211 INTRODUCING NEW GENETICS INTO A CLOSED BIOSECURE HERD OF DAIRY GOATS
D. Melican A , S. Blash A and W. Gavin AGTC Biotherapeutics, Framingham, MA, USA
Reproduction, Fertility and Development 20(1) 185-185 https://doi.org/10.1071/RDv20n1Ab211
Published: 12 December 2007
Abstract
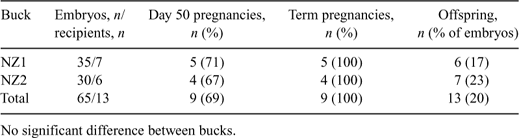
Transgenic dairy goats expressing recombinant molecules in their milk have been validated as a viable method for producing human therapeutic proteins. Although maintaining a closed herd ensures biosecurity within a facility, the ability to introduce new genetics into the herd can be difficult. In this work we determined the ability to use cryopreserved caprine semen, imported from New Zealand into the United States, for IVF as a method to increase the genetic diversity of the GTC Biotherapeutics closed caprine herd. Semen was collected from bucks owned by GTC Biotherapeutics and maintained in New Zealand. The bucks were serologically screened for goat pathogens prior to collection, and were maintained in quarantine during semen collection. Separate single experiments were performed using cryopreserved semen from each of 2 different bucks (NZ1 and NZ2). One or 2 straws of semen (107/0.25 mL straw) from each buck were thawed and then purified using a Percoll gradient. Ovulated oocytes surgically collected from superovulated does were co-incubated with sperm (5 × 105 mL–1) in Brackett-Oliphant medium supplemented with 10% fetal bovine serum, 7.7 mm calcium lactate plus 2.5 μg mL–1 of heparin for 18 h at 38°C. Presumptive zygotes were transferred to equilibrated SOF plus 0.8% BSA and cultured in vitro for 24 h. On Day 2 cleavage was determined and, as an added precaution, embryos selected for transfer were washed per the IETS protocol for the sanitary handling of embryos. Five 2-cell to 8-cell embryos from individual donors were surgically transferred to a single oviduct of each synchronized surrogate recipient. Pregnancies were determined by ultrasonography. Pregnancy rates for recipients at Day 50 of gestation (71 v. 67% pregnant), at term (100 v. 100%), and the proportion of offspring born from total embryos transferred (17 v. 23% offspring) were comparable for buck NZ1 and buck NZ2, respectively (P > 0.05). A total of 13 offspring (6 bucks and 7 does) were produced from 9 different oocyte donors. These results demonstrate that cryopreserved caprine semen, imported from New Zealand into the United States, can be used for IVF to introduce new genetics into a closed biosecure caprine herd. The use of IVF, compared with AI, allows more offspring to be produced per straw of semen. In addition, IVF offers the advantage of accelerated genetic gain by producing multiple offspring from elite does with more desirable lactation, reproduction, and conformation traits. Beyond the new F1 animals produced by IVF, several techniques (natural mating, AI, or IVF) can then be used to quickly disseminate the new genetics into both the nontransgenic and transgenic herds. Finally, skin cells obtained from the female IVF offspring or fetal cells derived from any pregnancies of the initial IVF offspring could also be used to generate transfected cells as karyoplast donors for future somatic cell nuclear transfer work.
|


