Coyote diet patterns in the Mojave Desert: implications for threatened desert tortoises
Brian L. Cypher A B , Erica C. Kelly A , Tory L. Westall A and Christine L. Van Horn Job AA Endangered Species Recovery Program, California State University – Stanislaus, One University Circle, Turlock, CA 95382, USA.
B Corresponding author. Email: bcypher@esrp.csustan.edu
Pacific Conservation Biology 24(1) 44-54 https://doi.org/10.1071/PC17039
Submitted: 21 October 2017 Accepted: 20 December 2017 Published: 23 January 2018
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Coyotes (Canis latrans) are generalist predators and are ubiquitous in North America. Occasionally, predation by coyotes can pose a threat to populations of rare species. We assessed diet patterns of coyotes over a 5-year period (2009–14) in a region of the Mojave Desert where high predation rates on threatened desert tortoises (Gopherus agassizii) had been reported. Our goal was to identify primary food items for coyotes and to assess the importance of desert tortoises in the diet. Coyotes primarily consumed rabbits and rodents with rabbits being consumed preferentially and rodents, along with secondary foods including various birds, reptiles, arthropods, and fruits, being consumed more opportunistically. In response to low annual precipitation in the last three years of the study, dietary diversity increased, as did use of anthropogenic food items by coyotes. However, coyotes did not seem to be dependent upon anthropogenic items. Remains of desert tortoises occurred in coyote scats at low frequencies (<6%) in all years and seasons, and use of tortoises appeared to be opportunistic as use varied with tortoise abundance. In the portion of the study area where 571 translocated desert tortoises had been released in 2008, the frequencies of tortoise remains in coyote scats were markedly higher in the two years following the releases (7.5% and 8.8%, respectively). The high predation rates on tortoises reported in this area may have resulted from focussed coyote foraging efforts due to the availability of vulnerable individuals (e.g. disoriented and displaced tortoises) as well as higher tortoise densities.
Additional keywords: California, food habits, hyperpredation, Mojave Desert, precipitation, predator subsidisation, prey availability
Introduction
Coyotes (Canis latrans) are ubiquitous in North America, and have increased both their range and abundance (Voigt and Berg 1987; Moore and Parker 1992), contrary to trends exhibited by many other species. Their success is a function of marked ecological plasticity, as exemplified by the diversity of habitats and food items used (Voigt and Berg 1987; Bekoff and Gese 2003). Coyotes are able to exploit a wide range of resources, and this sometimes includes species considered to be at risk of extinction. Examples of such species impacted by coyote predation include western snowy plovers (Charadrius alexandrinus nivosus) (Page et al. 1983; US Fish and Wildlife Service 2007), California least terns (Sterna antillarum browni) (Butchko 1990), San Joaquin kit foxes (Vulpes macrotis mutica) (Butchko 1990), swift foxes (V. velox) (McGee et al. 2006), and gopher tortoises (Gopherus polyphemus) (Moore et al. 2006). Predation pressure on these rare species is potentially exacerbated when local coyote populations are maintained at high levels due to an abundance of other foods, particularly anthropogenic sources, or when availability of primary food items declines, resulting in compensatory switching to secondary items that may include rare species.
Agassizi’s desert tortoise (Gopherus agassizii) occurs in the Mojave Desert of the United States. Desert tortoises in California are listed at both the federal and state levels as Threatened (California Department of Fish and Game 2008; US Fish and Wildlife Service 2011) due to numerous threats including habitat loss and direct mortality from humans, predators, and disease. Coyotes are among the many predators that kill desert tortoises (Woodbury and Hardy 1948; Esque et al. 2010; US Fish and Wildlife Service 2011; Lovich et al. 2014).
In the western Mojave Desert in California, annual mortality rates of desert tortoises in 2008 were found to be high, with rates exceeding 15% at six of nine study sites (Esque et al. 2010). Coyotes were determined to be the primary cause of mortality. Esque et al. (2010) hypothesised that low availability of other foods due to extended drought conditions may have been responsible for elevated predation rates on tortoises, and that predation may be enhanced in areas where coyotes are subsidised by anthropogenic foods.
Foraging dynamics of coyotes in the Mojave Desert have not been investigated. Ferrel et al. (1953) reported that rabbits (Lepus californicus and Sylvilagus nuttallii), rodents (primarily Microtus spp., Neotoma spp., and Otospermophilus beechyi), deer (Odocoileus hemionus), and birds were the most frequently occurring items in coyote stomachs from a region that included the Mojave Desert. However, most of the stomachs were collected in areas other than the Mojave Desert. Coyotes are foraging generalists that opportunistically exploit resources based on resource abundance and foraging efficiency. Therefore, predation by coyotes on desert tortoises likely varies with the availability and use of other food items.
From 2009 to 2014, we investigated patterns of food item use by coyotes in the western Mojave Desert relative to the annual availability of primary prey items, and we examined the effects of these patterns on use of desert tortoises by coyotes. Specifically, we (1) quantified annual and seasonal use of food items by coyotes, (2) determined the relationship between annual item use and abundance indices for primary prey items, (3) determined whether use of desert tortoises by coyotes varied in response to use and availability of other food items, and (4) assessed the effects of a tortoise translocation on use of tortoises by coyotes.
