143 IN VITRO DEVELOPMENT OF OVINE OOCYTES FROM EWES OF CONTRASTING VITAMIN B12 STATUS
L.M. Mitchell A , G. McCallum A , K. Mackie A , M. Ewen A , A. Ainslie A and T.G. McEvoy AAScottish Agricultural College, Aberdeen, AB21 9YA, UK. Email: l.mitchell@ab.sac.ac.uk
Reproduction, Fertility and Development 17(2) 222-222 https://doi.org/10.1071/RDv17n2Ab143
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
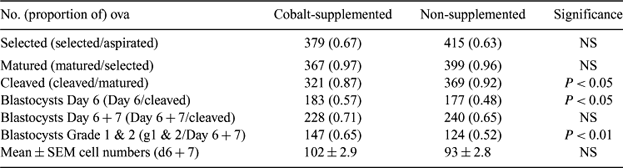
Suboptimal circulating concentrations of vitamin B12 are commonly found in cattle and sheep grazing cobalt-deficient pastures. Vitamin B12 is a co-factor for enzymes involved in energy metabolism (methylmalonyl CoA mutase) and DNA synthesis/methylation (methionine synthase), and vitamin B12 status may therefore impact on cell division and gene expression in early embryos. The aim of this study was to determine the effect of vitamin B12 status on the in vitro development of ovine oocytes to the blastocyst stage. Mature Scottish Blackface ewes from cobalt-deficient farms were housed for ∼ four months and fed a cobalt-deficient diet (0.06 mg cobalt kg DM−1). At housing, 55 of the ewes were given an intra-ruminal slow-release cobalt bolus to compensate for the dietary deficit, and 52 remained untreated. The ovaries of all ewes were recovered at slaughter within the natural breeding season. Oocytes were aspirated and those with evenly granulated cytoplasm and >3 layers of cumulus cells were pooled according to ewe cobalt treatment, matured, fertilized, and cultured in vitro (∼20 oocytes per 50-μL drop under mineral oil). Oocytes were matured for 24 h in M199 + 10% fetal calf serum at 38.5°C in a humidified atmosphere of 5% CO2 in air prior to co-incubation for 18 h with frozen-thawed semen from a single ejaculate (1 × 106 live sperm mL−1). Presumptive zygotes were cultured for 7 Days in synthetic oviduct fluid + 0.4% fatty acid-free BSA (5% CO2, 5% O2, 90% N). Blastocysts formed at the end of the culture period were fixed and stained (Hoechst 33258) to count cell numbers. Data were analyzed by ANOVA and chi-square. For cobalt-supplemented and non-supplemented ewes, circulating concentrations of vitamin B12 at the time of slaughter were 1244 ± 52.5 and 372 ± 27.9 pmol L−1 (P < 0.001), respectively. Numbers of small (<5 mm) follicles per ewe were 17.6 ± 1.22 and 17.1 ± 1.31, and large (>5 mm) follicles per ewe were 1.8 ± 0.16 and 1.6 ± 0.18, respectively (NS). Cobalt-supplemented ewes yielded a lower proportion of matured oocytes that cleaved but an increased proportion of cleaved oocytes that formed blastocysts by Day 6 of the culture period (Table 1). The proportion of grade 1 and 2 blastocysts was also increased but cobalt treatment did not affect blastocyst cell numbers. In conclusion, results suggest that cleaved eggs derived from ewes of adequate, compared to suboptimal vitamin B12 status have improved developmental competence in vitro.
|
This work was funded by the Scottish Executive Environment and Rural Affairs Department.


