Reduced efficacy of baiting programs for invasive species: some mechanisms and management implications
Sinéad E. Allsop A , Shannon J. Dundas A , Peter J. Adams A , Tracey L. Kreplins A , Philip W. Bateman B and Patricia A. Fleming A CA School of Veterinary and Life Sciences, Murdoch University, South Street, Murdoch, WA 6152, Australia.
B Department of Environment and Agriculture, Curtin University, Kent Street, Bentley, WA 6120, Australia.
C Corresponding author. Email: t.fleming@murdoch.edu.au
All authors contributed to the ideas and concept of this review; SA and PF led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Pacific Conservation Biology 23(3) 240-257 https://doi.org/10.1071/PC17006
Submitted: 3 March 2017 Accepted: 9 August 2017 Published: 5 September 2017
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
‘Bait-resistance’ is defined as progressive decreases in bait efficacy in controlled pest species populations. Understanding the mechanisms by which bait-resistance can develop is important for the sustainable control of pests worldwide, for both wildlife conservation programs and agricultural production. Bait-resistance is influenced by both behavioural (innate and learned bait-avoidance behaviour) and physiological aspects of the target pest species (its natural diet, its body mass, the mode of action of the toxin, and the animal’s ability to biochemically break down the toxin). In this review, we summarise the scientific literature, discuss factors that can lead to innate and learned aversion to baits, as well as physiological tolerance. We address the question of whether bait avoidance or tolerance to 1080 could develop in the red fox (Vulpes vulpes), an introduced predator of significant economic and environmental importance in Australia. Sublethal poisoning has been identified as the primary cause of both bait avoidance and increased toxin-tolerance, and so, finally, we provide examples of how management actions can minimise the risk of sublethal baits in pest species populations.
Additional keywords: 1080, bait avoidance, bait-shyness, control, innate behaviour, learned aversion, mammal, personality, pest, physiology, resistance, review, temperament, tolerance, vertebrate
Introduction
Poison baiting is the method of choice for vertebrate pest control in many parts of the world, being easy to use, cheap, and resulting in rapid knockdown (e.g. Innes et al. 1995; Gentle et al. 2007b; Howald et al. 2007). Baiting is the most cost-effective method for decreasing the population size of introduced pest species on a large scale, as a means of protecting livestock, increasing landscape production, and reducing predation pressure on native species (Armstrong 2004; Wright 2011). However, many pest control operations have shown significant decreases in the success of baiting over time (e.g. Quy et al. 1992; Hickling et al. 1999; Twigg et al. 2002), due to behavioural or physiological responses in the target pest population.
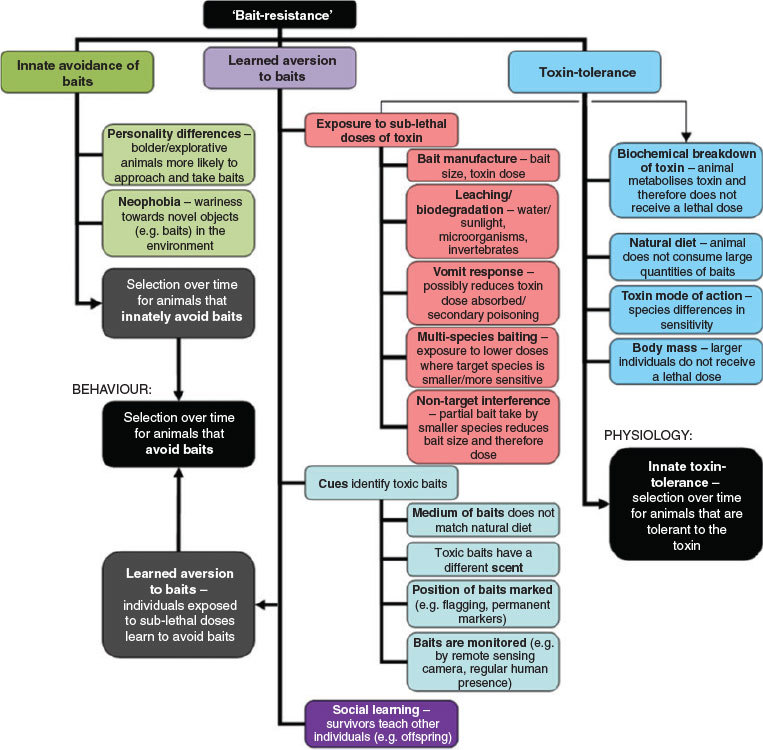
Baiting relies on the target species finding a bait (which, in turn, depends on the attractiveness of the bait) and then consuming it (which depends on the ‘acceptability’ or palatability of bait) and therefore ingesting sufficient quantities of toxin to provide a lethal dose (which depends on the amount of toxin in the bait and the animal’s metabolic capacity to deal with the toxin) (Saunders and McLeod 2007). Assuming that animals find baits, they may still avoid consuming them; they may not recognise the bait as food, they may show innate behavioural avoidance of baits, or show learned aversion behaviour based on previous experience or social learning. If target animals consume sublethal baits, they can show increasing physiological capacity to deal with the toxin present over subsequent generations, leading to populations exposed to baiting showing greater toxin-tolerance over time. We note that although we separately discuss behavioural and physiological mechanisms for bait-resistance, in some instances both bait avoidance and toxin-tolerance have been recorded (e.g. Quy et al. 1992).
Understanding bait-resistance is important for the sustainable control of pests worldwide, to protect wildlife and agricultural production (Hickling et al. 1999; Twigg et al. 2002; Twigg 2014). In this review, we summarise what is currently known about bait-resistance (both behavioural and physiological) in vertebrate pest species of significant economic impact in Australia and New Zealand (Table 1) and provide examples of how management actions can maximise efficacy of baiting programs. We examine the use of sodium fluoroacetate (hereafter ‘1080’; Box 1) baiting in Australia and New Zealand. We address the question of whether behavioural and physiological bait-resistance could develop in the red fox (Vulpes vulpes), an introduced predator of significant economic and environmental importance in Australia (Box 2).
|
| Box 1. Sodium fluoroacetate (hereafter ‘1080’) |
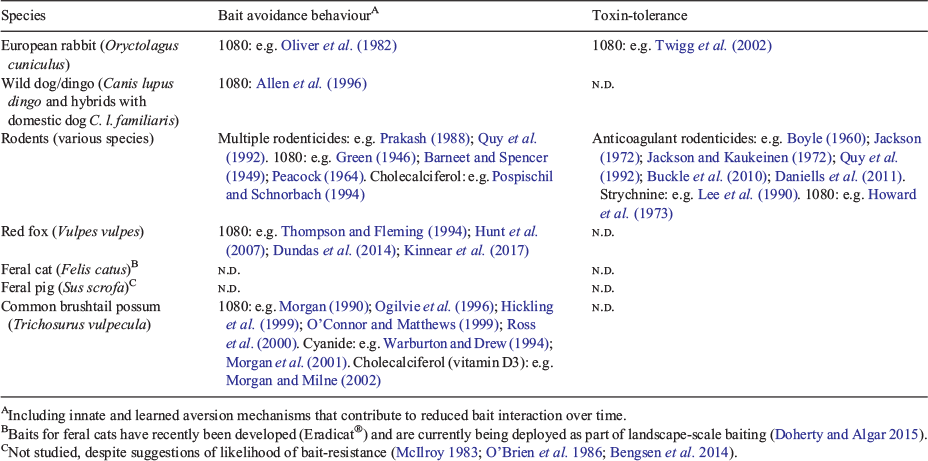
| A tasteless, odourless, and water soluble toxin, 1080 has been the preferred toxin for pest control for many years (McIlroy 1981b; Glen et al. 2007). Fluoroacetate is produced naturally in plant species found in Africa, South America and Australia (Twigg and King 1991). In Australia, fluoroacetate is expressed by indigenous plants of three Fabaceae genera (especially Gastrolobium, but also Acacia and Oxylobium: Oliver et al. 1977). Fluoroacetate-producing plants are common across south-west Western Australia, but are also represented patchily in the Northern Territory and Queensland; fluoroacetate-bearing vegetation does not occur in south-eastern Australia (see fig. 1 in Twigg and King 1991). Due to natural exposure to fluoroacetate through their diet, local native animal species consequently have a high level of 1080-tolerance, with tolerance greatest in herbivores and least for carnivorous species (Twigg and King 1991; Martin and Twigg 2002). One of the major benefits of using 1080 in baits, therefore, is the high susceptibility of introduced pest species relative to that of many native species (McIlroy 1981b, 1986). |
| 1080 is currently used in Australia, New Zealand, Mexico, Japan, the United States and Israel. 1080 was first used in the USA in rodenticides and then for predator control with the first field trials in 1944 targeting coyotes (Canis latrans) (Calver and King 1986); today, its use in the USA is restricted to livestock-protection collars, which protect sheep and cattle from coyote predation (Eason 2002). 1080 was introduced to Australia and New Zealand in the 1950s for European rabbit (Oryctolagus cuniculus) control. 1080 is used extensively today for control of rabbits, canids (red fox, wild dog Canis lupus dingo and hybrids with domestic dog), feral cats (Felis catus), feral pigs (Sus scrofa), mustelids, and a range of mammal species introduced into New Zealand, including common brushtail possums (Trichosurus vulpecula) (Calver and King 1986; Innes and Barker 1999; Eason 2002). 1080 baiting is the principal pest control tool in New Zealand, where 2–4 tons of 1080 concentrate per year are deployed (Innes and Barker 1999). This compares with only 200 kg per year for all of Australia (Anon. 2016). |
| Mode of action |
1080 is an acute metabolic poison without antidote (although there are poisoning treatment regimes and some suggested antidotes worthy of further research – see Twigg and Parker 2010). 1080 is particularly toxic to canids (Saunders and McLeod 2007). The mode of action of 1080 is extensively reviewed elsewhere (Twigg and King 1991; Twigg and Parker 2010). As a close analogue of sodium acetate, sodium fluoroacetate can take its place in biochemical pathways, the most notable being the citric acid (Krebs) cycle. Fluoroacetate is not itself toxic but, once ingested, it is metabolised in the body to fluorocitrate, which disrupts this central metabolic pathway by inhibiting the mitochondrial enzyme aconitate hydratase, leading to citrate accumulation. This results in an accumulation of citrate in the tissues and blood, energy deprivation, and gross organ dysfunction, which ultimately leads to death (Atzert 1971). Additionally, effects on citrate transport into and out of mitochondria and on some neurotransmitters have been reported (Twigg and King 1991; Twigg and Parker 2010). |
There are varied neurological, cardiac, and respiratory responses to 1080 intoxication, with death often occurring within 2–4 h of overt signs of poisoning becoming evident (Twigg and Parker 2010). The lethal effects of 1080 are not experienced immediately, as time is required for the 1080 to be absorbed, for the synthesis of fluorocitrate, and for the disruption of cellular processes to occur. In mammals, time until sign of toxicosis varies markedly between species, from 30 min to 3 h (Twigg and Parker 2010) (also see data reviewed by Sherley 2007). |
Most 1080-tolerant individuals tested have a very quick metabolism of 1080, with a half-life of only 1–14 h (Twigg and King 1991; Eason et al. 1993; Gooneratne et al. 1995). Repeated doses can lead to accumulation and toxicosis (Rowley 1963a), but small amounts of 1080 are rapidly detoxified, and sublethal doses are completely excreted within 7 days. The biochemical mechanisms responsible for large differences in 1080-tolerance are poorly understood; species differences suggest that a range of tolerance mechanisms have evolved (Twigg and King 1991; Twigg and Parker 2010). |
| Box 2. Could the red fox develop resistance to 1080 baits? |
| The red fox in Australia |
The red fox has contributed to the extinction of more than 25 mammal species and subspecies in Australia, and is recognised as a significant threat to many extant Australian species (Saunders et al. 1995; Woinarski et al. 2014). Foxes also threaten small livestock (e.g. lambs, chickens, piglets), causing significant losses (Saunders et al. 1995; Fleming et al. 2016), and it is estimated that foxes cost Australia $29 million annually in terms of losses to agriculture and costs of fox control actions, including baiting programs, predator-proof fencing, and monitoring of conservation estates (Gong et al. 2009). |
The instigation of broad-scale fox control in Australia in the 1980s has arguably prevented the extinction of many native species (Abbott 2008), with marked increases in native populations at baited sites (Saunders et al. 1995; Kinnear et al. 1998, 2010; Burrows and Christensen 2002). The success of translocations has also been strongly linked with predator control (Short et al. 1992; Risbey et al. 2000; Moseby et al. 2009; de Tores and Marlow 2012). Since the late 1960s, 1080 has been the poison of choice, delivered with a meat matrix because of its palatability to foxes and relatively high target specificity (Saunders and McLeod 2007). |
| Control of the red fox in Australia |
The food caching behaviour of foxes (when foxes take a bait and bury it in a new location for later consumption) is likely to decrease the amount of 1080 in baits (Gustavson 1977; Kay et al. 1999). In most cases, foxes retrieve their buried catches after short periods (Scott 1943; Macdonald 1976; Henry 1977; Macdonald et al. 1994; Kay et al. 1999); however, there are also scenarios where they are not retrieved for several months (Tinbergen 1972; Frank 1979; Kay et al. 1999). As these uneaten baits remain in the environment for long periods, it increases the likelihood of the water-soluble 1080 leaching into the soil such that the baits decrease in toxicity, with greater decreases over longer periods. Bait caching behaviour therefore represents an issue for fox baiting programs (e.g. Towerton et al. 2016). |
Although there is evidence for a decrease in fox density following baiting (e.g. Berry et al. 2013), the possibility of avoidance of baits by foxes has also been raised by various authors (e.g. van Polanen Petel et al. 2001; Dundas et al. 2014). Innate avoidance of baits can be selected for over successive baiting runs, which will quickly remove bold/explorative individuals from the population, rapidly shifting the population average towards more neophobic animals. Recent data indicate sex and age differences in diet, which suggests substantial variation in location of foraging activities within populations (Forbes-Harper et al. 2017). |
There is ample experimental evidence showing that foxes can develop learned aversion to untreated foods subsequent to exposure to foods treated with bitterants (e.g. Bitrex: Macdonald and Baker 2004) or other aversive agents (Ziram: Baker et al. 2007; Levamisole: Massei et al. 2002; Gentle et al. 2004). These studies suggest that aversive behaviour can last for more than five months after a single dose. |
Toxin-tolerance is likely to take longer than behavioural changes, but the possibility of 1080-tolerance in foxes has also been raised (Twigg 2014). Twigg (2014) also raised the issue of whether the average mass of animals has changed since regulations for the dose of 1080 in baits were established. However, a recent study of 540 foxes sampled across agricultural areas in south-west Western Australia (Forbes-Harper et al. 2017) recorded an average (±s.d.) mass of 5.3 ± 1.1 kg (below the mean of 6 kg used by McIlroy and King 1990 in their calculations), although males were larger than females and reached up to 8.9 kg. |
