272 BIRTH OF A CALF AFTER INTRACYTOPLASMIC SPERM INJECTION WITH FROZEN–THAWED EPIDIDYMAL SPERM FROM A POSTMORTEM BULL
C. A. Guerrero A , J. Smith A , J. W. Lynn B , K. R. Bondioli A and R. A. Godke AA Embyro Biotechnology Laboratory, School of Animal Science, Louisiana State University Agricultural Center, Baton Rouge, LA, USA;
B Department of Biological Sciences, Louisiana State University, Baton Rouge, LA, USA
Reproduction, Fertility and Development 20(1) 216-216 https://doi.org/10.1071/RDv20n1Ab272
Published: 12 December 2007
Abstract
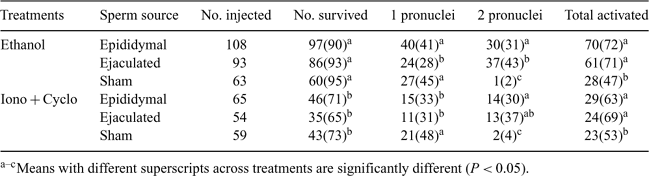
The use of postmortem epididymal sperm for intracytoplasmic sperm injection (ICSI) will allow a more effective use of valuable gametes if a breeding male dies unexpectedly. The objective of this study was to determine pronuclear formation and embryo development rates of frozen–thawed bovine epididymal sperm-injected oocytes. Epididymal sperm were harvested by multiple incisions in the cauda epididymides of an abattoir-derived mature, mixed breed beef bull within 5 h postmortem and frozen in 7% glycerol. Oocytes were matured in vitro for 21 h, selected for extrusion of the first polar body, and centrifuged at 6000g to assist in visualizing the microinjection procedure. Oocytes were injected with either frozen–thawed epididymal sperm, frozen–thawed ejaculated sperm (laboratory control), or were sham-injected (control). Piezo-injected oocytes were chemically activated 4 h post-injection in 7% ethanol for 5 min (Treatment A) or exposure to 5 μm ionomycin for 5 min followed by incubation in 10 μg mL–1 of cycloheximide for 5 h (Treatment B). The sperm-injected oocytes were cultured in CR1aa medium from day 0 to day 3 post-injection and then in CR1aa medium supplemented with 5% fetal bovine serum from day 3 to day 8 of in vitro culture. Pronuclear formation was assessed 18 to 20 h after sperm injection. A summary of oocyte activation by treatments indicated that ethanol was more successful than the ionomycin + cycloheximide treatment (Table 1). Cleavage and blastocyst rates were assessed on day 3 and day 8 of culture, respectively. A significantly higher (P ≤ 0.05) fertilization rate was achieved when ejaculated (43%) rather than epididymal (31%) sperm was used in the ICSI procedure. However, this difference in fertilization rate was only noted when ethanol was used for the exogenous activation. Furthermore, the blastocyst rate for epididymal sperm-injected oocytes was significantly greater when using ethanol (14%) compared with ionomycin followed by cycloheximide (4%). The birth of a live bull calf (42.2 kg; 292-day gestation) resulted from the nonsurgical transfer of 2 ethanol-activated Grade 1 day ICSI blastocysts into each of 2 beef recipient females (50%). To our knowledge, this is the first calf produced by piezo ICSI using cryopreserved bovine caudal epididymal sperm. We can conclude that postmortem epididymal sperm can be collected from genetically valuable males and used for the production of offspring using piezo ICSI.
|