Methods
Study area
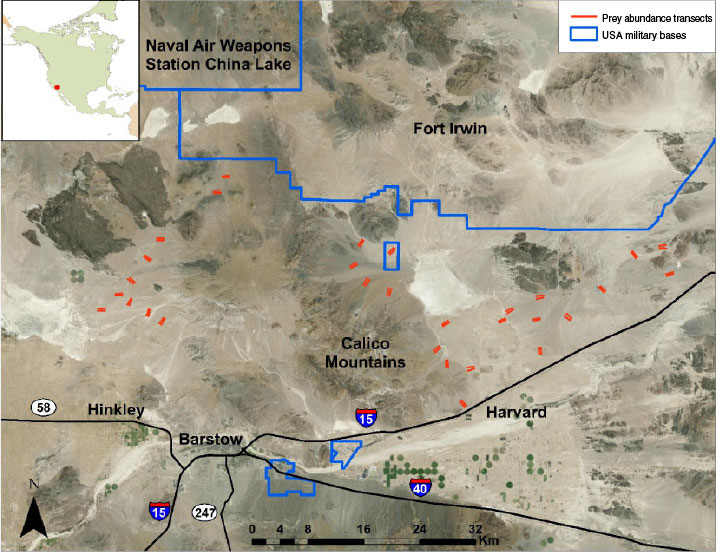
We conducted our investigation in an area encompassing ~1500 km2 north of Barstow, California (Fig. 1). The area is bounded on the north by Fort Irwin and the China Lake Naval Air Weapons Station, and on the south by Interstate 15 and State Route 58. The study area was characterised as typical Mojave Desert scrub vegetation (Turner 1994) dominated primarily by creosote bush (Larrea tridentata) with ground cover ranging from 1 to 29% and consisting of a diversity of forbs and grasses (Esque et al. 2010). Elevation ranged from 500 to 900 m, and the varied terrain included flat dry lake beds, gentle alluvial fans, and steep, rugged hills. Mean annual precipitation for Barstow, California, was 134 mm (US Climate Data 2014). Much of this area comprises public lands managed by the USA Bureau of Land Management with interspersed, mostly uninhabited, private lands. Human densities and influences were greatest around Barstow and the small towns of Hinkley and Harvard, and declined quickly with distance from these towns.
The study area was within the Western Mojave Recovery Unit of Critical Habitat for desert tortoises (US Fish and Wildlife Service 1994). In response to plans to expand Fort Irwin, which is an active training site for the US Army, 571 tortoises were captured and removed from proposed base-expansion lands in spring 2008 (Esque et al. 2005, 2009, 2010). The tortoises were released at 14 locations (Esque et al. 2010), all of which were within the eastern half of our study area (east of the Calico Mountains) (Fig. 1).
Study design
Particularly in arid environments, the annual availability of natural foods generally fluctuates with precipitation received during the wet season, which in the Mojave Desert occurs from autumn through spring. Thus, to better pair coyote foraging patterns with annual prey availability, years were defined as October to September. We determined annual precipitation totals for these intervals using data from US Climate Data (2014). Seasons were defined as autumn (October–December), winter (January–March), spring (April–June), and summer (July–September).
Use of food items by coyotes was determined by examining scat (faecal) samples. Each season, we traveled along unpaved roads at slow speed (i.e. 5–15 km h−1) while observers searched for coyote scats on and alongside the road. The total distance searched varied among seasons, but generally was ~225 km (e.g. the distance was greater if scats were less abundant). Scats also were collected opportunistically during other field activities (e.g. prey assessment transects). Scats were distinguished from those of other co-occurring predators (e.g. kit fox (Vulpes macrotis), bobcat (Lynx rufus), domestic dog (Canis familiaris)) based on size, shape, and composition. All scats were placed in separate paper bags, and the collection date and location (UTM coordinates) were recorded. Scats were air-dried and then stored in insect-proof storage containers.
Prior to analysis, scats were placed in a drying oven at 60°C for ≥24 h to destroy any zoonotic parasites (e.g. hydatid tapeworm; Echinococcus multilocularis). Scats then were placed in nylon pouches, washed in a washing machine to remove soluble material, and dried in a tumble dryer. The remaining undigested material was examined to identify items consumed by coyotes. Mammalian remains were identified by examining macroscopic (e.g. length, texture, colour, banding patterns) and microscopic (e.g. cuticular scale patterns) characteristics of hairs (Moore et al. 1974), and by comparing teeth and bone fragments to published guides (Glass 1981; Roest 1986) and reference specimens. Attempts were made to identify other vertebrate (i.e. bird, reptile) and all invertebrate (e.g. insects, arachnids) remains at least to order based on feathers, scales, and exoskeletons. Any fleshy fruits consumed were identified at least to genus based on seed characteristics (Young and Young 1992). Anthropogenic items were identified by the presence of domestic animal remains or incidentally ingested items (e.g. plastic, paper, cloth).
Leporids (primarily black-tailed jackrabbits) and rodents (primarily kangaroo rats (Dipodomys spp.), pocket mice (Perognathus spp., Chaetodipus spp.), squirrels (Xerospermophilus spp., Ammospermophilus leucurus), and desert woodrats (Neotoma lepida)) were expected to constitute primary prey items for coyotes in the study area. To assess annual abundance of primary prey, we established 60 1-km transects (Fig. 1) throughout the study area on public lands (USA Bureau of Land Management or California Department of Fish and Game). Transects began ~25 m from an unpaved road and were oriented approximately perpendicular to the road. To increase sampling efficiency, transects were established in pairs with the transects oriented parallel to each other and separated by 250 m. Pairs of transects were spaced at least 2 km apart and located in areas with typical habitat conditions. The beginning and end of each transect was marked with a wooden stake.
Assessments of prey abundance were conducted once each year in the spring. Assessments were conducted by two observers slowly walking along each transect. The first observer used a global positioning system unit to navigate to the end of the transect and also counted all active rodent burrows within 1 m either side of the transect. Burrows were characterised as ‘large’ (burrow opening ≥3 cm) or ‘small’ (burrow opening <3 cm). Large burrows are typical of those used by kangaroo rats or ground squirrels while small burrows are typical of those used by pocket mice or other mice. Burrows with openings obstructed by vegetation or spider webs were not considered active and were not counted. The second observer followed behind the first and counted all fresh rabbit pellets within 1 m either side of the transect and recorded data. Fresh pellets were characterised by a golden to dark brown colour and a smooth surface whereas old pellets were characterised by a grey colour and rough surface due to weathering.
