rationalised in terms of steric and/or electronic effects. Flash vacuum thermolysis of the products obtained gave β-substituted cyclopentenoids.
CH14191An Unexpected Coupling Reaction of 8-Quinolinolate at Elevated Temperature
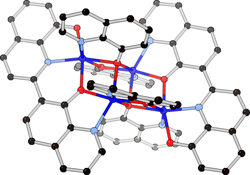
Reactions of 8-hydroxyquinoline (HOQ) with elemental rare-earth and transition metal combinations or alloys at 200–300°C yield a variety of complexes containing an unexpected 2,7′-biquinoline-8,8′-diolate ligand.
CH14121The Preparation of Macrocyclic Calpain Inhibitors by Ring Closing Metathesis and Cross Metathesis
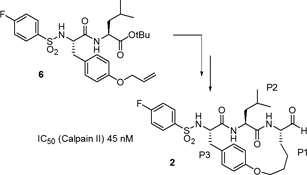
Two approaches to a new macrocyclic calpain inhibitor are reported. The inhibitor contains an N-terminal 4-fluoro-phenyl-sulfonyl group found in acyclic inhibitors and its backbone is constrained into a β-strand geometry known to favour active site binding. Compound 2 is a potent inhibitor of calpain II.
CH14128Crown Ether Derivatised Pyromellitic Diimides
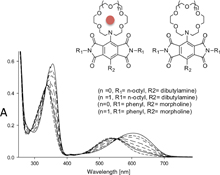
Pyromellitic diimide functionalised azacrown ethers that show dramatic changes in their colour by the addition of alkali metal have been investigated.
CH14135Synthesis, Structures, and Conformations of Linked Bis-Glyoxylamides Derived from Bis-Acylisatins
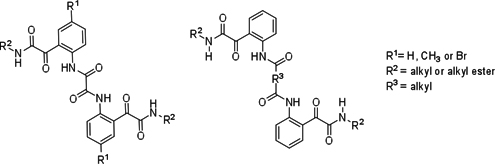
A series of bis-glyoxylamides possessing hydrophobic alkyl chains was successfully synthesised by ring opening of bis-acylisatins with amines or amino acid alkyl esters. The crystal structures revealed the interplay of intra- and intermolecular interactions (NH···O and C=O···C=O interactions) and the conformations of these long molecules.
CH14171Probing Mechanisms of Aryl–Aryl Bond Cleavages under Flash Vacuum Pyrolysis Conditions
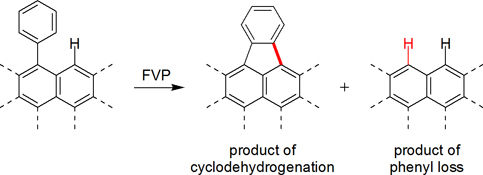
Flash vacuum pyrolysis (FVP) of phenyl-substituted polycyclic aromatic hydrocarbons (PAHs) promotes the formation of new rings by thermal cyclodehydrogenation reactions; however, such reactions are often accompanied by competitive loss of the phenyl group. Such ‘phenyl loss’ reactions cleave strong aryl–aryl bonds and are therefore important to understand. As probes for the mechanism(s) involved in the ‘phenyl loss’ reaction, FVP experiments were conducted with several phenyl-substituted PAHs and larger biaryls.
CH14155Unexpected Pyrolytic Behaviour of Substituted Benzo[c]thiopyran and Thieno[2,3-c]thiopyran S,S-dioxides
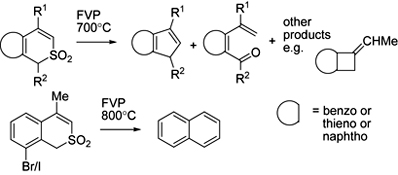
Flash vacuum pyrolysis of eight variously substituted ring-fused thiopyran S,S-dioxides results mainly in fragmentation via loss of SO from ring-expanded 7-membered ring sultines and subsequent rearrangement of the resulting diradicals to give a variety of novel products.
CH14244Origins of Stabilization and Evidence for Charge Delocalization in the Bicyclo[3.2.1]octadienyl Anion and Related Species
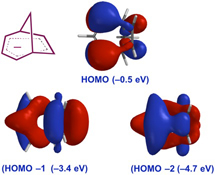
The status quo for stabilization of cyclopentadiene-related bridged allylic anions has been assessed and new DFT calculations carried out to facilitate comparisons between different systems. This work supports the presence of homoaromaticity in the bicyclo[3.2.1]octadienyl anion and closely related species, augmented by inductive effects and ion-pairing.
CH14238Microwave Flash Pyrolysis: C9H8 Interconversions and Dimerisations
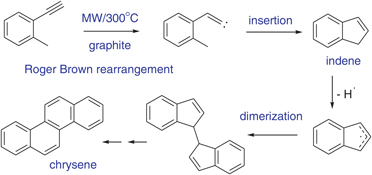
Roger Brown rearrangement of 2-ethynyltoluene leads to indene, which dimerises to chrysene. A similar dimerisation pathway is demonstrated for fluorene.
CH14217Nonstabilised Azomethine Ylids from N-Oxides: Unravelling the Deprotonation of N-Methylmorpholine N-Oxide
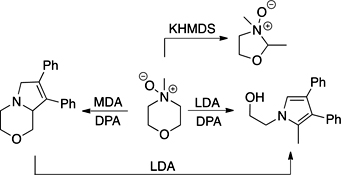
NAY SAYers. Those wishing to utilise nonstabilised azomethine ylids have the right to complain about reaction condition choice. Higher temperature conditions predominate over lower temperature conditions, which limits the application of azomethine cycloadditions to robust dipolarophiles. We present a mechanistic analysis of the application of the Roussi reaction for low-temperature generation of nonstabilised azomethine ylids to the challenging substrate N-methylmorpholine N-oxide.