A review of red fox control programs in Australia (Saunders and McLeod 2007) indicated red fox population reductions of 31–97% as a result of various baiting protocols (Thomson and Algar 2000; Thomson et al. 2000; Bengsen 2014). Greater baiting efficacy is achieved for bait-naïve fox populations (i.e. animals not previously exposed to persecution/baiting, and/or consisting of predominantly yearling dispersers), suggesting that long-term programs, using the same approach consistently, artificially select for innate bait avoidance traits in red foxes. Dundas et al. (2014) reported foxes passing baits on camera on multiple occasions, suggesting that, even though they had located baits, they did not take them. A similar finding has been reported by Kinnear et al. (2017). |
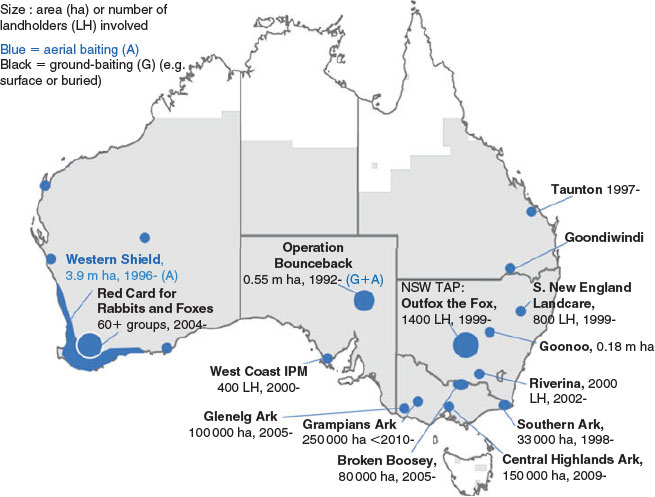
Across Australia, there are several large landscape-scale fox baiting programs, many that have been operating for decades (Fig. 1). Such wide-scale and long-term baiting is likely to have produced strong selective pressure for bait-resistance in foxes. Few baiting programs monitor their outcomes (Reddiex and Forsyth 2006), and reporting for many predator control programs ‘remains circumstantial and out of the scientific press (or in many cases not reported at all)’ (Saunders and McLeod 2007). Comparing bait interactions between long-term baited sites and those where baiting has not been routinely carried out would provide important understanding of whether bait-resistance is present, and therefore highlight management actions to minimise this situation. |
|
Innate avoidance of baits
Innate aversion is non-learned instinctive aversion that is a result of selection pressures on a species and refers to heritable characteristics of individuals within the target population. ‘Temperament’ refers to relatively consistent individual dispositions that underlie and modulate the expression of behaviour, resulting from genetic, epigenetic (i.e. developmental), and environmental effects (McDougall et al. 2006). The rapidly growing behavioural research field of animal personality/temperament recognises six main traits across species and studies: shyness–boldness, emotional reactivity/fearfulness, exploration–avoidance, activity, sociability, and aggressiveness (Boissy 1995; McDougall et al. 2006; Réale et al. 2007; Stamps and Swaisgood 2007). Temperament affects fitness through influences on predation rates, competition for mates and resources, and social interactions (reviewed by Smith and Blumstein 2008; May et al. 2016). For example, variation in neophobia has been linked with reproductive output in farmed silver foxes (V. vulpes) (Korhonen and Niemelä 1996), and variation in boldness has been linked with post-translocation survival in swift foxes (Vulpes velox) (Bremner-Harrison et al. 2004).
Because personality traits are heritable, baiting can result in artificial selection for particular personality traits across the population. Variation in temperament along a bold–shy or neophobia–exploration continuum results in differences in risk-prone behaviour amongst individuals of any given species, based on trade-offs between foraging gains and associated risks (Sloan Wilson et al. 1994; Ioannou et al. 2008). Such variation in behaviour influences latency to approach novel objects, latency to eat novel foods, or responses to threat stimuli (McDougall et al. 2006) and could therefore influence selective survival of individuals targeted through baiting programs. The degree of explorative behaviour could also influence vulnerability to taking a bait, with less exploratory individuals having reduced chance of encountering baits (Travaini et al. 2013).
The most marked examples of baiting influencing the temperament of animal populations are recorded in rodents. Neophobia is wariness expressed by an individual towards unfamiliar objects or foods in their familiar environment, which can result in cautious feeding strategies (Cowan 1977; Sunnucks 1998). After repeated baiting operations using toxic baits, there would be selection for survival of these neophobic individuals, thus decreasing the efficiency of control. Cowan (1977) demonstrated extreme forms of neophobia in commensal Rattus species that are likely to be exposed to traps or food containing poisons, and where avoidance of unfamiliar objects (including bait) obviously has survival value. Repeated poisoning regimes have led to selection for extremely neophobic brown rat (Rattus norvegicus) populations (Quy et al. 1992; Brunton et al. 1993).
Neophobia has also been recorded in Australian populations of the European rabbit (Oryctolagus cuniculus). 1080 baiting was first introduced to control rabbits in the 1950s. There was a major decrease in the number of rabbit kills for 1080 baiting trials between 1958–62 and 1971–75, although there was no significant increase in toxin-tolerance between 1955 and 1977 and bait-aversion was suggested as a possible reason (Oliver et al. 1982). Wariness towards 1080 baits in some Australian rabbit populations was recorded in the early 1960s (Poole 1963; Rowley 1963b), and several studies have directly observed individuals avoiding correctly presented baits (Carrick 1957; Rowley 1958). The selection for more neophobic individuals through repeated baiting was suggested as a likely cause for reduced bait efficacy recorded (Oliver et al. 1982). Similarly, rabbits were identified as showing cautious behaviour towards both non-toxic and toxic baits on initial exposure in New Zealand (Bell 1975).
The common brushtail possum (Trichosurus vulpecula) is regarded as New Zealand’s most detrimental vertebrate pest. Bait aversion due to neophobia has also been identified in New Zealand possums, with ~20% of bait-naïve possums avoiding non-toxic baits (O’Connor and Matthews 1999; Morgan 2004). These animals would therefore have greater chances of surviving exposure to toxic baits.
Learned aversion to baits (‘bait shyness’)
At an individual level, animals could learn to avoid baits if they survive an initial exposure to toxic baits and create an association of its ill effects with a sensory aspect of toxic bait – such as appearance, taste, or smell (Gustavson 1977). This is termed ‘learned aversion’, and can develop rapidly, after even a single or small number of doses (Morgan et al. 1996), but can persist within populations across multiple baiting events (Cowan 1977; O’Connor and Matthews 1996). Learned aversion threatens the current control programs for rodents (e.g. Quy et al. 1992; Brunton et al. 1993) and the common brushtail possum (e.g. Morgan et al. 1996; Ogilvie et al. 2000). Potential development of learned aversion towards baits has also been raised for foxes (Kay et al. 1999; van Polanen Petel et al. 2001; Carter and Luck 2013) and feral pigs (McIlroy 1983; O’Brien et al. 1986).
Learned aversion to poison baits has long been recognised and studied in rodent populations (see references in Table 1). Aversion can develop rapidly in rodents, with individuals learning to avoid particular flavours after a single flavour or toxicoses experience (Kalat 1974). Many rat populations have developed learned aversion to multiple rodenticide baits, making it increasingly hard to control them, as new baits to present these poisons must be developed (Prakash 1988). Rats can also develop aversion to 1080 baits if they ingest sublethal 1080 doses (Green 1946; Barneet and Spencer 1949; Peacock 1964).
An increase in the frequency and intensity of 1080 baiting to control common brushtail possums in New Zealand has been associated with a marked decrease in the success of possum kills (Hickling 1994; Moss et al. 1998). It is suspected that surviving possums develop learned aversion towards 1080 baits after consuming sublethal doses (e.g. Morgan 1990; Ogilvie et al. 1996), and it was predicted that providing a LD25 dose would rapidly generate aversion in survivors (Morgan et al. 1996). In a study area baited for three consecutive years, 63% of possums avoided baits, and aversion was still evident in individual possums several years after completion of the study (O’Connor and Matthews 1999). Possums ate less non-toxic carrot baits in areas where toxic baiting with carrots was carried out six months previously (compared with unbaited controls) (Hickling et al. 1999).
Social learning influences movement patterns, predator avoidance, mate choice, foraging decisions and food choice across a wide range of taxa (Galef and Laland 2005). Through teaching or observation of conspecifics, social learning increases the likelihood and rate of transmission of behaviour through a group of animals (Galef 1985; Frost et al. 2007). In rodents and European rabbits, feeding behaviour of juveniles is influenced by that of their older conspecifics (Galef 1990). Adults can potentially instil avoidance behaviour in their offspring during foraging though demonstration (e.g. Shier and Owings 2007). Social learning could also occur where conspecifics observe the results of other individuals consuming a bait. Personality traits or temperament also play a part in learned aversion through social learning (Ross 1999): when bold individuals observe their shy conspecifics, they become more cautious, although shy individuals observing bold conspecifics show no change and remain cautious (Frost et al. 2007). This illustrates that even in cases where shyness has not been inherited, parents can teach their young to be more wary of their environment.
Physiological mechanisms of toxin-tolerance
An animal’s physiology may protect it from the impacts of baiting. First, animals may have limited exposure to the toxins in baits due to their natural diet. Herbivores are generally assumed to avoid taking meat baits, although there is plenty of evidence that they do (e.g. Dundas et al. 2014). Second, species may also have different toxin tolerance by virtue of the mode of action of the toxin. For example, Tiliqua rugosa skinks have exceptionally high tolerance of 1080 (Twigg and King 1991), possibly because the mode of action (reducing ATP availability) has a minimal effect on ectothermic animals that already have a low metabolic rate. Third, the body mass of animals may confer an advantage, with larger individuals not receiving a lethal dose for their mass. Finally, animals may have the capacity to biochemically metabolise the toxin, removing it from their system. This final mechanism can potentially explain the increased physiological tolerance within populations of a target species over time, and we discuss aspects of biochemical toxin-tolerance below.
Heritable physiologically-based tolerance to toxins can threaten the efficacy of some baiting operations (Hickling 1994; Hickling et al. 1999). The physiological mechanisms for the breakdown and excretion of toxins can be artificially selected for by exposing populations to sublethal doses. Because of the marked selective pressure for tolerance to develop, toxin-tolerance can become established relatively rapidly. For example, anticoagulant rodenticides were developed in the early 1950s, but brown rat and house mouse (Mus musculus) populations resistant to various ‘first generation’ anticoagulant poisons (e.g. Warfarin) were already identified throughout the United Kingdom by the late 1950s to early 1960s (Buckle et al. 2010). More potent anticoagulant rodenticides (e.g. Difenacoum and Bromadiolone) were swiftly brought to the market, but heritable tolerance to the ‘second generation’ anticoagulant rodenticide Diphacinone was already recognised in 1958 in a Scottish routine field trial (Buckle et al. 2010). Toxin-tolerance was identified in some areas within a few years of the introduction of these more potent toxins (Boyle 1960; Jackson 1972; Jackson and Kaukeinen 1972; Buckle et al. 2010; Daniells et al. 2011). After initial exposure to anticoagulant poisons, some groups of individuals showed survival rates of almost 90% in subsequent feeding tests (Buckle et al. 2010).
The 1080-tolerance in Australian native species that coexist with fluoroacetate-bearing plants (Box 1) demonstrates that development of tolerance to this chemical can develop, given sufficient time. Because there is no reversal of this selection pressure (i.e. there is no cost to maintain the capacity to metabolise and excrete these compounds), in many cases these tolerances persist over 70–100 centuries after isolation from the toxic plants (Oliver et al. 1977; Eisler 1995). Comparing data for controlled, formal toxicity trials, the 1080 LD50 dose in European rabbits doubled (from 0.34–0.46 mg to 0.744–1.019 mg pure 1080 kg−1) over a period of 25 years for three Australian field sites that had been regularly baited since the introduction of 1080 baits in the early 1950s, with a non-significant increase in LD50 for a fourth site that had the least exposure to 1080 baiting (Wheeler and Hart 1979; Twigg et al. 2002). There was also a positive correlation between the degree of 1080-tolerance and the length of exposure to the toxin, and Twigg et al. (2002) suggested that continuous ingestion of sublethal doses of 1080 was the most likely mechanism for increased toxin-tolerance. Laboratory studies have also demonstrated rapid development of 1080-tolerance (Tahori 1963; Howard et al. 1973). Laboratory rats (Rattus norvegicus) given sublethal (LD75–LD90) doses of 1080 over successive generations developed a 1.8-fold increase in LD50 levels in five generations (Howard et al. 1973), while house flies (Musca domestica) showed a 7-fold increase over 25 generations (Tahori 1963).