Density estimates of desert tortoises were provided by the US Fish and Wildlife Service (L. Allison, US Fish and Wildlife Service, unpubl. data). These density estimates were derived from data collected during standardised transect surveys for tortoises that are conducted annually throughout the Mojave Desert (US Fish and Wildlife Service 2016). The transects are used to calculate a density estimate within large regions or ‘strata’, and our study area was located within one of these strata (the Superior-Cronese).
Analyses
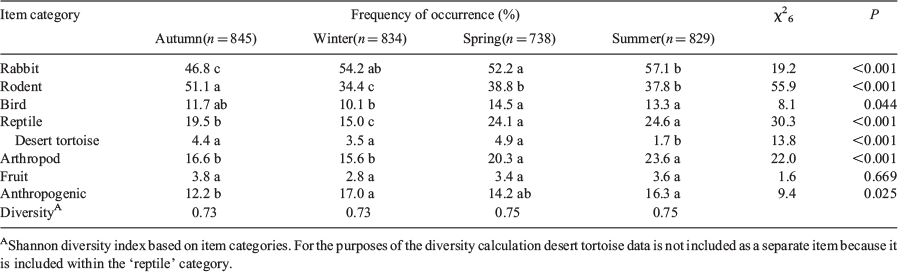
Frequency of occurrence of each item (number of scats with the item divided by the total number of scats) was determined for each season and year. For statistical analyses, items were grouped into seven categories: rabbit, rodent, bird, reptile, invertebrate, fruit, and anthropogenic foods. To compare the rankings of categories among seasons and among years, we calculated a Kendall’s coefficient of concordance (W). To compare use of a particular item category among seasons or years, we conducted contingency table analyses employing a Chi-square test for heterogeneity on the number of scats with and without the item (Zar 1984). If proportions of scats with an item varied among seasons or years, then the test was repeated for each pair of seasons or years. To help control for Type I errors resulting from multiple comparisons, P values were adjusted using a method described by Legendre and Legendre (1998). Shannon diversity indices (H′) were calculated for seasonal and annual diets using the equation:
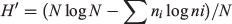
where N is the total number of occurrences of all items and ni is the number of occurrences of item i (Brower and Zar 1984).
Spearman-rank correlation analysis (Zar 1984) was used to examine the relationship between: annual precipitation and the frequency of occurrence of rabbits, kangaroo rats, and pocket mice in coyote scats; annual precipitation and mean annual rabbit pellet counts, large rodent burrows, and small rodent burrows; and annual precipitation and annual dietary diversity indices (H′).
To assess the relationship between coyote foraging patterns and desert tortoises, the frequency of occurrence of tortoise in coyote scats was compared among years and seasons by conducting contingency table analyses employing a Chi-square test for heterogeneity on the number of scats with and without tortoise. If proportions of scats with tortoise varied among seasons or years, then the test was repeated for each pair of seasons or years. Spearman-rank correlation analysis was used to examine the relationship between: the annual frequency of occurrence of tortoise in coyote scats and the frequency of occurrence of rabbits, kangaroo rats, pocket mice, and anthropogenic items in scats; the frequency of occurrence of tortoise and annual coyote dietary diversity (H′), annual precipitation, and annual tortoise density estimates; and annual precipitation and annual tortoise density estimates.
To determine whether the relocation of desert tortoises from Fort Irwin into release sites influenced use of tortoises by coyotes, the study area was divided into two regions separated by the Calico Mountains (Fig. 1). The eastern region encompassed the release sites for relocated tortoises. In the western region, coyote scats primarily were collected from areas located at least 20 km from the nearest desert tortoise release site. We compared the occurrence of desert tortoise in coyote scats between regions by conducting a contingency table analysis employing a Chi-square test for heterogeneity on the number of scats with and without tortoise.
For all statistical analyses, P-values were considered significant at α ≤ 0.1. We chose a more relaxed α value to reduce the risk of committing a Type II error, which tends to be high with small sample sizes like those in this study (Alldredge and Ratti 1986). Detecting trends with ecological data can be challenging because all potential confounding factors cannot be controlled (Germano et al. 2012). By reducing the Type II error rate, we were more likely to detect potential relationships that can be further investigated (Rotenberry and Wiens 1985; Taylor and Gerrodette 1993; Steidl et al. 1997; Di Stefano 2003; Scherer and Tracey 2011).
