239 EMBRYOTOXICITY ASSAY FOR ANTIVIRAL COMPOUND BPIP (5-[(4-BROMOPHENYL)METHYL]-2-PHENYL-5H-IMIDAZO[4,5-c]PYRIDINE) IN BOVINE IN VITRO-PRODUCED EMBRYOS
J. Mestach A , J. Paeshuyse B , J. Neyts B , H. J. Nauwynck A , D. Maes A and A. Van Soom AA Faculty of Veterinary Medicine, Merelbeke, Belgium
B Rega Institute for Medical Research, Leuven, Belgium
Reproduction, Fertility and Development 19(1) 235-236 https://doi.org/10.1071/RDv19n1Ab239
Submitted: 12 October 2006 Accepted: 12 October 2006 Published: 12 December 2006
Abstract
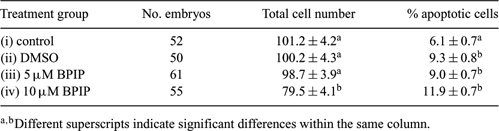
Bovine viral diarrhea virus (BVDV) causes serious economic losses in the cattle industry. Evidence exists that only zona pellucida (ZP)-free bovine embryos are susceptible to BVDV infection (Vanroose et al. 1998 Biol. Reprod. 58, 857–866); however, BVDV can adhere to and therefore ‘infect’ both in vivo-(Waldrop et al. 2004 Theriogenology 62, 387–397) and in vitro-produced ZP intact embryos (Stringfellow et al. 2000 Theriogenology 53, 827–839). To eliminate these sanitary risks, pre-treatment of embryos with antiviral compounds may be a promising approach (Givens et al. 2006 Theriogenology 65, 344–355). BPIP (5-[(4-bromophenyl)methyl]-2-phenyl-5H-imidazo[4,5-c]pyridine) has been reported to display antiviral activity against BVDV, with a 50% effective inhibition of BVDV-induced cytopathic effect formation at a concentration of 0.04 µM (Paeshuyse et al. 2006 J. Virol. 80, 149–160). However, since the short- and long-term effects of BPIP have not been described, the aim of the current study was to assess whether addition of BPIP for 2 days at a concentration of 5 µM is toxic for ZP-free cattle embryos. Oocytes were aspirated from 3–6-mm follicles of cattle ovaries, matured for 24 h, and subsequently co-incubated with 1 × 106 sperm cells mL−1 in IVF-TALP with 20 µg/mL−1 heparin for 24 h at 39°C and 5% CO2 in air. After fertilization, presumptive zygotes were put in groups of 25 into 50-µL droplets of SOF under oil in 5% CO2, 5% O2, and 90% N2 for 6 days. Afterwards, morulae and blastocysts were collected, rendered ZP-free by means of pronase treatment, and divided into 4 groups: (i) ZP-free control group, (ii) ZP-free control group treated with a volume of DMSO equal to condition (iv), (iii) ZP-free group treated with 5 µM BPIP in DMSO, and (iv) ZP-free group treated with 10 µM BPIP in DMSO. Because BPIP is a fat-soluble molecule, embryos were cultured in 0.5 mL SOF without oil for 2 days. At Day 8, all embryos were fixed, TUNEL-stained, and analyzed for total cell number and percentage of apoptotic cells. Three independent replicates were performed. Results are shown in Table 1 and were analyzed by means of ANOVA. Only group iv showed a significant decrease in total cell number, indicating that at 10 µM BPIP may negatively influence embryo development. At both 5 and 10 µM, BPIP treatment resulted in an increase in percentage of apoptotic cells compared to the control group. However, a similar increase was observed using DMSO alone (group ii), indicating that the apoptotic effect may be due solely to the DMSO. In conclusion, BPIP does not appear to cause embryo toxicity at 5 µM, but an alternative, less toxic, dissolving agent may be considered.
|