Certain traits of target species are likely to increase the rate of development of toxin-tolerance. Species with r-adapted life strategies tend to be relatively successful in adapting to changes within their environment (Jackson 1972; Corbet and Harris 1991; Lynch and Lande 1993; Kolar and Lodge 2001); the relatively rapid development of 1080-tolerant populations of rodents (Howard et al. 1973) and European rabbits (Twigg et al. 2002) is likely to be facilitated by their high reproductive potential (Silver 1924; Kolar and Lodge 2001). Development of 1080-tolerance may be much slower for animals that show k-adapted life strategies, such as canids and mustelids (Twigg et al. 2002). Furthermore, presumably because reproductive tissues have high energy demands and thus require adequate supply of ATP, sublethal doses of fluoroacetate temporarily suppress fertility of a range of animals, including birds, mammals and reptiles (reviewed by Twigg and King 1991); this effect is also likely to have greater impact on k-adapted species, which have fewer reproductive opportunities.
Management actions to reduce development of 1080 bait-resistance
The widespread use of successive 1080 baiting, particularly in Australia and New Zealand (Box 1), has meant that populations of target species have been repeatedly exposed to the toxin, increasing the risk for developing bait-resistance through either bait avoidance or toxin-tolerance. Bait-resistance could therefore represent a major challenge to on-going control programs, potentially reducing the effectiveness of these costly enterprises. This situation highlights the need for proactive management operations to minimise conditions that contribute to development of bait-resistance.
Increasing the toxin concentration in baits is often the strategy considered first to ensure a high proportion of successful target kills; however, this would increase risk to non-target species. Furthermore, it risks the baits becoming less palatable to target species, increasing development of bait-resistance (Staples 1969; Frampton et al. 1999), particularly if impurities in the toxin decrease palatability as well (e.g. Atzert 1971; Morgan 1982; Morgan et al. 1987). The following sections discuss management actions that may serve to reduce risk of bait-resistance without relying on increasing toxin dose.
Reduce incidence of sublethal 1080 baiting
Sublethal poisoning has been recognised as the primary cause of both learned aversion towards baits and development of toxin-tolerance (e.g. Morgan et al. 1987, 1996; Warburton and Drew 1994; Hickling 1994; Hickling et al. 1999; Saunders et al. 2000; Morgan 2004). Sublethal doses could ultimately result in target animal populations becoming less sensitive to the toxin as the larger-bodied and less sensitive individuals within the population are likely to survive. As Kinnear et al. (2017) puts it, ‘sub-lethal baits caused by microbial activity (e.g. following bait caching or rainfall) can potentially create bait-shy predators who become intractable killers’. Thus, management strategies should focus on preventing the development of sublethal baits, and also prevent target species encountering them.
Ensure bait quality at manufacture
Baits being presented in control operations can be of poor quality: unattractive, unpalatable, or with the incorrect toxin levels to deliver a lethal dose (Cook 1999). Dried meat baits are usually cut by hand and consequently can vary markedly in size and consistency. Substandard bait matrix can also easily be broken down into fragments, increasing the likelihood of consumption of inadequate toxin quantities (Nugent et al. 2010). There is also a 10% ‘allowable variation’ in toxin dose (Twigg 2014), with variation between baits due to the mechanical action of withdrawing the needle after each bait is injected.
Consider non-target interference and multispecies baiting
Because the lethal toxin level for a specific baiting program is usually determined based on the susceptibility of only one target species, sublethal poisoning can occur if another pest species, which is larger-bodied or has a higher 1080-tolerance, is also present (Hickling et al. 1999). For example, feral cats have almost four times greater tolerance (average LD50: 0.40 mg kg−1) of 1080 than canids (LD50 of red fox: 0.15 mg kg−1; LD50 of wild dog: 0.11 mg kg−1) (McIlroy 1981; McIlroy and King 1990), and a 3.0-mg 1080 bait aimed at foxes is hypothetically sufficient to provide a lethal dose in only half of feral cats weighing >7.5 kg (Twigg 2014). In New Zealand farmlands, baiting is carried out for both the common brushtail possum and the European rabbit, where possums have LD50 toxin levels that are at least three times higher than those of rabbits (Hickling et al. 1999). Consequently, possums are exposed to sublethal 1080 doses when they take baits intended for rabbits.
Due to partial consumption (Twigg et al. 2002; Dundas et al. 2014), many smaller animals that cannot consume a whole bait can interfere with the toxin dose present. For example, rodents may gnaw at baits, while invertebrates such as ants can also consume parts of the bait (Merks and Calver 1989). Target animals taking the partial remains of baits are therefore more likely to ingest sublethal doses.
Reduce leaching and biodegradation
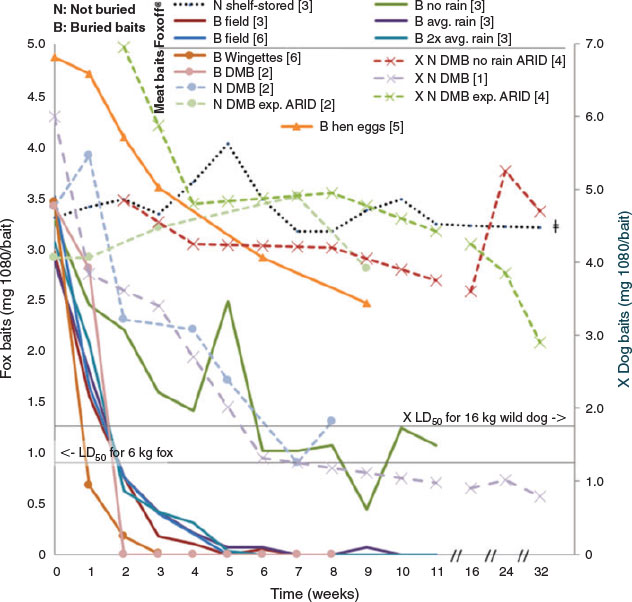
While there are multiple explanations for development of sublethal baits (Fig. 2), in most cases sublethal baits are probably the result of environmental exposure (Twigg et al. 2002). 1080 is highly water-soluble and, over time, leaches out of baits (Twigg et al. 2002); even humid environments can cause the toxicity of uneaten baits to steadily decrease (Staples et al. 1995). Thus rainfall, soil moisture, and temperature play an important role in the longevity of 1080 in baits, both directly and indirectly affecting the activity levels of microorganisms and invertebrates (e.g. blowfly larvae) (Twigg et al. 2002; Saunders and McLeod 2007; APVMA 2008). The bait matrix (especially the ‘skin’ of the bait) influences water penetration and therefore bait longevity. Consequently, bait longevity varies markedly between different bait matrices as well as with the conditions in which they are placed (Fig. 3).
|
Burial of fox baits is mandatory across much of eastern Australia in order to reduce non-target impacts (Saunders and McLeod 2007). With no strict guidelines in place there are many interpretations of the term ‘buried’, ranging from the bait being covered with a thin layer or clod, to shallow depressions, to burial 10–15 cm deep (Saunders and McLeod 2007). However, burial in soil, particularly deep burial (>10 cm), significantly shortens the effective life of 1080 meat baits (APVMA 2008) due to leaching as well as biodegradation of the toxin by bacteria and fungi (Twigg and Socha 2001), increasing likelihood that the bait will contain a sublethal dose. Some baits last only one week before the 1080 has sufficiently degraded that the bait is no longer toxic to the target species (Fig. 3 and references therein). Baits that have been left in the field longer than these average times would therefore contain a sublethal dose; assuming the bait remains attractive and palatable, this can increase the risk of learned aversion developing. Buried meat baits are also taken significantly less often by foxes than are baits placed on the surface (Thomson and Kok 2002). The potential risk of non-target take can be reduced by placing baits on the soil surface and lightly covering them with leaves, sticks, or twigs, or placing them under logs or in a shallow depression. At the very least, any buried 1080 bait should be removed and replaced every seven days; Saunders and McLeod (2007) present a decision tree guiding bait choice in scenarios where replacement is warranted.
The timing of baiting also needs to be considered carefully. While standard operating procedures for the deployment of 1080 baits recommend that baits not be deployed under wet conditions, it would be important to have some idea of how long baits are likely to last in the environment to ensure that they are not deployed before significant rainfall events. Although it is impossible to retrieve grain-based baits used for rabbit and feral pig control, the importance of retrieving larger baits after control operations has been broadly recognised (APVMA 2008) to minimise the time baits remain in the environment to avoid becoming sublethal.
Reduce vomiting
Incorporation of 1080 by an individual (i.e. fluoroacetate intoxication) can initiate a vomit response, most commonly observed in carnivores and feral pigs (e.g. McIlroy 1981; Sinclair and Bird 1984; O’Brien et al. 1986; O’Brien and Lukins 1988; Choquenot et al. 1996). Pen trials investigating the toxicity of 1080 to feral pigs observed high rates of vomiting in individuals that ingested 1080, and some pigs that vomited survived very high 1080 doses (Hone and Kleba 1984), which has raised concerns that sublethal dosing due to vomit responses can possibly lead to subsequent aversion (McIlroy 1983; O’Brien et al. 1986). However, additional trials, more closely simulating field conditions, demonstrated that while vomiting frequently occurred in feral pigs poisoned with 1080, it does not improve their survival as sufficient fluoroacetate had been absorbed before emesis (O’Brien 1988; O’Brien and Lukins 1988; Twigg et al. 2005). Similarly, the mortality of fat-tailed dunnarts (Sminthopsis crassicaudata) offered meat containing an estimated LD90 of 1080 was only one-tenth of that in animals orally administered the same dose in water, presumably due to vomiting (Sinclair and Bird 1984). However, although all dunnarts that ingested meat laced with 1080 vomited, two individuals that vomited 100% of the poisoned meat volume still died, indicating that they had already assimilated a lethal dose (Sinclair and Bird 1984).
Consumption of vomitus by feral pigs can potentially increase the chances of sublethal dosing through secondary poisoning (McIlroy 1983). While the amount of 1080 in vomitus is likely to be similar to that of the bait itself (Gentle et al. 2005), relatively small amounts of this material (~50 g) are likely to occur in the environment (Twigg et al. 2005). There is also the potential for non-target species to ingest vomitus and therefore be exposed to poisoning, a reasonable consideration given the large 1080 doses in feral pig baits. However, it should be noted that vomiting has not been commonly recorded in studies of 1080 baiting of free-ranging feral pigs (Twigg et al. 2005), which suggests that there may be differences between caged and field experiments in the amount of bait consumed while in traps compared with their natural diet.
In the case of feral pigs, management of their vomiting behaviour after poisoning (e.g. by including an antiemetic agent that prevents vomiting: McIlroy 1983) could help to reduce learned aversion developed as a consequence of regular exposure to sublethal doses of a toxin. While Rathore (1985) found such agents to be successful in preventing vomiting, Hone and Kleba (1984) reported that the majority (70%) of pigs still vomited, although kill rate was increased (O’Brien et al. 1986; Choquenot et al. 1996). Twigg and Parker (2010) discusses the potential implications of any bait additives for non-target animals that might ingest baits.
Reduce cues that identify toxic baits
Learned aversion to baits requires that the ill-effects of the toxin have an immediate effect on the target animals, thus allowing them to develop the association with the bait itself. 1080 is a relatively fast-acting toxin. Eastern quolls (Dasyurus viverrinus) show a vomit response within 13–49 min (mean: 26 min) of ingesting 1080, while in Tasmanian devils (Sarcophilus harrisii) it is 18–82 min (mean: 55 min) (McIlroy 1981). In foxes, symptoms are evident 30 min after bait ingestion (Saunders and McLeod 2007). These times may be sufficiently short for animals to associate symptoms of fluoroacetate intoxication with the last food item they consumed.
Learned aversion to baits also requires that the baits are sufficiently distinctive (e.g. through novelty or some distinguishing feature) for the target animal to be able to distinguish and therefore reject them. Several studies indicate that animals can discern fluoroacetate in their meals. Dietary studies indicate that crested pigeons (Ocyphaps lophotes) and grey kangaroos (Macropus fuliginosus) are able to discriminate between highly toxic and less toxic seeds/plant species, consuming less of the former, suggesting that they discern fluoroacetate (Twigg and King 1991). Two native carnivore species distinguish between baits or meat with and without 1080 added (spotted-tailed quoll, Dasyurus maculatus: Körtner and Watson 2005; fat-tailed dunnart, Sinclair and Bird 1984). Four rodent species show reduced intake of foods dosed with 1080 (Calver et al. 1989). Pen trials with feral pigs showed a significant reduction in their daily consumption of wheat when 0.05% 1080 was added, with intake returning to prepoison levels after three days (Hone and Kleba 1984). Similarly, Morgan et al. (1987) reported that while less than 5% of common brushtail possums avoided non-toxic baits, 17–22% avoided baits after 1080 was added. Such studies indicate that differences in the smell and/or taste of non-toxic and toxic baits can influence whether they are eaten or not.
Match the medium of baits to natural diet
The quality and suitability (acceptance and palatability) of the bait matrix can influence bait take by the target species (Twigg 2014). Oliver et al. (1982, p. 132) pointed out that ‘neophobia is likely to affect adversely all rabbit control by poison baiting whatever bait or poison is used, unless the poison can safely be incorporated into an existing and familiar food’. For example, baiting programs using dried meat baits use large quantities of meat and therefore often import meat (e.g. horse meat) slaughtered elsewhere to the site where baits are produced. Where this meat is different from the target species’ natural diet, then this will result in the bait matrix being novel in comparison to other potential foods in their environment. By virtue of their production, manufactured baits are particularly likely to trigger neophobic reactions.
Consider the scent of toxic baits
While 1080 in its pure form is odourless and tasteless (Atzert 1971), in commercial form it has been identified as sometimes containing an acetic acid impurity, giving it a faint vinegar smell (Morgan 2004).
Avoid ‘marking’ positions of baits
Many programs have permanent baiting sites marked in some manner to enable recovery of untaken baits at the end of a baiting cycle or redeployment at fixed locations over successive baiting cycles. Animals may learn to avoid these marked positions or animals may be alerted to the presence of baits by regular human presence at those sites. Animals that show aversion to human presence or disturbance will therefore be alerted to the presence of baits and avoidance of the general area could increase. For example, Rattus norvegicus demonstrate latency to pick up food presented in bait stations, thereby reducing effective administration of toxic baits via bait stations (Stryjek and Modlinska 2016). Baits are often also monitored by infrared camera traps, enabling identification of the species taking baits (e.g. Dundas et al. 2014), but also potentially alerting the target animals to their presence (Meek et al. 2014, 2016). In a trial on agricultural sites in south-west Western Australia, Dundas et al. (unpubl. data) found that 30% of 33 dried kangaroo meat baits monitored by camera were still present at 45 days compared with only 16% of 401 baits that were not monitored by camera.