Results
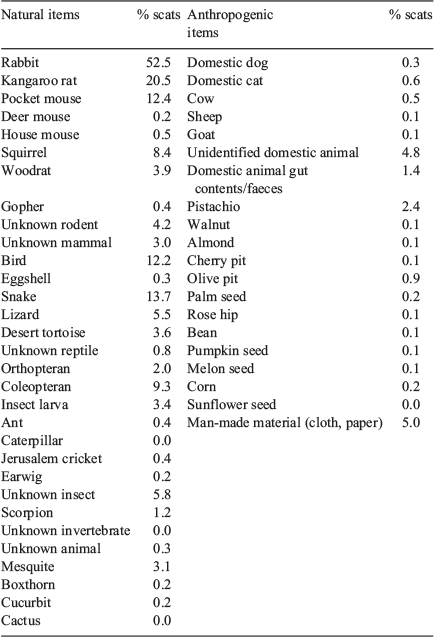
During the five years of the study, 3246 coyote scats were collected and analysed (range = 474–801 per year). On the basis of preliminary analyses (Cypher, unpubl. data), we found that a sample size of ~30 was usually sufficient to detect ≥90% of the items consumed in a given season. Approximately 50 different items were identified in the scats, including a considerable number of anthropogenic items (Table 1). Rabbits, kangaroo rats, snakes, pocket mice, and birds appeared to be the more frequently occurring food items in coyote scats (frequency of occurrence for all years combined >10%). The rabbit in scats likely was primarily black-tailed jackrabbit, which was the species most frequently observed in the study area. Desert cottontails (Sylvilagus audubonii) also are present in the area but appeared to have a more localised distribution, particularly near water sources. Kangaroo rat species potentially occurring in the study area included desert (Dipodomys deserti), Merriam’s (Dipodomys merriami), and chisel-tooth (Dipodomys microps) kangaroo rats. Potential pocket mouse species included desert (Chaetodipus pencillatus), long-tailed (Chaetodipus formosus), and little (Perognathus longimembris) pocket mice. Squirrels in the study area include round-tailed squirrels (Xerospermophilus tereticaudus), Mohave ground squirrels (Xerospermophilus mohavensis), and white-tailed antelope squirrels (Ammospermophilus leucurus). Other rodents found in scats included pocket gopher (Thomomys bottae) and desert woodrat. Birds and snakes and arthropods were also commonly consumed but usually could not be identified to species. Arthropods included beetles (Order Coleoptera) and beetle larvae, Jerusalem crickets (Family Stenopelmatidae), grasshoppers (Order Orthoptera), earwigs (Forficula auricularia), and scorpions (Order Scorpiones). Fruits found in scats included mesquite (Prosopsis spp.), wolfberry (Lycium spp.), and coyote melon (Cucurbita palmata). Anthropogenic remains identified in scats included cat (Felis catus), dog, livestock, entrails from butchered animals, domestic animal waste, and various crops including walnuts (Juglans regia), almonds (Prunus dulcis), pistachios (Pistacia vera), olives (Olea europaea), pumpkin (Cucurbita pepo), melon (Cucurbitaceae), corn (Zea mays), and beans (Fabaceae). Other non-food anthropogenic items included pieces of cloth, paper, plastic, leather, cartridge casings, and other materials. Several other items appeared to be ingested incidentally and included twigs, grass, other vegetation, pebbles, and soil.
|
When items were grouped into broader food categories, rodents were the most frequently occurring items in Years 1 and 2 while rabbits were the most frequently occurring items in Years 3–5 (Table 2). The occurrence of birds, reptiles, invertebrates, and fruits in coyote scats was relatively consistent among years. The occurrence of anthropogenic items increased over the last three years of the study and these items were found in over a quarter of all scats by the last year. According to the Shannon diversity indices, coyote diets were more diverse in the latter years of the study (Table 2).
Among years, the ranks of item categories exhibited significant concordance (W6 = 0.903, r = 0.879, P < 0.001), indicating that relative use of items was similar across years. However, the proportional use of each of the item categories varied among years (Table 2).
The occurrence of rabbit in coyote scats was consistently high in each season (Table 3). The occurrence of birds, reptiles, invertebrates, fruit, and anthropogenic items also was relatively consistent among seasons, and occurrence for all of these items was considerably lower than that for rabbits. The occurrence of rodents in scats was slightly higher than that of rabbits in fall, but was noticeably lower in other seasons. Coyote diets were slightly more diverse in spring and summer (Table 3).
Among seasons, the ranks of item categories exhibited significant concordance (W6 = 0.911, r = 0.881, P = 0.001), indicating that relative use of items was similar across seasons. However, the proportional use of each of the item categories except for fruit varied among seasons (Table 3).
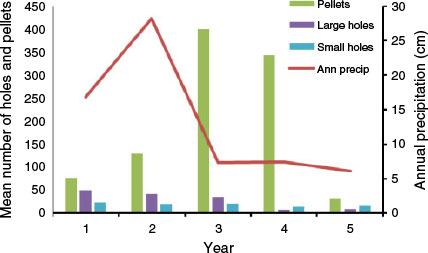
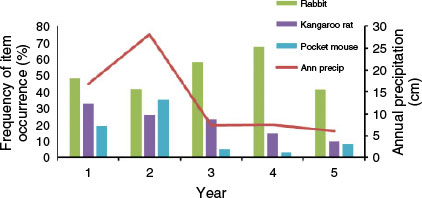
Precipitation totals during the five years of the study were 169 mm, 282 mm, 73 mm, 75 mm, and 61 mm, respectively. Thus, precipitation was above the annual mean (134 mm) during the first two years but considerably below the mean in the last three years. No statistically significant relationships were found between annual precipitation and the annual mean numbers of large rodent holes (r = 0.6, P = 0.285), small rodent holes (r = 0.3, P = 0.624), or rabbit pellets (r = 0.1, P = 0.873) (Fig. 2). Likewise, no statistically significant relationships were found between annual precipitation and the annual frequency of occurrence in coyote scats of kangaroo rats (r = 0.8, P = 0.104), pocket mice (r = 0.6, P = 0.285), or rabbits (r = 0.1, P = 0.873) (Fig. 3). However, dietary diversity was negatively related to annual precipitation (r = −1.0, P < 0.001).
|
|
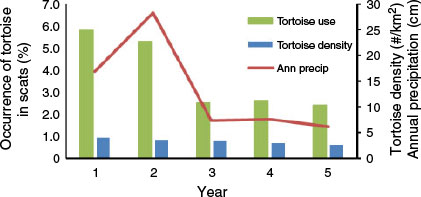
Estimated numbers of desert tortoises per square kilometre in the study area region were 3.9, 3.5, 3.2, 2.9 and 2.6 in Years 1–5, respectively. The occurrence of desert tortoise in coyote scats was relatively low and never exceeded 5.9% in any year. However, occurrence did vary among years (Table 2). It was highest during the first two years of the study and then significantly lower during the last three. The occurrence of tortoise in scats also varied among seasons and was significantly lower in summer than in the other seasons (Table 3).
