Predator-baiting experiments for the conservation of rock-wallabies in Western Australia: a 25-year review with recent advances
J. E. Kinnear A F , C. J. Krebs B , C. Pentland C , P. Orell D , C. Holme E and R. Karvinen EA Number 9, Valley Road, Wembley Downs, WA 6019, Australia.
B Institute for Applied Ecology, University of Canberra, Canberra, ACT 2601, Australia.
C School of Natural Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup, WA 6027, Australia.
D Environmental Management Branch, Department of Environment and Conservation, Dick Perry Avenue, Kensington, WA 6150, Australia.
E School of Computer & Information Science, Edith Cowan University, 2 Bradford Street, Mount Lawley, WA 6050, Australia.
F Corresponding author. Email: jakinn2@bigpond.com
Wildlife Research 37(1) 57-67 https://doi.org/10.1071/WR09046
Submitted: 16 April 2009 Accepted: 12 December 2009 Published: 1 March 2010
Abstract
Predation is widely believed to be the main threatening process for many native vertebrates in Australia. For 25 years, predator-baiting experiments have been used in the Western Australian Central Wheatbelt to control red fox predation on rock-wallabies and other endangered marsupial prey elsewhere. We review here the history of a series of baiting experiments designed to protect rock-wallaby colonies by controlling red foxes with 1080 poison baits. We continue to support the conclusion that red foxes can reduce or exterminate rock-wallaby populations in Western Australia. Research trials from 1990 to 2008 have uniformly shown a dramatic recovery of rock-wallaby populations once red foxes are baited. Baiting experiments are often black boxes and their success should not blind us to their weaknesses. Ideally, what we would like to measure are the functional responses of predators to prey abundance directly. As a contribution towards this goal, we describe new technology that enables one to determine which predator killed which prey, at exactly what time, with improved research and management outcomes.
Acknowledgements
A project of this nature and longevity (1978–2007) situated in the Western Australian Wheatbelt would not have been possible without the cooperation and goodwill of the farming families whose properties contained or abutted the rock-wallaby sites. We once again thank the McDonald, Canova, Sales, Hammond and Crooke families for allowing unfettered access to their properties, while tolerating our coming and goings often at the oddest of hours. We also acknowledge the major contributions of Senior Technical Officer Michael Onus, a founding member of the project, Rowan Inglis, and the volunteers who assisted with the trapping during 2006/2007. We are grateful to A. Kinnear for her many contributions. The interest and collaboration of Mark Eldridge is much appreciated. The ethics approval numbers granted by the WA Department of Environment and Conservation Animal Ethics Committee (formerly CALM) are: CALM AEC 2001/08, CALM AEC 2003/27, CALM AEC 2003/30, and DEC AEC 2006/36. The Edith Cowan AEC approval code for C. Pentland’s research is 03-A18.
This paper is dedicated to the memory of Howard Robinson, in recognition of his contributions to rock-wallaby conservation.
Banks, P. B. , Dickman, C. R. , and Newsome, A. E. (1998). Ecological costs of feral predator control: foxes and rabbits. Journal of Wildlife Management 62, 766–772.
| Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A. , and McKenzie, N. L. (1989). Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143–198.
| Crossref | GoogleScholarGoogle Scholar |
Christensen, P. E. S. (1980). A sad day for the fauna. Forest Focus 23, 3–12.
Hone, J. (1999). Fox control and rock-wallaby population dynamics – assumptions and hypotheses. Wildlife Research 26, 671–673.
| Crossref | GoogleScholarGoogle Scholar |
Molsher, R. , Newsome, A. , and Dickman, C. (1999). Feeding ecology and population dynamics of the feral cat (Felis catus) in relation to the availability of prey in central-eastern New South Wales. Wildlife Research 26, 593–607.
| Crossref | GoogleScholarGoogle Scholar |
Short, J. , Kinnear, J. E. , and Robley, A. (2002). Surplus killing by introduced predators in Australia – evidence for ineffective anti-predator adaptations in native prey species? Biological Conservation 103, 283–301.
| Crossref | GoogleScholarGoogle Scholar |
Spencer, P. B. S. (1991). Evidence of predation by a feral cat, Felis catus (Carnivora: Felidae) on an isolated rock-wallaby colony in tropical Queensland. Australian Mammalogy 14, 143–144.
Appendix 1
Potential predation rates and sustainability of Wheatbelt rock-wallaby populations
Two populations of rock-wallabies (RW), Nangeen Hill and Mount Caroline, predator-baited from 1982 have stabilised at carrying capacity (K). This is evident from the census data and obvious signs of overgrazing (C. Pentland and R. Inglis, unpubl. data). Furthermore, a dispersal event was detected and linked to Mount Caroline.
On Mount Caroline from 2001 to 2007, 136 RW were trapped and translocated to sites where they formerly occurred (Table 1). This was done in an effort to mitigate the damage to nearby crops caused by RW; however, despite these removals, the population by 2009 (300+, N. Willers, pers. comm.) was comparable to the 1998 census of 296.
These removals, in effect, can be treated as an example of ‘management predation’, and therefore, an average of 19 wallabies per year were removed during a 7-year period, without precipitating a permanent population decline. Given this 25-year dataset, we pose the following question: what is the theoretical level of predation that can be sustained by a population of n RW individuals? We treat this as an exercise in determining the maximum sustainable harvest for a population that grows according to the logistic model as outlined in Skalski et al. (2005). Logistic growth is never perfect in small populations, but is an approximation that can provide some guidance for managers.
We apply the following formula (Skalski et al. 2005: p. 20):
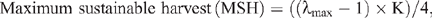
where, ln λmax = r is the annual growth-rate estimate calculated from the early stages of the population recoveries.
Note that λmax is derived from λt =Nt/No, where Nt is the population size at time t, and No is the population size at the commencement of predator baiting. For Mount Caroline, No is 13 (1982 census), Nt is 42 (1986), t = 4 (years) and K is 300 (Kinnear et al. 1988, and see text). Values of λmax = 1.34 and MSH = 26 imply a maximum sustained harvest of 26 RWs and this would be achieved by a Mount Caroline population of 150 individuals (K/2). We note that the average management predation rate during 2001–07 was 19, which was too low to effect a sustained population reduction. For Nangeen Hill, the data are No = 29 (1982), Nt=75 (1986) and t = 4 (ibid). λmax is found to be 1.27 and MSH = 8.
It is instructive to calculate the values for MSH, given a range of RW population sizes. For example, taking the average of λmax values (1.305, and assuming logistic growth) and if the RW population is 10, the MSH is 0.76; if it is 20, the MSH is 1.5. To enable population growth by small populations (i.e. 25 or less), effective predator control thus needs to be virtually absolute initially. This does not necessarily mean that every fox needs to be killed immediately after a baiting because they might be killed subsequently before any damage has occurred (see Thomson et al. 2000). It is also possible that only certain foxes are doing the killing, and only they need to be killed.
To summarise, this exercise highlights the following three points: (1) by inference, the RW fox-baiting protocol must be extraordinarily effective to allow population growth of small populations which has been repeatedly observed; (2) larger populations can tolerate a finite level of mortality caused by predators; and (3) the potential of contact telemetry to resolve such issues.


