8 THE USE OF ANNEXIN V MAGNETIC-ACTIVATED CELL SORTING TO SEPARATE APOPTOTIC SPERM FROM THE EJACULATE OF STALLIONS
M. A. Coutinho da Silva A , C. R. F. Pinto A , J. M. Young A and K. Cole AThe Ohio State University, Columbus, OH, USA
Reproduction, Fertility and Development 23(1) 110-111 https://doi.org/10.1071/RDv23n1Ab8
Published: 7 December 2010
Abstract
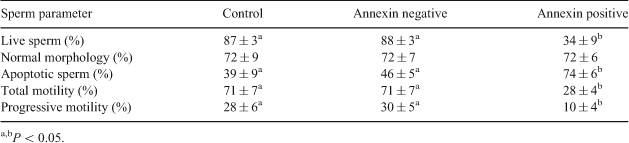
Magnetic-activated cell sorting (MACS) has been used successfully in humans to remove apoptotic sperm from the ejaculate. Annexin V-conjugated microbeads recognise sperm with externalized phosphatidylserine, which is considered one of the features of apoptosis, and the labelled sperm is separated by MACS. The goals of the study were to determine if MACS can be used to separate apoptotic sperm from the ejaculate of stallions; and to determine if removal of apoptotic sperm improves the quality of stallion sperm. Our hypothesis was that MACS would improve semen quality by removing apoptotic sperm, resulting in samples with higher motility and viability. Two ejaculates from three different stallions of good fertility were used. Sperm were diluted with Tyrode’s albumin lactate pyruvate (TALP) and incubated with annexin V-conjugated microbeads for 15 min at 37°C. Control samples were incubated in the absence of annexin V microbeads. The suspension was then loaded into the separation column containing iron globes, which were fitted in a magnet (MiniMACS; Miltenyi Biotec Inc., Auburn, CA, USA). The effluent sample containing annexin-negative sperm was collected and then, the column was removed from the magnetic field and rinsed with TALP to collect the annexin-positive cells. Sperm viability, motility, morphology and caspase activation were determined in all three samples: control, annexin-negative, and annexin-positive. Data were evaluated by ANOVA and individual comparisons were performed by Tukey’s hsd test. Significance was set at P < 0.05 and data is presented as means ± SEM (Table 1). The main effect of stallion was significant only for sperm motility parameters. Sperm recovery rate following MACS was 46 ± 3%. In conclusion, the use of MACS was effective in removing apoptotic sperm from the ejaculate. The annexin-positive population displayed a higher proportion of sperm with activated caspases and lower membrane integrity and motility. However, removal of apoptotic sperm from the ejaculate did not improve sperm parameters in the annexin-negative group compared to control group. In addition, sperm morphology was not affected by MACS. Further studies are necessary to determine if MACS could be used successfully to improve sperm quality from subfertile stallions and frozen semen.
|
The authors are thankful to Mark Williams at Miltenyi Biotec Inc. for providing supplies; and Dr Ashok Agarwal at The Center for Reproductive Medicine, Cleveland Clinic, for scientific input.