The annual occurrence of desert tortoise in coyote scats was positively related to the occurrence of kangaroo rats in scats (r = 0.9, P = 0.037) and negatively related to the occurrence of anthropogenic items (r = −0.9, P = 0.037), but was not related to use of rabbits (r = 0.2, P = 0.747) or use of pocket mice (r = 0.5, P = 0.391) (Fig. 4). The annual occurrence of desert tortoise in coyote scats was negatively related to dietary diversity (r = −0.9, P = 0.037), and was positively related to both annual precipitation (r = 0.9, P = 0.037) and annual desert tortoise abundance estimates (r = 0.9, P = 0.037). However, annual tortoise abundance was not related to annual precipitation (r = 0.8, P = 0.104) (Fig. 4).
|
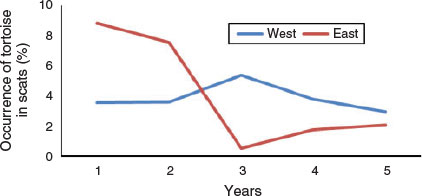
Frequency of occurrence of desert tortoise in coyote scats varied between the east (n = 1801) and west regions (n = 1435) of the study area (Fig. 5). Occurrence was significantly higher in the east region in Years 1 and 2 (χ21 = 6.87, P = 0.009 and χ21 = 3.12, P = 0.078, respectively), significantly lower in Years 3 and 4 (χ21 = 13.59, P < 0.001 and χ21 = 2.95, P = 0.086, respectively), and similar in Year 5 (χ21 = 0.48, P = 0.488).
|
Discussion
Coyote foraging patterns
Coyotes commonly are characterised as opportunistic foraging generalists (Bekoff and Gese 2003). On our Mojave Desert study site, they consumed a wide diversity of items. Despite being generalists, coyotes can exhibit preferences for particular items. On our study site, rabbits appeared to be a preferred food item. Rabbits were the most frequently occurring individual item in all years and seasons. (Rodents as a category were the most frequently occurring items in Years 1 and 2 and in autumn, but this included all rodent species combined.) On the basis of the annual pellet counts, rabbit abundance varied markedly during the five years of the study, but use by coyotes was consistently high (see Figs 2, 3). A preference for rabbits also has been reported from other arid locations (Clark 1972; MacCracken and Hansen 1987), and rabbits commonly are a primary prey item for coyotes in California (Ferrel et al. 1953; Cypher et al. 1994).
Rodents, particularly heteromyids (e.g. kangaroo rats and pocket mice) and squirrels, were also commonly consumed by coyotes. Indeed, as a category, rodents were the most frequently occurring items in coyote scats in the first two years of the study. Use of rodents appeared to vary with their relative availability. On the basis of annual burrow counts, rodent abundance declined during the course of the study coincident with lower annual precipitation. Concomitantly, use of rodents by coyotes declined as well.
Use of most other items, such as birds, reptiles, invertebrates and fruits, likely was more opportunistic, as indicated by their relatively low frequencies of occurrence in coyote scats. Coyotes probably did not actively search for these items, but instead consumed them as they encountered them while hunting for preferred items such as rabbits and rodents. Use of these other items increased in the latter years of the study, particularly the last year. This increase likely was associated with declining abundance of primary foods, particularly rabbits and rodents. Increased use of secondary food items as primary items decline is consistent with optimal foraging theory predictions for a foraging generalist (Pianka 1978; MacCracken and Hansen 1987).
A variety of anthropogenic items was found in coyote scats. Some of these items were apparently consumed for nutritional purposes (e.g. domestic animals, crops), but it is unclear why other items were consumed (e.g. cartridge casings, dog leash, rope). Domestic animal remains found in coyote scats could have resulted from depredation on live animals or scavenging on dead animals. Throughout the study area, we occasionally found carcasses of domestic animals that either had gotten lost and died or that were ‘dumped’ after death. Likewise, crops in scats may have been a result of depredation or scavenging from garbage. As with animal carcasses, we occasionally found sites where trash had been dumped. Such dumping is common near areas of human habitation in the Mojave Desert (Berry et al. 2006; Boarman et al. 2006).
The increased use of anthropogenic items across years likely resulted from declining abundance of natural foods due to below-average precipitation in the latter three years of the study. Increased use of anthropogenic items during droughts has also been documented for coyotes in the San Joaquin Valley of California (Cypher et al. 1994), dingoes (Canis lupus dingo) in Australia (Corbett 1995), golden jackals (Canis aureus) in India (Aiyadurai and Jhala 2006), and kit foxes in the Mojave Desert (Kelly 2017). Anthropogenic items can be an important supplemental food source for coyotes (Danner and Smith 1980; McClure et al. 1995) and potentially could sustain coyote abundance through periods of low availability of natural foods (e.g. Fedriani et al. 2001). Such anthropogenic subsidisation could result in increased foraging pressure on remaining natural food items, including desert tortoises (Esque et al. 2010). In Australia, cattle carcasses sustained dingo abundance during drought years and this exacerbated predation pressure on already depressed native species populations (Corbett 1995; Allen et al. 2013).