Increase bait uptake
Maximising bait uptake within days of bait deployment, reducing the amount of time that toxic baits are left in the environment and therefore subject to leaching and biodegradation, will therefore reduce the likelihood of sublethal toxin doses. Maximising bait uptake by the target species also serves to minimise risk to non-target species. Several methods have been proposed to increase rapid bait uptake.
Bait additives
Bait additives – in the form of dyes or other cues that can mask signals used by target animals to identify them – can disrupt the development of learned bait aversion (e.g. Thomas et al. 1996). For example, masking the smell and/or taste of the toxin with cinnamon and orange flavours was successful in masking the presence of 1080 in common brushtail possum baits (Morgan 2004). Microencapsulation to disguise the bitter taste of some toxins has been trialled (e.g. Shapiro et al. 2016).
Additives that disrupt the possibility of learned aversion have also been trialled. For example, Cook (1999) tested corticosterone glucocorticoids (which is released in response to stress) and mifepristone (another hormone that inhibits the actions of glucocorticoids) bait additives in rats, and found that high doses of mifepristone increased bait consumption and decreased aversion towards baits. Devine and Cook (1998) showed that the addition of neurotransmitter antagonists reduced development of learned aversion to baits by European rabbits.
There are several considerations in the decision of whether or not to pursue bait additives. With any additives, it is important to first establish the need to make existing baits more attractive and then test the effect of these attractants under field conditions to warrant the additional costs (Saunders and McLeod 2007). There is a risk that attractants may prolong attractiveness of baits, so that they are still attractive when they are sublethal, hence increasing risk of developing aversion. Some bait additives can have adverse effects on the mode of action of the toxin (e.g. the administration of diazepam together with 1080 resulted in decreased sensitivity of foxes to 1080: Marks et al. 2009; Twigg and Parker 2010). The implication of using bait additives also needs to be considered for non-target animals (Twigg and Parker 2010).
In addition to extra costs due to the additive itself, the inclusion of any new bait additive requires regulatory approval (e.g. Australian Pesticides and Veterinary Medicines Authority (APVMA); New Zealand Food Safety Authority (NZFSA)), which ultimately adds to the cost of bait manufacture, and therefore baiting. Although ‘evaluations of variations to available products can be shorter’ than evaluations for completely new products, the processes are acknowledged to ‘be lengthy [with] timeframes prescribed in the legislation’ (Anon 2017) (see also Springer 2011).
Free-feeding
A period of adequate free-feeding, preceding or after non-toxic baiting, could minimise development of learned aversion (Choquenot et al. 1996; Ross et al. 2000). With prefeeding, animals are attracted to bait stations and become habituated to the scent associated with a non-lethal reward, reducing neophobia (Ross 1999); toxic baits can then be deployed (using the same scent) once the target species has become acclimated to the feeding site (Choquenot et al. 1996). Prefeeding can also increase the amount of bait consumed by the target species, reducing the likelihood of sublethal doses. Prefeeding is a regulatory prerequisite for baiting feral pigs and rabbits (unless ‘one-shot’ oats are used for rabbit-baiting – as standard practice in Western Australia: Oliver et al. 1982).
Bait avoidance in common brushtail possums has been largely minimised with prefeeding where the prefed and toxic baits were similar, reducing the ability of possums to use novel cues to form associations between the toxic bait and its ill effects (Moss et al. 1998). Ross et al. (2000) found that while postfeeding was relatively ineffective in reducing aversion development in possums, prefeeding had a significant effect: 97% of non-prefed possums developed aversion compared with only 22% of prefed possums.
Free-feeding is not recommended for all vertebrate pests. Because foxes cache baits (van Polanen Petel et al. 2001), prefeeding can have negative consequences on toxic bait uptake (Gentle 2005). Additionally, free-feeding can also train non-targets to feed on the bait trails or stations and substantially increases the cost of control programs (Saunders and McLeod 2007).
Timing of baiting
Consideration should also be given to the timing of baiting, because seasonal saturation of an environment with an abundant, palatable and easily acquired food source is likely to make baits less desirable (Saunders and McLeod 2007), and hence increase the length of time that they are left in the environment exposed to leaching and biodegradation.
Taking advantage of peak food demands for the target species may help to improve effectiveness of baiting programs, especially where the target species shows strong seasonal reproductive cycles, such as the fox (Saunders and McLeod 2007). In New South Wales, the greatest decline in fox body condition for both sexes is recorded between August and November, coinciding with the peak birth and cub raising period (Saunders and McLeod 2007). This is also the time of year when grass cover is an impediment to shooting. Late summer (February–May) coincides with the dispersal of naïve individuals (i.e. young) into the population, which are more likely to take baits than wary adults. Twice-yearly baiting has been shown in some studies to keep fox population densities low all year round (Saunders and McLeod 2007), although other studies indicate that bait coverage, even under biannual programs, can still be insufficient for effective fox control (Bengsen 2014).
Avoid over-reliance on a single method of control
A general recommendation evident in the literature is the need for variation in the control methods used, avoiding over-reliance on a single control technique (e.g. Hickling et al. 1999; Kinnear et al. 2017).
Variation in bait presentation and toxin
Using the same bait type repeatedly could increase the likelihood of animals developing bait avoidance associated with the particular appearance of baits. Switching between bait types has been recommended to reduce target species becoming habituated to avoiding baits (APVMA 2008). There may be some capacity for bait switching: although virtually all rabbit control is undertaken using oat products, there are grain and manufactured baits for feral pigs and several manufactured bait types for canids, which offer the opportunity for alternating bait types. Varying the appearance or smell of baits (e.g. using different meat sources for dried meat baits) could increase bait take by individuals that have developed learned aversion to a specific bait. Cage (Morgan et al. 1996) and field-based (Ogilvie et al. 1996) studies show that altering the bait matrix can partially overcome aversive behaviour. Marlow et al. (2015) found that changing from dried meat baits to Probait® increased uptake by foxes in two Western Australian reserves. Switching between a cereal bait and gel increased efficacy in common brushtail possums (Ross et al. 2000). As well as palatability of the bait, longevity of different bait types under the specific climatic conditions experienced should be considered as part of the decision around which baits to use. For example, where the baits need to be buried to reduce non-target interference, chicken egg baits might offer a better option than meat baits (Fig. 3).
Alternating toxins may slow development of toxin-tolerance. While 1080 is a relatively fast-acting toxin, and thus allows target species to associate it or the bait with its symptoms if they do not die after ingestion, anticoagulants are comparatively slow, minimising the development of any association between consumption and adverse consequences (Henderson et al. 1997; Ross et al. 1997). Ross et al. (1997) demonstrated that while 1080 baits killed only 40% of 1080-avoiding possums, 70% were killed when alternative toxins (Brodifacoum and Cholecalciferol) were used, and 100% when bait type was also varied. Switching toxins could be the most promising technique to overcome aversion problems in common brushtail possums (O’Connor and Matthews 1999), while switching both toxin and bait matrix improved outcomes of baiting California voles (Microtus californicus) (Baldwin et al. 2016).
Several alternative toxins have been used or tested for use in Australia. Para-aminopropiophenone (PAPP) has been approved for use by the APVMA (January 2016) and PAPP baits targeting wild dogs (DOGABAIT®) and foxes (FOXECUTE®) are now commercially available in some states (Pestsmart Connect 2016a). This toxin has an antidote, reversing the effect of the compound if administered within 30 min. The toxin has some species selectivity, with carnivores (both native and introduced) more sensitive to PAPP than non-carnivore species (birds and humans) (reviewed by Eason et al. 2014). Considerable progress has been made with developing sodium nitrite baits for controlling feral pigs (Staples 2017). Other bait toxins used in Australia and New Zealand include strychnine, cyanide, and zinc phosphide (Eason et al. 2008).
Using alternative poisons may come with limitations. While 1080 can kill in a single dose, large and multiple doses of some alternative poisons are often needed to accumulate to a lethal level. Anticoagulants provide a promising direction for future research to overcome aversion in introduced pest species (Henderson et al. 1997), although Brodifacoum is associated with increasing primary and secondary non-target risks (Eason and Spurr 1995) and is relatively costly (Henderson et al. 1997). Considerable effort goes into developing products for vertebrate control, ensuring that they are efficacious, safe for the user and the environment, relatively humane and provide cost-effective control before they can be considered for registration. While the number of available population control options needs to be maximised to ensure that our long-term ability to reduce the impacts of vertebrate pests is maintained, bait preparation and presentation standards should remain first priority, preventing sublethal dose baits.
Variation in control methods used
Alternative delivery mechanisms for toxins, delivering lethal doses that would reduce opportunity for learned aversion, still require further investigation (Saunders and McLeod 2007), particularly in the field. Spring-loaded mechanical ejectors (known as M-44 ejectors or canid pest ejectors) were registered for use in Australia in 2016 (ACTA, 2016a); the device is triggered by a minimum force required to release the toxin, i.e. by an animal pulling on the trigger with its teeth (Osborne et al. 2014). Feral cat grooming traps (e.g. ‘Spitfire’, ‘Felixer’) use a combination of criteria based around body size and habits (e.g. scent marking) to trigger a lethal dose of toxin squirted onto the animal’s pelt which it then ingests when cleaning itself (Murphy et al. 2014; Read et al. 2014; Sjoberg et al. 2014). Similarly, a range of novel devices have been designed for rats (e.g. Murphy et al. 2014), common brushtail possums (e.g. Blackie et al. 2014), and mustelids (e.g. Morriss and Warburton 2014). The efficacy of these alternative control tools relies on the behavioural patterns of the target species (i.e. biting and pulling for the canid pest ejectors, lure investigation and scent marking, etc.), and, to varying degrees, such devices could also impose selection on the populations being targeted. Delivery systems specific to the target species (e.g. feral pig feeder: ACTA 2016b) will reduce non-target exposure to toxin that could contribute development of bait-resistance, although such devices may accelerate selection for neophobic individuals in the target species population.
The key to targeted baiting without developing bait-resistance is likely to lie in maintaining a multimodal approach to reduce development of aversion responses. A combination of pest control techniques such as baiting, shooting and trapping is recommended in rotation with baiting practises (Warburton and Drew 1994; Morgan et al. 1996). For example, the Red Card for Rabbits and Foxes program in the Western Australian wheatbelt (http://www.redcard.org.au/; last accessed 14 August 2017) has been successfully coordinating baiting and shooting across private land (Fig. 4). Such efforts can show success in terms of perceived knockdown and livestock protection (McLeod et al. 2011), although long-term success of such programs relies on continued funding and community engagement.

|
Conclusions
-
Development of bait avoidance and toxin-tolerance are inherent in any long-term baiting program, threatening current and future control of introduced pest species, and making strategic choices around the most effective management options a pressing issue.
-
Increasing toxin-tolerance over time has been recorded in one field study (European rabbits: Twigg et al. 2002); by contrast, there have been many cases demonstrating innate and learned aversion towards baits (Table 1). Learned aversion to baits can take place after even a single bait dose, while innate avoidance responses at the population level, and the build-up of toxin-tolerance, take place over longer, evolutionary time scales, and therefore require long-term monitoring data to fully understand.
-
There is limited evidence for bait-resistance in many introduced pest species. However, a potential for bait-resistance to develop has been identified through learned aversion behaviour in the feral pig (McIlroy 1983; O’Brien et al. 1986) and through both behavioural and physiological resistance in the red fox (e.g. van Polanen Petel et al. 2001; Gentle et al. 2007a). In both of these species, the likelihood of sublethal dosing is enhanced due to baiting practices and caching behaviour in foxes.
-
It has long been recognised that baiting is the most cost-effective method of controlling many vertebrate pest species. In addition to the constraints of funding and non-target interference (Staples 1969; Frampton et al. 1999), the development of bait-resistance also needs to be considered as part of designing pest control actions. If appropriate action is not taken, it is possible for bait-resistance to increase, resulting in bait-shy populations that we would be unable to control (O’Connor and Matthews 1996). There is clearly a need for more research if we are to maintain the efficacy of current pest control methods into the future.
Glossary of terms
-
Bait avoidance: reduced interactions with bait over time, either due to innate characteristics or learned aversion.
-
Bait-efficacy: achieving the desired outcome of progressive decreases in pest species population being controlled.
-
Bait-resistance: the measure of difficulty that a chemical or strategy has at controlling a specific pest population under practical conditions (Blaszkowicz 2016).
-
Behavioural resistance: where the animal alters its behaviour and therefore increases its likelihood of survival (Blaszkowicz 2016).
-
Innate bait avoidance: non-learned, instinctive avoidance to approaching or consuming baits, due to natural differences in behavioural responses, e.g. neophobia, wariness.
-
LD dose: toxin dose required to kill a portion of the population; e.g. LD50 is the amount of poison that will kill 50% of animals in a sample, LD25 the dose that will kill 25% of animals in a sample.
-
Learned bait aversion/bait-shyness: acquired aversion behaviour to approaching or consuming baits due to association of a sensory aspect of toxic bait – such as appearance, taste, or smell – with its ill effects. Thus, if poisons are used for control they must provide no sensation of illness following ingestion.
-
Physiological resistance: where aspects of an animal’s physiology enable it to survive what would normally be considered a lethal dose, due to its body mass or its natural diet precluding consumption of a lethal dose, the mode of action of the toxin, or the animal’s ability to break down the toxin.
-
Sublethal dose of toxin: a dose that does not kill the animal.
-
Toxin-tolerance: physiological (biochemical) capacity to metabolise or excrete the toxin.
Conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgements
We acknowledge Murdoch University for funding this project.
