299 BIOACTIVE, BACTERIAL-DERIVED RECOMBINANT BOVINE FOLLICLE-STIMULATING HORMONE
M. E. Wilson A , J. C. Morris A and J. R. Gibbons AClemson University, Clemson, SC, USA
Reproduction, Fertility and Development 21(1) 246-247 https://doi.org/10.1071/RDv21n1Ab299
Published: 9 December 2008
Abstract
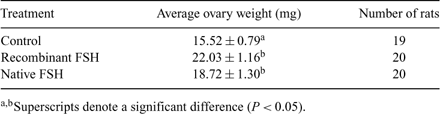
Follicle-stimulating hormone is a pituitary-derived glycoprotein hormone comprising an α and β subunit produced from different genes. A suitable recombinant form of FSH is not readily available in the cattle industry, but would completely revamp current superovulation protocols and eliminate any opportunity for disease transmission. It is widely believed that post-translational glycosylations to the FSH molecule are important for in vivo half-life and must be present on the recombinant form to be most effective as a treatment. There is also speculation that this recombinant protein must be made in a tethered fashion to be most effective; furthermore, there is concern about which subunit must be engineered first in the fused product, and whether there should be some sort of linker sequence between the two subunits. The objective of this research was to produce a tethered, recombinant bovine FSH from Escherichia coli and evaluate its bioactivity compared with a marketed native FSH preparation. This recombinant protein has the β subunit fused upstream of the α subunit with no linker sequence. The pQE30 expression system was used to clone the desired gene, followed by protein expression induced by 1 m IPTG in M15pREP4 E. coli cells. The FSH protein was purified by using a C-terminal 6-histidine tag (provided by the pQE30 plasmid) in conjunction with nickel affinity chromatography. Subsequently, the Steelman-Pohley assay was used to evaluate bioactivity; briefly, 20-day-old Sprague-Dawley female rats were given 3 i.p. injections of a saline (used as a control), native, or bacterial recombinant FSH treatment every 24 h. Additionally, each treatment contained 50 μg of human chorionic gonadotropin. The 2 FSH treatments included the same amount of protein on a dry weight basis over the 3 injections adjusted for predicted FSH protein purity. Rats were sacrificed and ovaries were weighed 72 h after the first injection. Average ovarian weights were compared using a Student’s t-test and significance was defined as P < 0.05 (see Table 1). Results indicated that the recombinant FSH treatment group and the native FSH treatment group had heavier ovaries compared with the controls, but the recombinant FSH did not differ from the native FSH. Representative nonadjacent, midcortical ovarian histological sections (12 sections per treatment group) showed 5.6 ± 0.9, 11.3 ± 1.8, and 8.1 ± 1.6 antral follicles greater than 150 μm (mean ± SEM) for the control, recombinant FSH, and native FSH treatments, respectively; in addition, 0.8 ± 0.2, 1.2 ± 0.4, and 0.7 ± 0.2 corpa lutea were observed for the control, recombinant FSH, and native FSH treatments, respectively. These results suggest that a nonglycosylated bacterial-derived form of recombinant bovine FSH may be used to stimulate ovarian development in rats; however, more experiments must be conducted to evaluate in vivo half-life, bioactivity dynamics, and ultimately the capacity of this FSH preparation as a hyperstimulatory drug.
|


