102 VITRIFICATION OF IN VITRO-PRODUCED BOVINE AND OVINE EMBRYOS USING THE MINIMUM VOLUME COOLING CRYOTOP METHOD
J.M. Kelly A , D.O. Kleemann A , M. Kuwayama B and S.K. Walker AA South Australian Research and Development Institute, Adelaide, Australia email: kelly.jen@saugov.sa.gov.au;
B Kato Ladies’ Clinic, Tokyo, Japan.
Reproduction, Fertility and Development 16(2) 172-173 https://doi.org/10.1071/RDv16n1Ab102
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
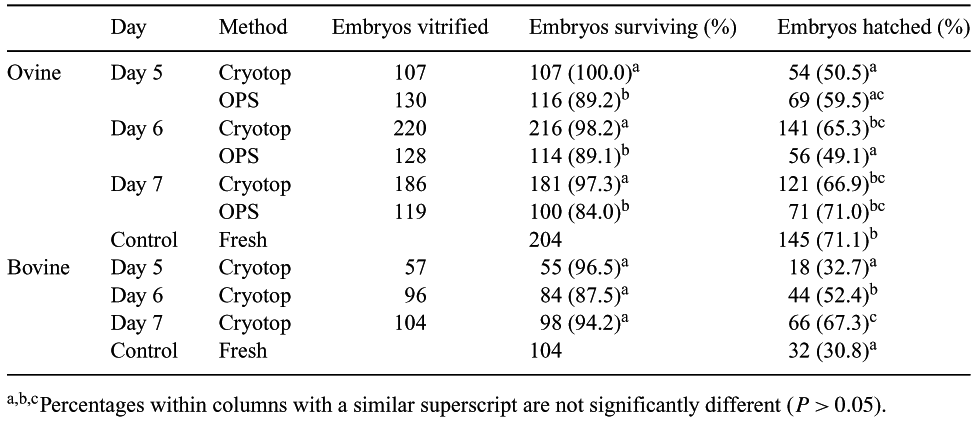
Considerable progress has been achieved in the cryopreservation of mammalian embryos. The use of vitrification minimizes chilling injuries by increasing cooling and warming rates. This study assesses the effect of vitrification using the minimum volume cooling (MVC) method (Kuwayama & Kato 2000 J. Assist. Reprod. Genet. 17, 477) on in vitro-produced bovine and ovine embryos. A total of 1756 ovine and 753 bovine cumulus-oocyte complexes were obtained from the abattoir and matured, fertilized (Day 0) and cultured in vitro (Walker et al., 1996 Biol. Reprod. 55, 703–708, Kelly et al., 1997 Theriogenology 47, 291). Overall cleavage rates were 93.7% and 80.5% respectively. Embryos were vitrified (OPS or MVC method) on Days 5 (morula, compact morula), 6 (expanded blastocyst, blastocyst, compact morula) or 7 (hatched and hatching blastocysts, expanded blastocyst, blastocyst). Embryos were equilibrated with 7.5% ethylene glycol (EG) and 7.5% dimethyl sulfoxide (DMSO) for 3 min and then exposed to 16.5% EG, 16.5% DMSO, 0.5 M sucrose and 20% FCS for 30 s. Embryos were loaded onto either an MVC plate (Cryotop, Kitazato Supply Co, Toyko, Japan) or open pulled straw (OPS) and plunged into liquid nitrogen. After 5 days, embryos were thawed directly into 1.25 M sucrose solution at 38.5°C, followed by stepwise dilution of the cryoprotectants. Embryo survival was assessed by culture to Day 8 and compared to the development of non-vitrified control embryos (Table 1). Variables were assessed using procedure CATMOD in SAS. The Cryotop method yielded a significantly higher percentage of viable ovine embryos after thawing compared with OPS (P < 0.0001); neither day nor treatment x day interaction was significant (P > 0.05). A significant interaction between vitrification treatment and day (P < 0.007) indicated that the percentage of hatched embryos peaked at Day 6 using the Cryotop method compared with Day 7 for OPS. Hatching rates for fresh and vitrified embryos were similar at Day 7 and were independent of treatment. With the Cryotop method, day of vitrification did not influence the percentage of Days 6 and 7 bovine embryos that hatched after thawing but, on each day, this figure was significantly higher (P < 0.003 and P < 0.0001, respectively) than that obtained with fresh embryos. To further assess embryo viability, 36 fresh, 52 OPS and 56 Cryotop vitrified Day-6 in vitro-produced ovine embryos were transferred to synchronized recipients. Survival rates to Day 13 were 29/33 (87.9%), 23/36 (63.9%) and 42/51 (82.4%), respectively (P < 0.05). This study demonstrates that using the MVC Cryotop method, the viability of vitrified embryos, as assessed at Days 8 and 13, is similar to that obtained with fresh embryos.
|