References
Abbott, I. (2008). Historical perspectives of the ecology of some conspicuous vertebrate species in south-west Western Australia. Conservation Science Western Australia 6, 1–214.ACTA (2016a). Canid Pest Ejector. (Animal Control Technologies (Australia) Pty. Ltd.) Available at: http://www.animalcontrol.com.au/news/2015/20151112-2.htm [verified 14 August 2017].
ACTA (2016b). HOGHOPPERTM bait delivery system. Animal Control Technologies (Australia) Pty Ltd.
Allen, L., Engeman, R., and Krupa, H. (1996). Evaluation of three relative abundance indices for assessing dingo populations. Wildlife Research 23, 197–205.
| Evaluation of three relative abundance indices for assessing dingo populations.Crossref | GoogleScholarGoogle Scholar |
Anon. (2013). Threat Abatement Plan for predation by the European red fox (2008); five yearly review. Department of the Environment, Australian Government.
Anon. (2016). 1080 – characteristics and use. Western Australian Agriculture Authority, Department of Agriculture and Food, Government of Western Australia.
Anon. (2017). Regulation. The Australian Pesticides and Veterinary Medicines Authority (APVMA).
APVMA (2008). Review findings sodium fluoroacetate: Technical report. The reconsideration of registrations of products containing sodium fluoroacetate and approvals of their associated labels: environmental assessment. Australian Pesticides and Veterinary Medicines Authority, Canberra.
Armstrong, R. (2004). Baiting operations: Western Shield review – February 2003. Conservation Science Western Australia 5, 31–50.
Atzert, S. (1971). A review of sodium monofluoroacetate (compound 1080): its properties, toxicology, and use in predator and rodent control. US Fish and Wildlife Service, Washington, DC, USA.
Baker, S. E., Johnson, P. J., Slater, D., Watkins, R. W., and Macdonald, D. W. (2007). Learned food aversion with and without an odour cue for protecting untreated baits from wild mammal foraging. Applied Animal Behaviour Science 102, 410–428.
| Learned food aversion with and without an odour cue for protecting untreated baits from wild mammal foraging.Crossref | GoogleScholarGoogle Scholar |
Baldwin, R. A., Meinerz, R., and Witmer, G. W. (2016). Cholecalciferol plus diphacinone baits for vole control: a novel approach to a historic problem. Journal of Pest Science 89, 129–135.
| Cholecalciferol plus diphacinone baits for vole control: a novel approach to a historic problem.Crossref | GoogleScholarGoogle Scholar |
Barneet, S., and Spencer, M. M. (1949). Sodium fluoracetate (1080) as a rat poison. The Journal of Hygiene 47, 426–430.
| Sodium fluoracetate (1080) as a rat poison.Crossref | GoogleScholarGoogle Scholar |
Bell J. (1975). Search for causes of poison failures. APDC Newsletter of the Agricultural Pests Destruction Council Newsletter 1.
Bengsen, A. (2014). Effects of coordinated poison-baiting programs on survival and abundance in two red fox populations. Wildlife Research 41, 194–202.
| Effects of coordinated poison-baiting programs on survival and abundance in two red fox populations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsleitLvJ&md5=2f4c762570748d7468da7a7577f9b801CAS |
Bengsen, A. J., Gentle, M. N., Mitchell, J. L., Pearson, H. E., and Saunders, G. R. (2014). Impacts and management of wild pigs Sus scrofa in Australia. Mammal Review 44, 135–147.
| Impacts and management of wild pigs Sus scrofa in Australia.Crossref | GoogleScholarGoogle Scholar |
Berry, O., Tatler, J., Hamilton, N., Hilmer, S., Hitchen, Y., and Algar, D. (2013). Slow recruitment in a red-fox population following poison baiting: a non-invasive mark–recapture analysis. Wildlife Research 40, 615–623.
| Slow recruitment in a red-fox population following poison baiting: a non-invasive mark–recapture analysis.Crossref | GoogleScholarGoogle Scholar |
Blackie, H., Barrett, B., and MacKay, J. (2014). Novel long-term possum control tools in New Zealand. In ‘16th Australasian Vertebrate Pest Conference, Brisbane, Australia’. (Ed. M. Gentle) pp. 139. (Invasive Animals Cooperative Research Centre: Canberra)
Blaszkowicz, N. (2016). What is resistance (and why does it matter?). International Pest Control 58, 202.
Boissy, A. (1995). Fear and fearfulness in animals. The Quarterly Review of Biology 70, 165–191.
| Fear and fearfulness in animals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MzjsVGlsQ%3D%3D&md5=8a75239bb92c4901a757d0c63f8f8f5bCAS |
Boyle, M. C. (1960). A case of apparent resistance of Rattus norvegicus to anticoagulant poisons. Nature 188, 517.
| A case of apparent resistance of Rattus norvegicus to anticoagulant poisons.Crossref | GoogleScholarGoogle Scholar |
Bremner-Harrison, S., Prodohl, P. A., and Elwood, R. W. (2004). Behavioural trait assessment as a release criterion: boldness predicts early death in a reintroduction programme of captive‐bred swift fox (Vulpes velox). Animal Conservation 7, 313–320.
| Behavioural trait assessment as a release criterion: boldness predicts early death in a reintroduction programme of captive‐bred swift fox (Vulpes velox).Crossref | GoogleScholarGoogle Scholar |
Brunton, C. F. A., Macdonald, D. W., and Buckle, A. P. (1993). Behavioural resistance towards poison baits in brown rats, Rattus norvegicus. Applied Animal Behaviour Science 38, 159–174.
| Behavioural resistance towards poison baits in brown rats, Rattus norvegicus.Crossref | GoogleScholarGoogle Scholar |
Buckle, A., Charlton, J., Meyer, A., and Prescott, C. (2010). Anticoagulant resistance in the Norway rat and guidelines for the management of resistant rat infestations in the UK. Rodenticide Resistance Action Group, UK.
Burrows, N. D., and Christensen, P. E. S. (2002). Long-term trends in native mammal capture rates in a jarrah forest in south-western Australia. Australian Forestry 65, 211–219.
| Long-term trends in native mammal capture rates in a jarrah forest in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Calver, M., and King, D. (1986). Controlling vertebrate pests with fluoroacetate: lessons in wildlife management, bio-ethics, and co-evolution. Journal of Biological Education 20, 257–262.
| Controlling vertebrate pests with fluoroacetate: lessons in wildlife management, bio-ethics, and co-evolution.Crossref | GoogleScholarGoogle Scholar |
Calver, M. C., King, D. R., Bradley, J. S., Gardner, J. L., and Martin, G. (1989). An assessment of the potential target specificity of 1080 predator baiting in Western Australia. Wildlife Research 16, 625–638.
| An assessment of the potential target specificity of 1080 predator baiting in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Carrick, R. (1957). What is the best free-feeding system for furrow-poisoning the rabbit? Wildlife Research 2, 78–84.
| What is the best free-feeding system for furrow-poisoning the rabbit?Crossref | GoogleScholarGoogle Scholar |
Carter, A., and Luck, G. W. (2013). Fox baiting in agricultural landscapes: preliminary findings on the importance of bait-site selection. Wildlife Research 40, 184–195.
| Fox baiting in agricultural landscapes: preliminary findings on the importance of bait-site selection.Crossref | GoogleScholarGoogle Scholar |
Choquenot, D., McIlroy, J., and Korn, T. (1996). ‘Managing Vertebrate Pests: Feral Pigs.’ (Australian Government Publishing Service: Canberra.)
Cook, C. J. (1999). Smarter baits: the effects of stress on bait aversion and options to avoid the development of bait aversions. New Zealand Journal of Ecology 23, 275–279.
Corbet, G., and Harris, S. (1991). ‘The Handbook of British Animals.’ 3rd edn. (Blackwell Scientific Publications: Oxford.)
Cowan, P. E. (1977). Neophobia and neophilia: new object and new place reactions of three Rattus species. Journal of Comparative and Physiological Psychology 91, 63–71.
| Neophobia and neophilia: new object and new place reactions of three Rattus species.Crossref | GoogleScholarGoogle Scholar |
Daniells, L., Buckle, A., and Prescott, C. (2011). Resistance as a factor in environmental exposure of anticoagulant rodenticides – a modelling approach. Julius-Kühn-Archiv 432, 58.
de Tores, P. J., and Marlow, N. (2012). The relative merits of predator-exclusion fencing and repeated fox baiting for protection of native fauna: five case studies from Western Australia. In ‘Fencing for Conservation: Restriction of evolutionary potential or a riposte to threatening processes?’. (Eds M. J. Somers, and M. Hayward.) pp. 21–42. (Springer: New York.)
Devine, C. D., and Cook, C. J. (1998). Bait shyness and its prevention in the rabbit Oryctolagus cuniculus L. New Zealand Journal of Zoology 25, 223–229.
| Bait shyness and its prevention in the rabbit Oryctolagus cuniculus L.Crossref | GoogleScholarGoogle Scholar |
Doherty, T. S., and Algar, D. (2015). Response of feral cats to a track‐based baiting programme using Eradicat® baits. Ecological Management & Restoration 16, 124–130.
| Response of feral cats to a track‐based baiting programme using Eradicat® baits.Crossref | GoogleScholarGoogle Scholar |
Dundas, S. J., Adams, P. J., and Fleming, P. A. (2014). First in, first served: uptake of 1080 poison fox baits in south-west Western Australia. Wildlife Research 41, 117–126.
| First in, first served: uptake of 1080 poison fox baits in south-west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Eason, C. (2002). Sodium monofluoroacetate (1080) risk assessment and risk communication. Toxicology 181–182, 523–530.
| Sodium monofluoroacetate (1080) risk assessment and risk communication.Crossref | GoogleScholarGoogle Scholar |
Eason, C., and Spurr, E. (1995). Review of the toxicity and impacts of brodifacoum on non‐target wildlife in New Zealand. New Zealand Journal of Zoology 22, 371–379.
| Review of the toxicity and impacts of brodifacoum on non‐target wildlife in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Eason, C., Gooneratne, R., Wright, G., Pierce, R., and Frampton, C. (1993). The fate of sodium monofluoroacetate (1080) in water, mammals and invertebrates. In ‘Proceedings of the 46th New Zealand Plant Protection Conference’. (Ed. A. J. Popay) pp. 297–301. (New Zealand Plant Protection Society Inc.: New Zealand.)
Eason, C., Ogilvie, S., Miller, A., Henderson, R., Shapiro, L., Hix, S., MacMorran, D., and Murphy, E. (2008). Smarter pest control tools with low-residue and humane toxins. In ‘Proceedings of the 23rd Vertebrate Pest Conference’. (Eds R. M. Timm and M. B. Madon) pp. 148–153. (University of California: Davis, CA.)
Eason, C. T., Miller, A., MacMorran, D. B., and Murphy, E. C. (2014). Toxicology and ecotoxicology of para-aminopropiophenone (PAPP) – a new predator control tool for stoats and feral cats in New Zealand. New Zealand Journal of Ecology 38, 177–188.
Eisler, R. (1995). Sodium monofluoroacetate (1080) hazards to fish, wildlife, and invertebrates: a synoptic review. US Department of the Interior, National Biological Service, USA.
Fleming, P. A., and Bateman, P. W. (2016). The good, the bad and the ugly: which Australian terrestrial mammals attract most research? Mammal Review 46, 241–254.
| The good, the bad and the ugly: which Australian terrestrial mammals attract most research?Crossref | GoogleScholarGoogle Scholar |
Fleming, P. A., Dundas, S. J., Lau, Y. Y. W., and Pluske, J. R. (2016). Predation by red foxes (Vulpes vulpes) at an outdoor piggery. Animals (Basel) 6, 60.
| Predation by red foxes (Vulpes vulpes) at an outdoor piggery.Crossref | GoogleScholarGoogle Scholar |
Fleming, P. J. S., and Parker, R. W. (1991). Temporal decline of 1080 within meat baits used for control of wild dogs in New South Wales. Wildlife Research 18, 729–740.
| Temporal decline of 1080 within meat baits used for control of wild dogs in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Forbes-Harper, J. L., Crawford, H. M., Dundas, S. J., Warburton, N. M., Adams, P. J., Bateman, P. W., Calver, M. C., and Fleming, P. A. (2017). Diet and bite force in red foxes: ontogenetic and sex differences in an invasive carnivore. Journal of Zoology , .
| Diet and bite force in red foxes: ontogenetic and sex differences in an invasive carnivore.Crossref | GoogleScholarGoogle Scholar |
Frampton, C., Warburton, B., Henderson, R., and Morgan, D. (1999). Optimising bait size and 1080 concentration (sodium monofluoroacetate) for the control of brushtail possums Trichosurus vulpecula). Wildlife Research 26, 53–59.
| Optimising bait size and 1080 concentration (sodium monofluoroacetate) for the control of brushtail possums Trichosurus vulpecula).Crossref | GoogleScholarGoogle Scholar |
Frank, L. (1979). Selective predation and seasonal variation in the diet of the fox, Vulpes vulpes in N.E. Scotland. Journal of Zoology 189, 526–532.
| Selective predation and seasonal variation in the diet of the fox, Vulpes vulpes in N.E. Scotland.Crossref | GoogleScholarGoogle Scholar |
Frost, A. J., Winrow-Giffen, A., Ashley, P. J., and Sneddon, L. U. (2007). Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proceedings. Biological Sciences 274, 333–339.
| Plasticity in animal personality traits: does prior experience alter the degree of boldness?Crossref | GoogleScholarGoogle Scholar |
Galef, B. G. (1985). Direct and indirect behavioral pathways to the social transmission of food avoidance. Annals of the New York Academy of Sciences 443, 203–215.
| Direct and indirect behavioral pathways to the social transmission of food avoidance.Crossref | GoogleScholarGoogle Scholar |
Galef, B. G. (1990). A historical perspective on recent studies of social learning about foods by Norway rats. Canadian Journal of Psychology 44, 311.
| A historical perspective on recent studies of social learning about foods by Norway rats.Crossref | GoogleScholarGoogle Scholar |
Galef, B. G., and Laland, K. N. (2005). Social learning in animals: empirical studies and theoretical models. Bioscience 55, 489–499.
| Social learning in animals: empirical studies and theoretical models.Crossref | GoogleScholarGoogle Scholar |
Gentle, M. (2005). Factors affecting the efficiency of fox baiting practices on the Central Tablelands of New South Wales. PhD thesis, University of Canberra, Canberra.