The observed annual variation in use of foods by coyotes likely resulted from fluctuations in absolute as well as relative item abundance attributable to annual precipitation. In arid regions, annual precipitation is a significant ecosystem driver. In particular, precipitation directly affects primary production (i.e. plant growth), and this in turn affects the abundance of primary consumers, including herbivores such as rabbits and rodents (Gross et al. 1974; Kelt 2011) that constitute prey for coyotes. Abundance of other potential coyote foods, such as reptiles, is affected as well (Whitford and Creusere 1977). Although we found few statistical relationships, trends in abundance and use of some primary coyote foods, particularly rodents, generally followed precipitation patterns. The lack of statistical significance likely was a result of a small number of sample points (i.e. five) and possibly also due to time-lag responses. Such responses have been reported previously for rabbits and heteromyid rodents (Brown and Harney 1993; Otten and Holmstead 1996; Cypher et al. 2000). In our study, rabbit abundance exhibited a 1- to 2-year lag response to annual precipitation during years of above-average precipitation.
The significant inverse relationship between dietary diversity and annual precipitation reflected the expansion of dietary breadth as primary items became less abundant, resulting in a shift from a more specialised diet emphasising rabbits and rodents to a more generalised diet. Such a shift also is consistent with optimal foraging theory predictions (Pianka 1978; Stephens and Krebs 1986; MacCracken and Hansen 1987). Increased dietary breadth in response to a decline in preferred food items is a common functional response among medium-size carnivores and has been observed among coyotes elsewhere (MacCracken and Hansen 1987; Cypher et al. 1994), dingoes (Corbett 1995; Paltridge 2002), red foxes (Vulpes vulpes) (Errington 1937; Spencer et al. 2017), kit foxes (Kelly 2017), bobcats (Lynx rufus) (Beasom and Moore 1977; McKinney and Smith 2007), Iberian lynx (Lynx pardina) (Delibes 1980), and caracals (Felis caracal) (Palmer and Fairall 1988).
Although proportional use of individual food items varied among years, the rankings of items in the diet were similar among years, further indicating preferences by coyotes for certain items, particularly rabbits and rodents. Likewise, the rankings of items consumed by coyotes were similar among seasons, but proportional use of individual items varied. Seasonal differences in proportional use likely were due to seasonal variation in item availability. For example, use of reptiles and invertebrates were highest in spring and summer when these animals likely are more active due to warmer temperatures.
Coyote foraging and desert tortoises
The relatively low frequency of occurrence of desert tortoise remains in coyote scats suggests that tortoises are a secondary or incidental prey item and likely are consumed opportunistically. This is consistent with the fact that tortoises occur in low densities (<4 km−2 regionally during the study: US Fish and Wildlife Service, unpubl. data) and significantly reduce activity and retreat to burrows during very hot or cold temperatures. Thus, foraging specifically for such a low-density and widely dispersed item would be inconsistent with an optimal foraging strategy (Pianka 1978; Stephens and Krebs 1986). Furthermore, an important caveat is that predation could not be distinguished from scavenging. Desert tortoises can be killed by other species, such as badgers (Taxidea taxus), dogs, kit foxes, ravens (Corvus corax), and humans (Berry 1986; Boarman 1992; Kristan and Boarman 2003; Esque et al. 2010; Riedle et al. 2010). Also, some tortoises die of disease (e.g. upper respiratory tract disease, shell disease) and natural causes such as starvation and dehydration (Peterson 1994; US Fish and Wildlife Service 2011; Lovich et al. 2014). Starvation and dehydration are not uncommon during periods of drought (Peterson 1994; Longshore et al. 2003; Lovich et al. 2014).
The seasonal occurrence of desert tortoise remains in scats was consistent with seasonal patterns of tortoise activity. Remains were most frequent in spring and autumn when tortoises in the western Mojave Desert may be more vulnerable to predation because they commonly are out of their burrows seeking food or mates (Nagy and Medica 1986; Zimmerman et al. 1994). Remains were least frequent in summer when tortoises spend more time in their burrows to avoid extreme heat. Also, rabbits and rodents, the primary foods for coyotes, are abundant in summer following the spring reproductive pulse. Tortoises are likely to be less vulnerable when in their burrows although there are anecdotal reports of predators, possibly coyotes, digging tortoises out of their burrows (Esque et al. 2010).
The occurrence of desert tortoise remains in scats tracked annual precipitation and declined as annual rainfall declined. Concomitantly, on the basis of the US Fish and Wildlife Service density estimates, regional tortoise abundance also exhibited a gradual declining trend from Year 1 to Year 5. These results indicated that tortoise abundance may have been influenced by precipitation and that use by coyotes varied with tortoise abundance, further indicating that use was opportunistic. Desert tortoises are herbivores and reduced primary productivity in years of lower precipitation coupled with lack of free water for drinking potentially could result in fewer tortoises due to death by starvation or dehydration (Peterson 1994; Longshore et al. 2003; Lovich et al. 2014). Also, tortoises may reduce activity in order to conserve energy and water (Peterson 1994; Duda et al. 1999), and this lower above-ground activity would reduce tortoise vulnerability to predation. Thus, both reduced abundance and reduced activity could explain the decline in use of tortoises by coyotes across years.
Use of desert tortoises by coyotes did not appear to be inversely related to the availability of other prey on our study site. Increased predation on tortoises concurrent with declines in rabbits and rodents has been reported previously (Woodbury and Hardy 1948; Turner et al. 1984; Peterson 1994), although prey availabilities in those study sites was not quantified. In our study, as use of primary items such as rabbits and rodents declined across years, use of some secondary items (e.g. birds, reptiles, invertebrates, fruit, anthropogenic items) increased, but use of tortoises did not exhibit a similar increase. This would explain the inverse relationship between use of tortoises and item diversity in coyote diets.
The positive relationship between the occurrence of tortoise remains and the occurrence of kangaroo rats in coyote scats may indicate that the abundances of these food items were responding similarly to environmental conditions, particularly precipitation. This conclusion would also be consistent with the negative relationship between the occurrence of tortoise remains in scats and the occurrence of anthropogenic items. As the availability of natural food items, including tortoises, declined due to low precipitation, coyotes consumed more anthropogenic items, as discussed previously.
