69 A HOMEMADE N2 SUPERCOOLING DEVICE (NITROCOOLER) ENHANCES VIABILITY AFTER VITRIFICATION OF BOVINE OOCYTES BUT NOT OF IN VITRO-PRODUCED EMBRYOS
J. C. Mezzalira A , A. D. Vieira A , G. Zanardi A , M. C. Gonçalves A , M. F. Rodrigues A , L. T. Martins A , L. U. Ohlweiler A , M. Bertolini A and A. Mezzalira ACenter of Agroveterinarian Sciences, Santa Catarina State University, Lages, SC, Brazil
Reproduction, Fertility and Development 21(1) 134-135 https://doi.org/10.1071/RDv21n1Ab69
Published: 9 December 2008
Abstract
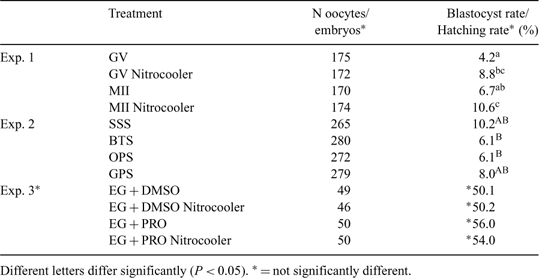
Increasing the cooling rate is a common strategy to enhance vitrification efficiency of bovine oocytes and in vitro-produced (IVP) embryos. Under vacuum conditions, liquid N2 (LN2) temperature decreases, increasing the cooling rate during the vitrification procedure. However, commercially available brands of equipment to supercool nitrogen are expensive. The aim of this study was to verify the effectiveness of a low-cost homemade nitrogen supercooling apparatus (Nitrocooler) for vitrification of bovine oocytes and IVP embryos. The device consists of a vacuum pump coupled to a close-tight-lidded flask with a styrofoam cup filled with 300 mL of LN2. After 5 to 7 min of vacuum pumping, LN2 goes through the slushing phenomenon, turning solid. After the lid is opened, the N2 turns liquid again, but in a stable, supercooled physical state lasting for approximately 10 min, boiling off when objects are plunged into it. Nitrocooler was tested for vitrified oocytes (Experiment I), vitrified oocytes in different containers (Experiment II), and embryos in 2 different vitrification solutions (Experiment III). In Experiment I (Ciência Rural, 2006 36, 1501–1506), immature (IM, n = 172) or in vitro-matured (IVM, n = 174) oocytes were exposed to 10% ethylene glycol (EG) + 10% DMSO for 30 s, followed by 20% EG + 20% DMSO + 0.5 m sucrose for 20 s, loaded in open-pulled straws (OPS), and plunged into LN2 or Nitrocooler-pumped LN2. For both IM (8.8%) and IVM (10.6%) oocytes, Nitrocooler-pumped LN2 increased blastocyst rates (Bonferroni, P < 0.05). In Experiment II, 1454 oocytes were vitrified using Nitrocooler after loading in different containers: stainless steel straws (SSS), beveled-tip straws (BTS), open-OPS, and glass-pulled straws (GPS). Greater blastocyst rates were obtained in the SSS (10.2%) and the GPS (8.0%) treatments, both being greater than the BTS (6.1%) and OPS (6.1%) containers (Tukey, P < 0.05). In Experiment III (Acta Sci. Vet. 2006 34, 77–82), 195 IVP bovine blastocysts were vitrified with or without Nitrocooler under 2 different cryoprotectant solutions: 10% EG + 10% DMSO or 10% EG + 10% propylene glycol (PROP). Hatching rates using Nitrocooler for EG + DMSO (50.2%) or EG + PROP (54.0%) were similar (ANOVA, P > 0.05) to normal atmosphere rates using EG + DMSO (50.1%) or EG + PROP (56.0%). Under these experimental conditions, our results suggest that the greater cooling rates obtained favored an increase in oocyte viability after vitrification, whereas no such beneficial effects were detected for vitrified IVP embryos. In conclusion, Nitrocooler was proven effective as a low-cost device to supercool LN2, increasing viability after vitrification of bovine oocytes, but not of IVP embryos.
|