Gentle, M., Massei, G., and Saunders, G. (2004). Levamisole can reduce bait monopolization in wild red foxes Vulpes vulpes. Mammal Review 34, 325–330.
| Levamisole can reduce bait monopolization in wild red foxes Vulpes vulpes.Crossref | GoogleScholarGoogle Scholar |
Gentle, M., Elsworth, P., and Parker, B. (2005). Sodium fluoroacetate residue in feral pigs (Sus scrofa) carcasses – is it a significant secondary poisoning hazard. In ‘13th Australasian Vertebrate Pest Conference, Wellington, New Zealand’. (Eds J. Parkes, M. Stahtam, G. Edwards) pp. 143–147. (Landcare Research: Lincoln, New Zealand.)
Gentle, M., Saunders, G., and Dickman, C. (2007a). Persistence of sodium monofluoroacetate (1080) in fox baits and implications for fox management in south-eastern Australia. Wildlife Research 34, 325–333.
| Persistence of sodium monofluoroacetate (1080) in fox baits and implications for fox management in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnsV2gsrc%3D&md5=22edf182d6f146856a9e3ca2bea68fcfCAS |
Gentle, M. N., Saunders, G. R., and Dickman, C. R. (2007b). Poisoning for production: how effective is fox baiting in south‐eastern Australia? Mammal Review 37, 177–190.
| Poisoning for production: how effective is fox baiting in south‐eastern Australia?Crossref | GoogleScholarGoogle Scholar |
Glen, A. S., Gentle, M. N., and Dickman, C. R. (2007). Non-target impacts of poison baiting for predator control in Australia. Mammal Review 37, 191–205.
| Non-target impacts of poison baiting for predator control in Australia.Crossref | GoogleScholarGoogle Scholar |
Gong, W., Sinden, J., Braysher, M. L., Jones, R., and Wales, N. S. (2009). The economic impacts of vertebrate pests in Australia. Invasive Animals Cooperative Research Centre: Canberra.
Gooneratne, S., Eason, C., Dickson, C., Fitzgerald, H., and Right, G. (1995). Persistence of sodium monofluoroacetate in rabbits and risks to non-target species. Human and Experimental Toxicology 14, 212–216.
| Persistence of sodium monofluoroacetate in rabbits and risks to non-target species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXkvVGmt7k%3D&md5=4e09076931be86947ab29e0828714b00CAS |
Green, D. (1946). Service policy on the use of compound 1080 (sodium fluoroacetate). US Fish and Wildlife Service, Division of Predator and Rodent Control, Memorandum No. 121 (revised).
Gustavson, C. R. (1977). Comparative and field aspects of learned food aversions. In ‘Learning Mechanisms in Food Selection’. (Eds L. Barker, B. Best, and M. Domjam.) pp. 22–44. (Baylor University Press: Waco, TX.)
Henderson, R., Morriss, G., and Morgan, D. (1997). The use of different types of toxic bait for sustained control of possums. In ‘Proceedings of the New Zealand Plant Protection Conference 50’. (Ed. M. O’Callaghan) pp. 382–390. (New Zealand Plant Protection Society Inc.: New Zealand.)
Henry, J. (1977). The use of urine marking in the scavenging behaviour of the red fox (Vulpes vulpes). Behaviour 61, 82–105.
| The use of urine marking in the scavenging behaviour of the red fox (Vulpes vulpes).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2s3gtFequw%3D%3D&md5=3bb4b67d5cc9e4a6b932feb143879c73CAS |
Hickling, G. J. (1994). Behavioural resistance by vertebrate pests to 1080 toxin: implications for sustainable pest management in New Zealand. In ‘Proceedings of the Science Workshop on 1080’. The Royal Society of New Zealand Miscellaneous Series. (Eds A. A. Seawright, and C. T. Eason.) pp. 151–158. (The Royal Society of New Zealand, Wellington: New Zealand.)
Hickling, G. J., Henderson, R. J., and Thomas, M. C. C. (1999). Poisoning mammalian pests can have unintended consequences for future control: two case studies. New Zealand Journal of Ecology 23, 267–273.
Hone, J., and Kleba, R. (1984). The toxicity and acceptability of warfarin and 1080 poison to penned feral pigs. Wildlife Research 11, 103–111.
| The toxicity and acceptability of warfarin and 1080 poison to penned feral pigs.Crossref | GoogleScholarGoogle Scholar |
Howald, G., Donlan, C., Galván, J. P., Russell, J. C., Parkes, J., Samaniego, A., Wang, Y., Veitch, D., Genovesi, P., and Pascal, M. (2007). Invasive rodent eradication on islands. Conservation Biology 21, 1258–1268.
| Invasive rodent eradication on islands.Crossref | GoogleScholarGoogle Scholar |
Howard, W., Marsh, R., and Palmateer, S. (1973). Selective breeding of rats for resistance to sodium monofluoroacetate. Journal of Applied Ecology 10, 731–735.
| Selective breeding of rats for resistance to sodium monofluoroacetate.Crossref | GoogleScholarGoogle Scholar |
Hunt, R. J., Dall, D. J., and Lapidge, S. J. (2007). Effect of a synthetic lure on site visitation and bait uptake by foxes (Vulpes vulpes) and wild dogs (Canis lupus dingo, Canis lupus familiaris). Wildlife Research 34, 461–466.
| Effect of a synthetic lure on site visitation and bait uptake by foxes (Vulpes vulpes) and wild dogs (Canis lupus dingo, Canis lupus familiaris).Crossref | GoogleScholarGoogle Scholar |
Innes, J., and Barker, G. (1999). Ecological consequences of toxin use for mammalian pest control in New Zealand – an overview. New Zealand Journal of Ecology 23, 111–127.
Innes, J., Warburton, B., Williams, D., Speed, H., and Bradfield, P. (1995). Large-scale poisoning of ship rats (Rattus rattus) in indigenous forests of the North Island, New Zealand. New Zealand Journal of Ecology 19, 5–17.
Ioannou, C., Payne, M., and Krause, J. (2008). Ecological consequences of the bold–shy continuum: the effect of predator boldness on prey risk. Oecologia 157, 177–182.
| Ecological consequences of the bold–shy continuum: the effect of predator boldness on prey risk.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cvjslKrsA%3D%3D&md5=e744ac994502a3f39a2b1724352e4343CAS |
Jackson, W. B. (1972). Biological and behavioural studies of rodents as a basis for control. Bulletin of the World Health Organization 47, 281.
| 1:STN:280:DyaE3s%2FptVKltg%3D%3D&md5=409a7530995f87d2e6e1089fafd0bb0aCAS |
Jackson, W. B., and Kaukeinen, D. (1972). Resistance of wild Norway rats in North Carolina to warfarin rodenticide. Science 176, 1343–1344.
| Resistance of wild Norway rats in North Carolina to warfarin rodenticide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38XkvFOktrg%3D&md5=ae31f3a824301073a5718dfd033865adCAS |
Kalat, J. (1974). Taste salience depends on novelty, not concentration, in taste-aversion learning in the rat. Journal of Comparative and Physiological Psychology 86, 47.
| Taste salience depends on novelty, not concentration, in taste-aversion learning in the rat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXhtVKmsbo%3D&md5=14562db2b2971d6a3b9cc3decd51ca65CAS |
Kay, B., Saunders, G., and McLeod, L. (1999). Caching of baits by foxes (Vulpes vulpes) on agricultural lands. Wildlife Research 26, 335–340.
| Caching of baits by foxes (Vulpes vulpes) on agricultural lands.Crossref | GoogleScholarGoogle Scholar |
Kinnear, J. E., Onus, M. L., and Sumner, N. R. (1998). Fox control and rock-wallaby population dynamics – II. An update. Wildlife Research 25, 81–88.
| Fox control and rock-wallaby population dynamics – II. An update.Crossref | GoogleScholarGoogle Scholar |
Kinnear, J. E., Krebs, C. J., Pentland, C., Orell, P., Holme, C., and Karvinen, R. (2010). Predator-baiting experiments for the conservation of rock-wallabies in Western Australia: a 25-year review with recent advances. Wildlife Research 37, 57–67.
| Predator-baiting experiments for the conservation of rock-wallabies in Western Australia: a 25-year review with recent advances.Crossref | GoogleScholarGoogle Scholar |
Kinnear, J. E., Pentland, C., Moore, N., and Krebs, C. J. (2017). Fox control and 1080 baiting conundrums: time to prepare for a CRISPR solution. Australian Mammalogy 39, 127–136.
| Fox control and 1080 baiting conundrums: time to prepare for a CRISPR solution.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, W. E. (1999). Assessment of sodium monofluroacetate (1080) in baits and its biodegradation by microorganisms. M.Sc. Thesis, Curtin University of Technology, Perth.
Kolar, C. S., and Lodge, D. M. (2001). Progress in invasion biology: predicting invaders. Trends in Ecology & Evolution 16, 199–204.
| Progress in invasion biology: predicting invaders.Crossref | GoogleScholarGoogle Scholar |
Korhonen, H., and Niemelä, P. (1996). Temperament and reproductive success in farmbred silver foxes housed with and without platforms. Journal of Animal Breeding and Genetics 113, 209–218.
| Temperament and reproductive success in farmbred silver foxes housed with and without platforms.Crossref | GoogleScholarGoogle Scholar |
Körtner, G., and Watson, P. (2005). The immediate impact of 1080 aerial baiting to control wild dogs on a spotted-tailed quoll population. Wildlife Research 32, 673–680.
| The immediate impact of 1080 aerial baiting to control wild dogs on a spotted-tailed quoll population.Crossref | GoogleScholarGoogle Scholar |
Lee, L. L., Howard, W. E., and Marsh, R. E. (1990). Acquired strychnine tolerance by pocket gophers. In ‘Proceedings of the 14th Vertebrate Pest Conference 1990’. (Eds L.R. Davis and R.E. Marsh) Paper 52. (University of California: Davis, CA)
Lynch, M., and Lande, R. (1993). Evolution and extinction in response to environmental change. In ‘Biotic Interactions and Global Change’. (Eds P. M. Kareiva, J. G. Kingsolver, and R. B. Huey.) pp. 234–250. (Sinauer Associates: Sunderland, MA.)
Macdonald, D. (1976). Food caching by red foxes and some other carnivores. Zeitschrift für Tierpsychologie 42, 170–185.
| Food caching by red foxes and some other carnivores.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2s%2Fotl2ntg%3D%3D&md5=bc6bbd0e0373391bb9d216403054ebb7CAS |
Macdonald, D., and Baker, S. (2004). Non-lethal control of fox predation: the potential of generalised aversion. Animal Welfare (South Mimms, England) 13, 77–85.
| 1:CAS:528:DC%2BD2cXhtFSnuro%3D&md5=4a470d4831c5b344d99dc8bc1c088ce4CAS |
Macdonald, D., Brown, L., Yerli, S., and Canbolat, A. (1994). Behaviour of red foxes, Vulpes vulpes, caching eggs of loggerhead turtles, Caretta caretta. Journal of Mammalogy 75, 985–988.
| Behaviour of red foxes, Vulpes vulpes, caching eggs of loggerhead turtles, Caretta caretta.Crossref | GoogleScholarGoogle Scholar |
Marks, C. A., Gigliotti, F., and Busana, F. (2009). Assuring that 1080 toxicosis in the red fox (Vulpes vulpes) is humane. II. Analgesic drugs produce better welfare outcomes. Wildlife Research 36, 98–105.
| Assuring that 1080 toxicosis in the red fox (Vulpes vulpes) is humane. II. Analgesic drugs produce better welfare outcomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitVyhtLc%3D&md5=e270b6e48b67dbf5286d0425b5f44763CAS |
Marlow, N. J., Thomas, N. D., Williams, A. A. E., Macmahon, B., Lawson, J., Hitchen, Y., Angus, J., and Berry, O. (2015). Lethal 1080 baiting continues to reduce European red fox (Vulpes vulpes) abundance after more than 25 years of continuous use in south‐west Western Australia. Ecological Management & Restoration 16, 131–141.
| Lethal 1080 baiting continues to reduce European red fox (Vulpes vulpes) abundance after more than 25 years of continuous use in south‐west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Martin, G. R., and Twigg, L. E. (2002). Sensitivity to sodium fluoroacetate (1080) of native animals from north-western Australia. Wildlife Research 29, 75–83.
| Sensitivity to sodium fluoroacetate (1080) of native animals from north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Massei, G., Lyon, A. J., and Cowan, D. P. (2002). Conditioned taste aversion can reduce egg predation by rats. Journal of Wildlife Management 66, 1134–1140.
| Conditioned taste aversion can reduce egg predation by rats.Crossref | GoogleScholarGoogle Scholar |
May, T. M., Page, M. J., and Fleming, P. A. (2016). Predicting survivors: animal temperament and translocation. Behavioural Ecology 27, 969–977.
| Predicting survivors: animal temperament and translocation.Crossref | GoogleScholarGoogle Scholar |
McDougall, P. T., Réale, D., Sol, D., and Reader, S. M. (2006). Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Animal Conservation 9, 39–48.
| Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations.Crossref | GoogleScholarGoogle Scholar |
McIlroy, J. C. (1981). The sensitivity of Australian animals to 1080 poison. II. Marsupial and eutherian carnivores. Wildlife Research 8, 385–399.
| The sensitivity of Australian animals to 1080 poison. II. Marsupial and eutherian carnivores.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XotFShtg%3D%3D&md5=cfff7a9ae18e531edbd6958d9e00d3edCAS |
McIlroy, J. C. (1983). The sensitivity of Australian animals to 1080 poison. V. The sensitivity of feral pigs, Sus scrofa, to 1080 and its implications for poisoning campaigns. Wildlife Research 10, 139–148.