A significant concern in desert tortoise conservation has been the potential anthropogenic subsidisation of tortoise predators (Berry et al. 2006; Esque et al. 2010). Such subsidisation could enhance coyote abundance or use of areas near anthropogenic food sources, resulting in increased predation on tortoises. Subsidised raven populations have been documented to enhance predation on hatchling tortoises (Boarman 1992; Kristan and Boarman 2003). Also, both Berry et al. (2006) and Esque et al. (2010) reported that mortality rates of transmittered desert tortoises were higher near areas that were human-occupied or anthropogenically disturbed (including the presence of trash piles). Similarly, dingoes in Australia that were feeding extensively on cattle carcasses significantly impacted populations of threatened rodents (Corbett 1995; Allen and Leung 2012) including dusky hopping-mice (Notomys fuscus), fawn hopping-mice (Notomys cervinus), and plains mice (Pseudomys australis). Coyotes on our study site did indeed consume anthropogenic food items. However, use of these items was low when natural foods were abundant in early years of the study, and only increased as these foods declined in abundance concomitant with below-average precipitation. Thus, it did not appear that coyotes were extensively using or were dependent on anthropogenic foods. Unfortunately, we did not have data on coyote abundance or movement patterns associated with increasing use of anthropogenic foods.
The occurrence of desert tortoise remains in coyote scats exhibited very different trends between the east and west regions of the study site. Occurrence was relatively consistent across years in the western region (3–5.4%) whereas in the eastern region, occurrence was markedly lower in the last three years (0.5–2.1%) compared with the first two years (7.5–8.8%). One potential explanation is that the trend observed in the eastern region simply reflected drier conditions in the last three years, but then a similar trend would be expected in the western region. Another potential explanation is that the trend observed in the eastern region was related to the presence of translocated tortoises. In 2008, the year before we initiated our study, 571 tortoises were released at 14 sites widely dispersed across the eastern region that were also occupied by resident tortoises (Esque et al. 2010). During the 2-year post-translocation monitoring period, mortality rates of tortoises with radio-transmitters were 26% for translocated tortoises (n = 357), 21% for resident tortoises (n = 140), and 19% for control (resident animals on reference sites located at least 1 km from areas where translocated animals were released) tortoises (n = 149). These rates were considered to be high and much of the mortality was attributed to coyotes (Esque et al. 2010).
The translocation effort could have resulted in higher predation rates on tortoises in several ways. Translocated tortoises could have been disoriented and resident tortoises could have been displaced, thereby increasing the vulnerability of both groups to predation. Coyotes may have been attracted to release sites due to the presence of vulnerable animals (or also possibly to increased activity by human researchers). Coyotes also could have been attracted simply by the increase in tortoise abundance on the sites. Esque et al. (2010) reported that each site was ~2.58 km2 in size, and that the number of translocated tortoises released on each site ranged from 10 to 50 (3.9–19.4 km−2). Using their estimated density of 7.5 km−2 for resident tortoises, the addition of the relocated tortoises would have increased the densities on the sites to 11.4–26.5 km−2, or 1.5–3.5 times higher than the original densities with just residents. Using the US Fish and Wildlife Service estimate of 3.9 km−2 in Year 1 of the study, the addition of the relocated tortoises would have increased the densities on the sites to 6.5–23.3 km−2, or 2.5–6.0 times higher than the original densities with just residents. Finally, if coyotes were attracted to the release sites for any reason, this would also likely have resulted in increased travel by coyotes through the areas between sites where control tortoises were located. This plausible scenario could have produced a hyperpredation event and the high mortality rates observed among all three tortoise groups as well as the higher occurrence of tortoise in coyote scats in the eastern region during the first two years of the study. Desert tortoise translocations are commonly conducted in association with development projects and the effects of coyotes on the efficacy of such translocations warrant further investigation.
On the basis of the results of our study, we conclude that (1) coyotes on our study site are primarily consuming rabbits and rodents, consistent with results elsewhere; (2) rabbits appeared to be preferentially consumed, with other items consumed more opportunistically (i.e. use more related to availability); (3) coyote dietary diversity increased with reductions in availability of primary prey related to lower precipitation; (4) coyotes used anthropogenic items, especially when availability of natural foods was lower, but did not appear dependent upon these items; (5) use of threatened desert tortoises by coyotes as a food source was generally infrequent and opportunistic, and use did not increase as availability of other foods declined; and (6) the release of a large number of translocated tortoises may have attracted coyotes to the release site region resulting in locally higher predation rates on tortoises.
Conflicts of interest
None of the authors of this paper have any conflicts of interest to declare.
Acknowledgements
Funding for this project was provided by the US Fish and Wildlife Service (cooperative agreement #CESU 81332-8-J005). We thank Catherine (Cat) Darst and Roy Averill-Murray for initiating the project and for administrative and technical support all throughout the project. Clarence Everly secured project funding from the Department of the Army and also provided much appreciated administrative and project support. We thank William Boarman, Andrew Walde, and Todd Esque for technical support and information sharing. We also thank Erin Tennant, Allie Madrid, Ellen Cypher, and Pat Kelly for field assistance and other support. Eric Somer and Santino Lauricella provided samples and valuable field observations. Scott Phillips provided graphics, database and other assistance throughout the project. William Boarman and two anonymous reviewers provided helpful comments that improved the manuscript.
