382 SYNCHRONIZING ACTIVATION TIME WITH MAXIMUM MATURATION PROMOTING FACTOR AND MITOGEN-ACTIVATED PROTEIN KINASE LEVELS DOES NOT IMPROVE BOVINE INTRACYTOPLASMIC SPERM INJECTION
B. J. Woodward A , W. E. Maalouf A and K. H. S. Campbell AASchool of Biosciences, University of Nottingham, Sutton Bonington, Leics, LE12 5RD, UK
Reproduction, Fertility and Development 19(1) 306-307 https://doi.org/10.1071/RDv19n1Ab382
Submitted: 12 October 2006 Accepted: 12 October 2006 Published: 12 December 2006
Abstract
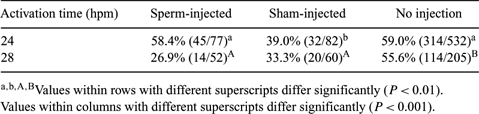
The success of bovine intracytoplasmic sperm injection (ICSI) remains low because of the tightly packed sperm nucleus failing to decondense and the need for additional external activation. Maturation promoting factor (MPF) and mitogen-activated protein kinase (MAPK) assist the breakdown of the nuclear envelope. Activities are high at the early metaphase II (MII) stage but decrease with time. We assessed MPF and MAPK dynamics during the MII stage from 24 h postmaturation (hpm) onward. We then tested the hypothesis that ICSI may be more successful if the external activation is performed following sperm exposure to maximum MPF and MAPK levels. Bovine oocytes were matured in M199 with 10% FCS, 5 µg mL−1 FSH, 5 µg mL−1 LH, 1 µg mL−1 estradiol, and 50 µL mL−1 gentamycin, and cumulus cells were removed at 18 hpm. Groups of 10 MII oocytes were sampled from 24 hpm and analyzed for MPF and MAPK activities as previously described (Ye et al. 2003 Reproduction 125, 645–656). For ICSI, MII oocytes were moved to HSOF supplemented with amino acids and BSA (HSOFaa) and injected with sperm from 19 to 22 hpm. Further MII oocytes were subjected to sham or no injections at this time. Injections were performed manually using a 1480-nm diode laser (XY-clone; Hamilton-Thorne, Beverly, MA, USA) to breach the zona pellucida. Because the maximum kinase activities were reached at 28 hpm, oocytes were activated in 2 groups: at either 24 or 28 hpm, using HSOFaa containing 7% ethanol followed by culture in mSOFaa with 10 µg mL−1 cycloheximide for 6 h, and then transferred to mSOFaa medium until cleavage assessment on Day 2. Differences in the percentage of cleaved oocytes in the different groups were analyzed by a chi-squared test. From 24 to 28 hpm, there was an increase in both MPF (24.6%) and MAPK (58.7%) levels, followed by a slow decline. There were no significant differences in cleavage rates for sham-injected or control oocytes at the different activation times (P < 0.05). However, a significantly higher cleavage rate was observed following ICSI with activation at 24 hpm compared with 28 hpm (P < 0.001), even though kinase levels appeared maximal at 28 hpm. The manual injection procedure appeared detrimental, because oocytes in the control group showed a significantly higher cleavage rate than sham-injected oocytes (P < 0.01). In conclusion, bovine oocytes subjected to ICSI showed a better cleavage rate if external activation was performed at 24 hpm rather than at 28 hpm, even though the MPF and MAPK levels were maximal at 28 hpm (P < 0.001), even though kinase levels appeared maximal at 28 hpm. The manual injection procedure appeared detrimental, because oocytes in the control group showed a significantly higher cleavage rate than sham-injected oocytes (P < 0.01). In conclusion, bovine oocytes subjected to ICSI showed a better cleavage rate if external activation was performed at 24 hpm rather than at 28 hpm, even though the MPF and MAPK levels were maximal at 28 hpm.
|