| The sensitivity of Australian animals to 1080 poison. V. The sensitivity of feral pigs, Sus scrofa, to 1080 and its implications for poisoning campaigns.Crossref | GoogleScholarGoogle Scholar |
McIlroy, J. C. (1986). The sensitivity of Australian animals to 1080 poison. IX. Comparisons between the major groups of animals, and the potential danger nontarget species face from 1080 poisoning campaigns. Wildlife Research 13, 39–48.
| The sensitivity of Australian animals to 1080 poison. IX. Comparisons between the major groups of animals, and the potential danger nontarget species face from 1080 poisoning campaigns.Crossref | GoogleScholarGoogle Scholar |
McIlroy, J. C., and King, D. R. (1990). Appropriate amounts of 1080 poison in baits to control foxes, Vulpes vulpes. Australian Wildlife Research 17, 11–13.
| Appropriate amounts of 1080 poison in baits to control foxes, Vulpes vulpes.Crossref | GoogleScholarGoogle Scholar |
McLeod, L. J., Saunders, G. R., and Miners, A. (2011). Can shooting be an effective management tool for foxes? Preliminary insights from a management programme. Ecological Management & Restoration 12, 224–226.
| Can shooting be an effective management tool for foxes? Preliminary insights from a management programme.Crossref | GoogleScholarGoogle Scholar |
Meek, P. D., Ballard, G.-A., Fleming, P. J. S., Schaefer, M., Williams, W., and Falzon, G. (2014). Camera traps can be heard and seen by animals. PLoS One 9, e110832.
| Camera traps can be heard and seen by animals.Crossref | GoogleScholarGoogle Scholar |
Meek, P., Ballard, G., Fleming, P., and Falzon, G. (2016). Are we getting the full picture? Animal responses to camera traps and implications for predator studies. Ecology and Evolution 6, 3216–3225.
| Are we getting the full picture? Animal responses to camera traps and implications for predator studies.Crossref | GoogleScholarGoogle Scholar |
Merks, P. F., and Calver, M. C. (1989). The effect of ant activity on meat baits impregnated with sodium monofluoroacetate (Compound 1080) used for the control of dingoes and wild dogs. The Rangeland Journal 11, 83–87.
| The effect of ant activity on meat baits impregnated with sodium monofluoroacetate (Compound 1080) used for the control of dingoes and wild dogs.Crossref | GoogleScholarGoogle Scholar |
Morgan, D. R. (1982). Field acceptance of non-toxic and toxic baits by populations of the brushtail possum (Trichosurus vulpecula Kerr). New Zealand Journal of Ecology 5, 36–43.
Morgan, D. (1990). Behavioral response of brushtail possums, Trichosurus vulpecula, to baits used in pest control. Wildlife Research 17, 601–613.
| Behavioral response of brushtail possums, Trichosurus vulpecula, to baits used in pest control.Crossref | GoogleScholarGoogle Scholar |
Morgan, D. R. (2004). Maximising the effectiveness of aerial 1080 control of possums (Trichosurus vulpecula). PhD Thesis, Lincoln University, New Zealand.
Morgan, D. R., and Milne, L. (2002). Cholecalciferol-induced bait shyness in possums (Trichosurus vulpecula). International Journal of Pest Management 48, 113–119.
| Cholecalciferol-induced bait shyness in possums (Trichosurus vulpecula).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xks1egtLg%3D&md5=bc74651765b1dbb13c824a2835519378CAS |
Morgan, D. R., Batcheler, C. L., and Peters, J. A. (1987). Why do possums survive aerial poisoning operations? In ‘Proceedings of the Twelfth Vertebrate Pest Conference (1986)’. (Ed. T. P. Salmon) pp. 210–214. (University of California: Davis, CA.)
Morgan, D. R., Morriss, G., and Hickling, G. J. (1996). Induced 1080 bait-shyness in captive brushtail possums and implications for management. Wildlife Research 23, 207–211.
| Induced 1080 bait-shyness in captive brushtail possums and implications for management.Crossref | GoogleScholarGoogle Scholar |
Morgan, D. R., Milne, L., O’Connor, C., and Ruscoe, W. A. (2001). Bait shyness in possums induced by sublethal doses of cyanide paste bait. International Journal of Pest Management 47, 277–284.
| Bait shyness in possums induced by sublethal doses of cyanide paste bait.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXoslamur0%3D&md5=2bc29d2a92c805757a84835067de0d33CAS |
Morriss, G., and Warburton, B. (2014). Modified Vistor® Easy Set® rat traps for trapping stoats and ship rats in New Zealand: pen and field trials. In ‘16th Australasian Vertebrate Pest Conference’. (Ed. M. Gentle) pp. 125. (Invasive Animals Cooperative Research Centre: Canberra)
Moseby, K. E., Hill, B. M., and Read, J. L. (2009). Arid Recovery – a comparison of reptile and small mammal populations inside and outside a large rabbit, cat and fox-proof exclosure in arid South Australia. Austral Ecology 34, 156–169.
| Arid Recovery – a comparison of reptile and small mammal populations inside and outside a large rabbit, cat and fox-proof exclosure in arid South Australia.Crossref | GoogleScholarGoogle Scholar |
Moss, Z. N., O’Connor, C. E., and Hickling, G. J. (1998). Implications of prefeeding for the development of bait aversions in brushtail possums (Trichosurus vulpecula). Wildlife Research 25, 133–138.
| Implications of prefeeding for the development of bait aversions in brushtail possums (Trichosurus vulpecula).Crossref | GoogleScholarGoogle Scholar |
Murphy, E., Sjoberg, T., Dilks, P., MacMorran, D., Eason, C., and Aylett, P. (2014). Development of re-setting toxin delivery devices and long-life lures for rats. In ‘16th Australasian Vertebrate Pest Conference’. (Ed. M. Gentle) pp. 129. (Invasive Animals Cooperative Research Centre: Canberra)
Nugent, G., Morgan, D., Clayton, R., and Warburton, B. (2010). Improving the efficacy of aerial poisoning of brushtail possums (Trichosurus vulpecula) through reduced fragmentation of bait. International Journal of Pest Management 57, 51–59.
| Improving the efficacy of aerial poisoning of brushtail possums (Trichosurus vulpecula) through reduced fragmentation of bait.Crossref | GoogleScholarGoogle Scholar |
O’Brien, P. H. (1988). The toxicity of sodium monofluoroacetate (Compound 1080) to captive feral pigs, Sus scrofa. Wildlife Research 15, 163–170.
| The toxicity of sodium monofluoroacetate (Compound 1080) to captive feral pigs, Sus scrofa.Crossref | GoogleScholarGoogle Scholar |
O’Brien, P. H., and Lukins, B. S. (1988). Factors influencing the intake of sodium monofluoroacetate (Compound-1080) by free-ranging feral pigs. Wildlife Research 15, 285–291.
| Factors influencing the intake of sodium monofluoroacetate (Compound-1080) by free-ranging feral pigs.Crossref | GoogleScholarGoogle Scholar |
O’Brien, P., Kleba, R., Beck, J., and Baker, P. (1986). Vomiting by feral pigs after 1080 intoxication: non-target hazard and influence of anti-emetics. Wildlife Society Bulletin 14, 425–432.
O’Connor, C., and Matthews, L. (1996). Behavioural mechanisms of bait and poison avoidance. Improving conventional control of possums. The Royal Society of New Zealand Miscellaneous Series 35, 51–53.
O’Connor, C., and Matthews, L. (1999). 1080-induced bait aversions in wild possums: influence of bait characteristics and prevalence. Wildlife Research 26, 375–381.
| 1080-induced bait aversions in wild possums: influence of bait characteristics and prevalence.Crossref | GoogleScholarGoogle Scholar |
Ogilvie, S., Thomas, M., Fitzgerald, H., and Morgan, D. (1996). Sodium monofluoroacetate (1080) bait-shyness in a wild brushtail possum (Trichosurus vulpecula) population. In ‘Proceedings of the New Zealand Plant Protection Conference 49 (1996)’. (Ed. M. O’Callaghan) pp. 143–146. (New Zealand Plant Protection Society Inc.: New Zealand.)
Ogilvie, S. C., Thomas, M. D., Morriss, G. A., Morgan, D. R., and Eason, C. T. (2000). Investigation of sodium monofluoroacetate (1080) bait shyness in wild brushtail possum (Trichosurus vulpecula) populations. International Journal of Pest Management 46, 77–80.
| Investigation of sodium monofluoroacetate (1080) bait shyness in wild brushtail possum (Trichosurus vulpecula) populations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktVClsro%3D&md5=6aceeb0b4416a500440161e79e03ea38CAS |
Oliver, A., King, D., and Mead, R. (1977). The evolution of resistance to fluoroacetate intoxication in mammals. Search 8, 130–132.
| 1:CAS:528:DyaE2sXltFaktr8%3D&md5=6b5061d2436f10eb60d5e732c744efc3CAS |
Oliver, A., Wheeler, S., and Gooding, C. (1982). Field evaluation of 1080 and pindone oat bait, and the possible decline in effectiveness of poison baiting for the control of the rabbit, Oryctolagus cuniculus. Wildlife Research 9, 125–134.
| Field evaluation of 1080 and pindone oat bait, and the possible decline in effectiveness of poison baiting for the control of the rabbit, Oryctolagus cuniculus.Crossref | GoogleScholarGoogle Scholar |
Osborne, E., Ballard, G., Vernes, K., and Fleming, P. (2014). M-44 ejector activated by red foxes (Vulpes vulpes) in agri-ecosystems. In ‘16th Australasian Vertebrate Pest Conference’. (Ed. M. Gentle) pp. 149. (Invasive Animals Cooperative Research Centre: Canberra)
Parkes, J. P., Robley, A., Forsyth, D. M., and Choquenot, D. (2006). Adaptive management experiments in vertebrate pest control in New Zealand and Australia. Wildlife Society Bulletin 34, 229–236.
| Adaptive management experiments in vertebrate pest control in New Zealand and Australia.Crossref | GoogleScholarGoogle Scholar |
Peacock, E. (1964). Sodium monofluoroacetate (compound 1080). US Bureau of Sport Fisheries and Wildlife, Division of Predator and Rodent Control, USA.
PestSmart Connect (2016a). PAPP for wild dog and fox control. Invasive Animals Cooperative Research Centre: Canberra.
PestSmart Connect (2016b). PestSmart Factsheet: European red fox. Invasive Animals Cooperative Research Centre: Canberra.
Poole, W. (1963). Field enclosure experiments on the technique of poisoning the rabbit, Oryctolagus cuniculus (L.). II. A study of territorial behaviour and the use of bait stations. Wildlife Research 8, 28–35.
| Field enclosure experiments on the technique of poisoning the rabbit, Oryctolagus cuniculus (L.). II. A study of territorial behaviour and the use of bait stations.Crossref | GoogleScholarGoogle Scholar |
Pospischil, R., and Schnorbach, H.-J. (1994). Racumin Plus, a new promising rodenticide against rats and mice. In ‘Proceedings of the 16th Vertebrate Pest Conference’. (Eds W. S. Halverson, and A. C. Crabb.) pp. 48. (University of California: Davis, CA.)
Prakash, I. (1988). Bait shyness and poison aversion. In ‘Rodent Pest Management’. (Ed. I. Prakash.) pp. 321–329. (CRC Press: Florida, USA.)
Quy, R. J., Shepherd, D. S., and Inglis, I. R. (1992). Bait avoidance and effectiveness of anticoagulant rodenticides against warfarin and difenacoum-resistant populations of Norway rats (Rattus norvegicus). Crop Protection 11, 14–20.
| Bait avoidance and effectiveness of anticoagulant rodenticides against warfarin and difenacoum-resistant populations of Norway rats (Rattus norvegicus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhvV2murk%3D&md5=608fed8111a96d0c90098fe7e448b9beCAS |
Rathore, A. K. (1985). Use of metoclopramide to prevent 1080-induced emesis in wild pigs. Journal of Wildlife Management 49, 55–56.
| Use of metoclopramide to prevent 1080-induced emesis in wild pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhvF2mtL4%3D&md5=ffe631e8389e08ea99bb9d360df14f90CAS |
Read, J., Gigliotti, F., Darby, S., and Lapidge, S. (2014). Dying to be clean: pen trials of novel cat and fox control devices. International Journal of Pest Management 60, 166–172.
| Dying to be clean: pen trials of novel cat and fox control devices.Crossref | GoogleScholarGoogle Scholar |
Réale, D., Reader, S. M., Sol, D., McDougall, P. T., and Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biological Reviews of the Cambridge Philosophical Society 82, 291–318.
| Integrating animal temperament within ecology and evolution.Crossref | GoogleScholarGoogle Scholar |
Reddiex, B., and Forsyth, D. M. (2006). Control of pest mammals for biodiversity protection in Australia. II. Reliability of knowledge. Wildlife Research 33, 711–717.
| Control of pest mammals for biodiversity protection in Australia. II. Reliability of knowledge.Crossref | GoogleScholarGoogle Scholar |
Risbey, D. A., Calver, M. C., Short, J., Bradley, J. S., and Wright, I. W. (2000). The impact of cats and foxes on the small vertebrate fauna of Heirisson Prong, Western Australia. II. A field experiment. Wildlife Research 27, 223–235.
| The impact of cats and foxes on the small vertebrate fauna of Heirisson Prong, Western Australia. II. A field experiment.Crossref | GoogleScholarGoogle Scholar |
Ross, J. G. (1999). Cost-effective control of 1080 bait-shy possums (Trichosurus vulpecula). PhD thesis, Lincoln University, Lincoln, New Zealand.
