137 EFFECT OF POST-IVF DEVELOPMENTAL KINETICS ON SURVIVAL AND QUALITY OF VITRIFIED–WARMED DOMESTIC CAT BLASTOCYSTS
T. Tsujioka A , C. Otzdorff B , J. Braun B and S. Hochi AA Graduate School of Science and Technology, Shinshu University, Nagano, Japan
B Veterinary College, Ludwig-Maximilians-Universität München, München, Germany
Reproduction, Fertility and Development 19(1) 186-186 https://doi.org/10.1071/RDv19n1Ab137
Submitted: 12 October 2006 Accepted: 12 October 2006 Published: 12 December 2006
Abstract
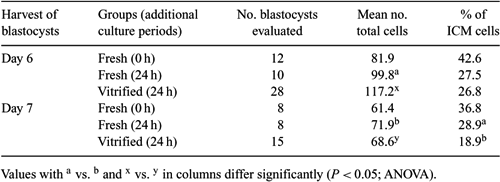
A limited number of reports are available for cryopreservation of IVF-derived cat blastocysts (Karja et al. 2006 Theriogenology 65, 415–423). In the present study, the IVF-derived domestic cat blastocysts exhibiting differences in their developmental kinetics were cryopreserved by vitrification. Pre- and post-warm blastocysts were examined for their cell number, and the survival rate was determined by in vitro culture of the post-warm embryos. Cumulus–oocyte complexes, recovered from the ovaries of post-pubertal queens, were matured for 24 h in TCM-199 supplemented with 3 mg mL−1 BSA, 1 µg mL−1 estradiol, 0.1 IU mL−1 FSH, and 0.0063 IU mL−1 LH. Oocytes were inseminated with 2 × 106 cells mL−1 cauda epididymal spermatozoa for 22 h in TALP solution. Presumptive zygotes were cultured in modified SOF medium at 38.5°C in 5% CO2 in air. Newly formed blastocysts were harvested 6 and 7 days post-IVF and subjected to vitrification (Hochi et al. 2004 Theriogenology 61, 267–275) by a minimum-volume cooling procedure using Cryotop (Kitazato Supply Co., Tokyo, Japan) as a cryodevice. The post-warm blastocysts were cultured for 24 h, and complete re-expansion was considered to be an indicator of survival. Embryos were differentially stained with Hoechst 33342 and propidium iodide to assess the total number of cells and the ICM ratio in the blastocysts. The cleavage rate of oocytes was 47.1% (314/666) and the percentage of oocytes developing to blastocysts on Day 6 and Day 7 was 9.8 and 9.5%, respectively (total blastocyst yield: 19.2%; 128/666). Post-warm in vitro survival rates of blastocysts harvested at Days 6 and 7 were 73.8% (31/42) and 66.7% (18/27), respectively (P = 0.59; exact probability test). There were no significant differences in the total number of cells and the ICM ratio between fresh control and vitrified blastocysts (P ≥ 0.05; ANOVA), although the ICM ratio of surviving Day 7 blastocysts was significantly smaller than that of fresh controls (18.9 vs. 28.9%; P = 0.03), as shown in Table 1. These results indicate that IVF-derived domestic cat blastocysts can survive vitrification by a minimum-volume cooling procedure without a reduction in the parameters studied, as long as the blastocysts are harvested on Day 6.
|


