81 MEMBRANE PERMEABILITY CHARACTERISTICS OF BOVINE OOCYTES IN THE PRESENCE OF DIFFERENT CRYOPROTECTANTS
X. Wang A B , A. Al Naib A , D.-W. Sun A and P. Lonergan AA University College Dublin, Ireland;
B University of Shanghai for Science and Technology, China
Reproduction, Fertility and Development 21(1) 141-141 https://doi.org/10.1071/RDv21n1Ab81
Published: 9 December 2008
Abstract
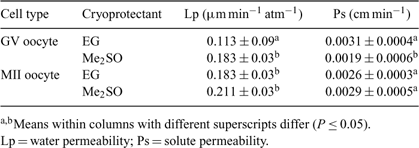
Development of effective cryopreservation protocols for mammalian oocytes depends on knowledge of fundamental cryobiologic characteristics of the oocyte membrane. The aim of the present work was to study the membrane permeability of immature (germinal vesicle, GV, n = 95) and IVM (metaphase II, MII, n = 104) bovine oocytes to water, dimethyl sulfoxide (Me2SO), or ethylene glycol (EG). Bovine ovaries were obtained from a local abattoir and oocytes were recovered by follicular aspiration. Volumetric changes of bovine oocytes in anisotonic solutions (osmolarity ranges from 130 to 1250 mOsm kg–1) were measured after 20 min of exposure at 24 ± 1°C and the osmotically inactive volume of the oocytes (Vb) was determined using the Boyle van’t Hoff relationship. Volumetric changes of oocytes during exposure to 1.8 m ethylene glycol (EG) or 1.4 m Me2SO at 24 ± 1°C were also measured. Volumetric change was recorded over a 20-min period using an inverted microscope and images were analyzed using the UTHSCSA ImageTool (Version 3.0 University of Texas Health Science Center, San Antonio, TX). The data were fitted to a 2-parameter model to determine the water (Lp) and solute permeability (Ps) of GV and MII stage bovine oocytes using the Runge–Kutta 4 method in Berkeley Madonna 8.3.11 (University of California, CA). Data were analyzed by ANOVA and are expressed as means ± SD. The osmotically inactive volume of GV and MII bovine oocytes were found to be 16.07 and 26.08% of the isotonic cell volume, respectively. In conclusion, these data highlight differences in sensitivity between GV and MII oocytes and provide the fundamental basis for the development of vitrification protocols for bovine oocytes.
|