Ross, J. G., Hickling, G. J., and Morgan, D. R. (1997). Use of subacute and chronic toxicants to control sodium monofluoroacetate (1080) bait shy possums. In ‘Proceedings of the New Zealand Plant Protection Conference 50 (1997)’. (Ed. M. O’Callaghan) pp. 397–400. (The New Zealand Plant Protection Society Inc.: Auckland, NZ)
Ross, J., Hickling, G., Morgan, D., and Eason, C. (2000). The role of non-toxic prefeed and postfeed in the development and maintenance of 1080 bait shyness in captive brushtail possums. Wildlife Research 27, 69–74.
| The role of non-toxic prefeed and postfeed in the development and maintenance of 1080 bait shyness in captive brushtail possums.Crossref | GoogleScholarGoogle Scholar |
Rowley, I. (1958). Behaviour of a natural rabbit population poisoned with ‘1080‘. Wildlife Research 3, 32–39.
| Behaviour of a natural rabbit population poisoned with ‘1080‘.Crossref | GoogleScholarGoogle Scholar |
Rowley, I. (1963a). The effect on rabbits of repeated sublethal doses of sodium fluoroacetate. Wildlife Research 8, 52–55.
| The effect on rabbits of repeated sublethal doses of sodium fluoroacetate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXosFOmtg%3D%3D&md5=cb52a0beb6f2ab58153caff10b95303aCAS |
Rowley, I. (1963b). Field enclosure experiments on the technique of poisoning the rabbit, Oryctolagus cuniculus (L.). IV. A study of shyness in wild rabbits subjected to ‘1080’poisoning. Wildlife Research 8, 142–153.
| Field enclosure experiments on the technique of poisoning the rabbit, Oryctolagus cuniculus (L.). IV. A study of shyness in wild rabbits subjected to ‘1080’poisoning.Crossref | GoogleScholarGoogle Scholar |
Saunders, G., and McLeod, L. (2007). Improving fox management strategies in Australia. Bureau of Rural Sciences, Canberra.
Saunders, G., Coman, B., Kinnear, J., and Braysher, M. (1995). ‘Managing Vertebrate Pests: Foxes.’ (Australian Government Publishing Service: Australia.)
Saunders, G., McLeod, S., and Kay, B. (2000). Degradation of sodium monofluoroacetate (1080) in buried fox baits. Wildlife Research 27, 129–135.
| Degradation of sodium monofluoroacetate (1080) in buried fox baits.Crossref | GoogleScholarGoogle Scholar |
Scott, T. (1943). Some food coactions of the Northern Plains red fox. Ecological Monographs 13, 427–479.
| Some food coactions of the Northern Plains red fox.Crossref | GoogleScholarGoogle Scholar |
Shapiro, L., Eason, C., Bunt, C., Hix, S., Aylett, P., and MacMorran, D. (2016). Efficacy of encapsulated sodium nitrite as a new tool for feral pig management. Journal of Pest Science 89, 489–495.
| Efficacy of encapsulated sodium nitrite as a new tool for feral pig management.Crossref | GoogleScholarGoogle Scholar |
Sherley, M. (2007). Is sodium fluoroacetate (1080) a humane poison? Animal Welfare (South Mimms, England) 16, 449–458.
| 1:CAS:528:DC%2BD2sXhtlSlu7fJ&md5=d77924b515554a6fbcd0df7cc6c3ca0aCAS |
Shier, D. M., and Owings, D. H. (2007). Effects of social learning on predator training and postrelease survival in juvenile black-tailed prairie dogs, Cynomys ludovicianus. Animal Behaviour 73, 567–577.
| Effects of social learning on predator training and postrelease survival in juvenile black-tailed prairie dogs, Cynomys ludovicianus.Crossref | GoogleScholarGoogle Scholar |
Short, J., Bradshaw, S. D., Giles, J., Prince, R. I. T., and Wilson, G. R. (1992). Reintroduction of macropods (Marsupialia: Macropodoidea) in Australia – a review. Biological Conservation 62, 189–204.
| Reintroduction of macropods (Marsupialia: Macropodoidea) in Australia – a review.Crossref | GoogleScholarGoogle Scholar |
Silver, I. (1924). The reproductive potential of rats. Journal of Mammalogy 5, 66–67.
| The reproductive potential of rats.Crossref | GoogleScholarGoogle Scholar |
Sinclair, R. G., and Bird, P. L. (1984). The reaction of Sminthopsis crassicaudata to meat baits containing 1080: implications for assessing risk to non-target species. Wildlife Research 11, 501–507.
| The reaction of Sminthopsis crassicaudata to meat baits containing 1080: implications for assessing risk to non-target species.Crossref | GoogleScholarGoogle Scholar |
Sjoberg, T., Murphy, E., Barun, A., MacMorran, D., Aylett, P., and Barret, B. (2014). Novel multiple-kill control devices for feral cats. In ‘16th Australasian Vertebrate Pest Conference’. (Ed. M. Gentle) pp. 154. (Invasive Animals Cooperative Research Centre: Canberra)
Sloan Wilson, D., Clark, A. B., Coleman, K., and Dearstyne, T. (1994). Shyness and boldness in humans and other animals. Trends in Ecology & Evolution 9, 442–446.
| Shyness and boldness in humans and other animals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itFWntA%3D%3D&md5=4072b668a816489ea15e15be6ff0c572CAS |
Smith, B. R., and Blumstein, D. T. (2008). Fitness consequences of personality: a meta-analysis. Behavioural Ecology 19, 448–455.
| Fitness consequences of personality: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Springer, K. (2011). Planning processes for eradication of multiple pest species on Macquarie Island – an Australian case study. In ‘Island Invasives: Eradication and Management’. (Eds C. R. Veitch, M. N. Clout, and D. R. Towns.) pp. 228–232. (IUCN: Gland, Switzerland.)
Stamps, J. A., and Swaisgood, R. R. (2007). Someplace like home: experience, habitat selection and conservation biology. Applied Animal Behaviour Science 102, 392–409.
| Someplace like home: experience, habitat selection and conservation biology.Crossref | GoogleScholarGoogle Scholar |
Staples, E. (1969). Absorption of sodium monofluoroacetate (“1080”) solution by carrot baits. New Zealand Journal of Agricultural Research 12, 783–788.
| Absorption of sodium monofluoroacetate (“1080”) solution by carrot baits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3cXovFanug%3D%3D&md5=bcbf271f14fb7f8dca2367b396bc8c4eCAS |
Staples, L. D. (2017). A story of challenges & teamwork: development of a new toxin and bait for feral pig management. In ‘Proceedings of the 17th Australian Vertebrate Pest Conference’. (Ed. T. Buckmaster) Oral presentation. (Invasive Animals Cooperative Research Centre: Canberra)
Staples, L. D., McPhee, S. R., Bloomfield, T., and Wight, G. (1995). Foxoff fox baits: stability, lethal efficiency and degradation in soil. In ‘Proceedings of the 10th Australian Vertebrate Pest Conference’. (Ed. M. Statham) pp. 444–445. (Dept. of Primary Industry and Fisheries: Hobart, Tasmania)
Stryjek, R., and Modlinska, K. (2016). Neophobia in wild rats is elicited by using bait stations but not bait trays. International Journal of Pest Management 62, 158–164.
| Neophobia in wild rats is elicited by using bait stations but not bait trays.Crossref | GoogleScholarGoogle Scholar |
Sunnucks, P. (1998). Avoidance of novel objects by rabbits (Oryctolagus cuniculus L.). Wildlife Research 25, 273–283.
| Avoidance of novel objects by rabbits (Oryctolagus cuniculus L.).Crossref | GoogleScholarGoogle Scholar |
Tahori, A. (1963). Selection for a fluoroacetate resistant strain of house flies and investigation of its resistance pattern. Journal of Economic Entomology 56, 67–69.
| Selection for a fluoroacetate resistant strain of house flies and investigation of its resistance pattern.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3sXmvVaruw%3D%3D&md5=9415648a6bb242db7d71d10851fe8634CAS |
Thomas, M., Henderson, R., and Hickling, G. (1996). Optimising the use of bait stations for possum control. The Royal Society of New Zealand Miscellaneous Series 35, 65–69.
Thompson, J. A., and Fleming, P. J. S. (1994). Evaluation of the efficacy of 1080 poisoning of red foxes using visitation to non-toxic baits as an index of fox abundance. Wildlife Research 21, 27–39.
| Evaluation of the efficacy of 1080 poisoning of red foxes using visitation to non-toxic baits as an index of fox abundance.Crossref | GoogleScholarGoogle Scholar |
Thomson, P. C., and Algar, D. (2000). The uptake of dried meat baits by foxes and investigations of baiting rates in Western Australia. Wildlife Research 27, 451–456.
| The uptake of dried meat baits by foxes and investigations of baiting rates in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Thomson, P. C., and Kok, N. E. (2002). The fate of dried meat baits laid for fox control: the effects of bait presentation on take by foxes and non-target species, and on caching by foxes. Wildlife Research 29, 371–377.
| The fate of dried meat baits laid for fox control: the effects of bait presentation on take by foxes and non-target species, and on caching by foxes.Crossref | GoogleScholarGoogle Scholar |
Thomson, P. C., Marlow, N. J., Rose, K., and Kok, N. E. (2000). The effectiveness of a large-scale baiting campaign and an evaluation of a buffer zone strategy for fox control. Wildlife Research 27, 465–472.
| The effectiveness of a large-scale baiting campaign and an evaluation of a buffer zone strategy for fox control.Crossref | GoogleScholarGoogle Scholar |
Tinbergen, N. (1972). ‘The Animal in its World. Field Studies.’ (Harvard University Press: Cambridge, MA.)
Towerton, A. L., Dickman, C. R., Kavanagh, R. P., and Penman, T. D. (2016). Control of the red fox in remnant forest habitats. Wildlife Research 43, 169–177.
| Control of the red fox in remnant forest habitats.Crossref | GoogleScholarGoogle Scholar |
Travaini, A., Vassallo, A. I., García, G. O., Echeverría, A. I., Zapata, S. C., and Nielsen, S. (2013). Evaluation of neophobia and its potential impact upon predator control techniques: a study on two sympatric foxes in southern Patagonia. Behavioural Processes 92, 79–87.
| Evaluation of neophobia and its potential impact upon predator control techniques: a study on two sympatric foxes in southern Patagonia.Crossref | GoogleScholarGoogle Scholar |
Twigg, L. E. (2014). 1080-baits for fox control: is everything all that it seems? Pacific Conservation Biology 20, 230–236.
| 1080-baits for fox control: is everything all that it seems?Crossref | GoogleScholarGoogle Scholar |
Twigg, L. E., and King, D. R. (1991). The impact of fluoroacetate-bearing vegetation on native Australian fauna: a review. Oikos 61, 412–430.
| The impact of fluoroacetate-bearing vegetation on native Australian fauna: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmslaktrs%3D&md5=e0cd928c7dddd8104a7d4afeb5431749CAS |
Twigg, L. E., and Parker, R. W. (2010). Is sodium fluoroacetate (1080) a humane poison? The influence of mode of action, physiological effects, and target specificity. Animal Welfare (South Mimms, England) 19, 249–263.
| 1:CAS:528:DC%2BC3cXhtVClurjK&md5=4c287ce7ec47df8a746a91de6f1d851bCAS |
Twigg, L. E., and Socha, L. V. (2001). Defluorination of sodium monofluoroacetate by soil microorganisms from central Australia. Soil Biology & Biochemistry 33, 227–234.
| Defluorination of sodium monofluoroacetate by soil microorganisms from central Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXht1Kmtro%3D&md5=3d0dd668bf6ba8f752b62a2184de388aCAS |
Twigg, L. E., Eldridge, S. R., Edwards, G. P., Shakeshaft, B. J., and Adams, N. (2000). The longevity and efficacy of 1080 meat baits used for dingo control in central Australia. Wildlife Research 27, 473–481.
| The longevity and efficacy of 1080 meat baits used for dingo control in central Australia.Crossref | GoogleScholarGoogle Scholar |
Twigg, L. E., Kok, N. E., Kirkpatrick, W. E., and Burrow, G. (2001). The longevity of 1080 egg-baits in a regularly baited nature reserve in south-western Australia. Wildlife Research 28, 607–618.
| The longevity of 1080 egg-baits in a regularly baited nature reserve in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Twigg, L. E., Martin, G. R., and Lowe, T. J. (2002). Evidence of pesticide resistance in medium‐sized mammalian pests: a case study with 1080 poison and Australian rabbits. Journal of Applied Ecology 39, 549–560.
| Evidence of pesticide resistance in medium‐sized mammalian pests: a case study with 1080 poison and Australian rabbits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XntVCis74%3D&md5=c8261e4aaaf8cae1103e0e8d64448818CAS |
Twigg, L. E., Lowe, T., Martin, G., and Everett, M. (2005). Feral pigs in north-western Australia: basic biology, bait consumption, and the efficacy of 1080 baits. Wildlife Research 32, 281–296.
| Feral pigs in north-western Australia: basic biology, bait consumption, and the efficacy of 1080 baits.Crossref | GoogleScholarGoogle Scholar |
van Polanen Petel, A. M., Marks, C. A., and Morgan, D. G. (2001). Bait palatability influences the caching behaviour of the red fox (Vulpes vulpes). Wildlife Research 28, 395–401.
| Bait palatability influences the caching behaviour of the red fox (Vulpes vulpes).Crossref | GoogleScholarGoogle Scholar |
Warburton, B., and Drew, K. W. (1994). Extent and nature of cyanide-shyness in some populations of Australian brushtail possums in New Zealand. Wildlife Research 21, 599–605.
| Extent and nature of cyanide-shyness in some populations of Australian brushtail possums in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Wheeler, S., and Hart, D. (1979). The toxicity of sodium monofluoroacetate to wild rabbits, Oryctolagus cuniculus (L.), from three sites in Western Australia. Wildlife Research 6, 57–62.
| The toxicity of sodium monofluoroacetate to wild rabbits, Oryctolagus cuniculus (L.), from three sites in Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXltVelu7o%3D&md5=350263c0d725d32b53ae1704e7e45057CAS |
Woinarski, J. C. Z., Burbidge, A. A., and Harrison, P. L. (2014). ‘The Action Plan for Australian Mammals 2012.’ (CSIRO Publishing: Melbourne.)
Wright, J. (2011) Evaluating the use of 1080: predators, poisons and silent forests. New Zealand Government Report. Parliamentary Commissioner for the Environment, Wellington, New Zealand.