CH14227Major Differences Between Mononuclear and Binuclear Manganese Carbonyl Cyanides and Isoelectronic Binary Chromium Carbonyls Arising from Basicity of the Cyanide Nitrogen Atom
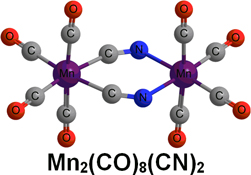
The C-bonded cyanide structure Mn(CO)5(CN) is preferred energetically by ~60 kJ mol–1 over the N-bonded isocyanide structure Mn(CO)5(NC). Two end-to-end bridging µ-CN groups are found in all of the low-energy Mn2(CO)n(CN)2 structures (n = 8, 7).
CH14149Pyridyl- and Pyridylperoxy Radicals – A Matrix Isolation Study
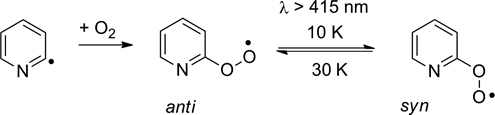
The three isomeric pyridyl radicals were synthesised by flash vacuum pyrolysis and their reaction with oxygen investigated by matrix isolation spectroscopy.
CH14057Solvatochromism in Diketopyrrolopyrrole Derivatives: Experimental and Computational Studies
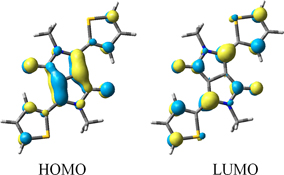
Negative solvatochromism in substituted derivatives of diketopyrrolopyrrole arises from significant charge transfer existing in the ground states of these molecules that is reduced on excitation.
CH14289Use of External Radical Sources in Flash Vacuum Pyrolysis to Facilitate Cyclodehydrogenation Reactions in Polycyclic Aromatic Hydrocarbons
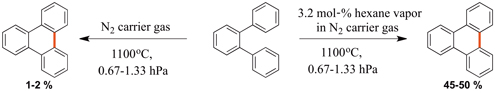
Flash vacuum pyrolysis (FVP) has become an integral part of the synthesis of both flat and curved polycyclic aromatic hydrocarbons (PAHs). We report a new process to facilitate the cyclodehydrogenation of PAHs using an external radical source. This process has been shown to greatly increase the conversion of PAHs to their cyclodehydrogenated products.
CH14270Studies of the Structure, Amidicity, and Reactivity of N-Chlorohydroxamic Esters and N-Chloro-β,β-dialkylhydrazides: Anomeric Amides with Low Resonance Energies
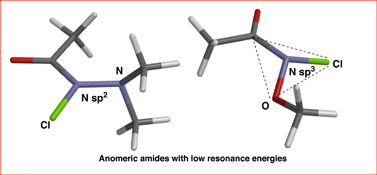
DFT with dispersion correction and expanded basis set computes N-chloro-N-dimethylaminoacetamide to be completely planar but with only 63 % the resonance energy of N,N-dimethylacetamide. N-chloro-N-methoxyacetamide has a much lower resonance (45 %) and it is pyramidal at nitrogen. These are unusual anomeric amides.
CH14211Enantioselective Pd-Catalysed Deallylative γ-Lactonisation of Propargyl Carbazolone Allyl Carbonates: Mechanistic Insight into their Decarboxylative Allylation
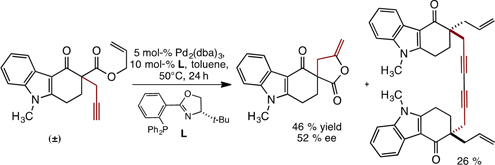
Subjection of N-methyl carbazolone allyl carbonates bearing a propargyl side chain to Pd0 catalysis leads to the formation of enantioenriched γ-lactones, rather than the expected products of decarboxylative allylation. Following lactonisation, the Pd0 catalyst is regenerated by PdII reductive alkyne coupling.
CH14176Functionalised 1-Alkynylarsines: Synthesis, Characterisation, and Attempts of Rearrangement into Functionalised Arsaalkynes

Functionalised 1-alkynylarsines, potential precursors of functionalised arsaalkynes, have been prepared and characterised by 1H and 13C NMR spectroscopy.
CH14242Solvent-Free, Microwave-Assisted Conversion of Tosylates into Iodides
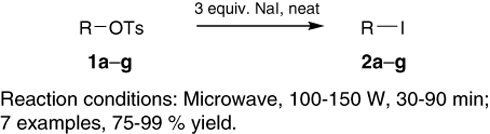
A highly efficient method for the conversion of tosylates into the corresponding iodides is outlined, involving heating a neat mixture of tosylates and sodium iodide in a microwave cavity. This procedure is especially useful for the in situ generation of volatile primary iodides.
CH14220Remembering Roger F. C. Brown: A Personal Note on the Development of Pyrolysis as a Synthetic and Mechanistic Technique in Organic Chemistry
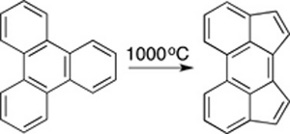
A personal note in honour of Professor Roger F. C. Brown with some memories of collaboration in the field of flash vacuum pyrolysis.