References
Aiyadurai, A., and Jhala, Y. V. (2006). Foraging and habitat use by golden jackals (Canis aureus) in the Bhal region, Gujarat, India. Journal of the Bombay Natural History Society 103, 5–12.Alldredge, J. R., and Ratti, J. T. (1986). Comparison of some statistical techniques for analysis of resource selection. Journal of Wildlife Management 50, 157–165.
| Comparison of some statistical techniques for analysis of resource selection.Crossref | GoogleScholarGoogle Scholar |
Allen, B. L., and Leung, L. K.-P. (2012). Assessing predation risk to threatened fauna from their prevalence in predator scats: dingoes and rodents in arid Australia. PLoS One 7, e36426.
| Assessing predation risk to threatened fauna from their prevalence in predator scats: dingoes and rodents in arid Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XntlWgsrg%3D&md5=8ce95cc122a05a19086b32c6c6361685CAS |
Allen, B. L., Fleming, P. J. S., Allen, L. R., Engeman, R. M., Ballard, G., and Leung, L. K.-P. (2013). As clear as mud: a critical review of evidence for the ecological roles of Australian dingoes. Biological Conservation 159, 158–174.
| As clear as mud: a critical review of evidence for the ecological roles of Australian dingoes.Crossref | GoogleScholarGoogle Scholar |
Beasom, S. L., and Moore, R. A. (1977). Bobcat food habit response to a change in prey abundance. The Southwestern Naturalist 21, 451–457.
Bekoff, M., and Gese, E. M. (2003). Coyotes. In ‘Wild Mammals of North America: Biology, Management, and Conservation’. (Eds G. A. Feldhamer, B. C. Thompson, and J. A. Chapman.) pp. 467–481. (The Johns Hopkins University Press: Baltimore, MD.)
Berry, K. H. (1986). Incidence of gunshot deaths in desert tortoise populations in California. Wildlife Society Bulletin 14, 127–132.
Berry, K. H., Bailey, T. Y., and Anderson, K. M. (2006). Attributes of desert tortoise populations at the National Training Center, central Mojave Desert, California, USA. Journal of Arid Environments 67, 165–191.
| Attributes of desert tortoise populations at the National Training Center, central Mojave Desert, California, USA.Crossref | GoogleScholarGoogle Scholar |
Boarman, W. I. (1992). Problems with management of a native predator on a threatened species: raven predation on desert tortoises. Proceedings of the Vertebrate Pest Conference 15, 48–52.
Boarman, W. I., Patten, M. A., Camp, R. J., and Collis, S. J. (2006). Ecology of a population of subsidized predators: common ravens in the central Mojave Desert, California. Journal of Arid Environments 67, 248–261.
| Ecology of a population of subsidized predators: common ravens in the central Mojave Desert, California.Crossref | GoogleScholarGoogle Scholar |
Brower, J. E., and Zar, J. H. (1984). ‘Field and Laboratory Methods for General Ecology.’ (Wm. C. Brown Publishers: Dubuque, IA.)
Brown, J. H., and Harney, B. A. (1993). Populations and community ecology of heteromyid rodents in temperate habitats. In ‘Biology of the Heteromyidae’. American Society of Mammalogists Special Publication No. 10. (Eds H. H. Genoways and J. H. Brown.) pp. 618–651. (Brigham Young University: Provo, UT.)
Butchko, P. H. (1990). Predator control for the protection of endangered species in California. Proceedings of the Vertebrate Pest Conference 14, 237–240.
California Department of Fish and Game (2008). California species of special concern. Available at: http://www.wildlife.ca.gov/Conservation/ SSC [accessed 25 September 2017].
Clark, F. W. (1972). Influence of jackrabbit density on coyote population change. Journal of Wildlife Management 36, 343–356.
| Influence of jackrabbit density on coyote population change.Crossref | GoogleScholarGoogle Scholar |
Corbett, L. (1995). ‘The Dingo.’ (Cornell University Press: Ithaca, NY.)
Cypher, B. L., Spencer, K. A., and Scrivner, J. H. (1994). Food-item use by coyotes at the Naval Petroleum Reserves in California. The Southwestern Naturalist 39, 91–95.
| Food-item use by coyotes at the Naval Petroleum Reserves in California.Crossref | GoogleScholarGoogle Scholar |
Cypher, B. L., Warrick, G. D., Otten, M. R. M., O’Farrell, T. P., Berry, W. H., Harris, C. E., Kato, T. T., McCue, P. M., Scrivner, J. H., and Zoellick, B. W. (2000). Population dynamics of San Joaquin kit foxes at the Naval Petroleum Reserves in California. Wildlife Monographs 45, 1–43.
Danner, D. A., and Smith, N. S. (1980). Coyote home range, movement, and relative abundance near a cattle feedyard. Journal of Wildlife Management 44, 484–487.
| Coyote home range, movement, and relative abundance near a cattle feedyard.Crossref | GoogleScholarGoogle Scholar |
Delibes, M. (1980). Feeding ecology of the Spanish lynx in the Coto Doñana. Acta Theriologica 25, 309–324.
| Feeding ecology of the Spanish lynx in the Coto Doñana.Crossref | GoogleScholarGoogle Scholar |
Di Stefano, J. (2003). How much power is enough? Against the development of an arbitrary convention for statistical power calculations. Functional Ecology 17, 707–709.
| How much power is enough? Against the development of an arbitrary convention for statistical power calculations.Crossref | GoogleScholarGoogle Scholar |
Duda, J. J., Krzysik, A. J., and Freilich, J. E. (1999). Effects of drought on desert tortoise movement and activity. Journal of Wildlife Management 63, 1181–1192.
| Effects of drought on desert tortoise movement and activity.Crossref | GoogleScholarGoogle Scholar |
Errington, P. L. (1937). Food habits of Iowa red foxes during a drought summer. Ecology 18, 53–61.
| Food habits of Iowa red foxes during a drought summer.Crossref | GoogleScholarGoogle Scholar |
Esque, T. C., Nussear, K. E., and Medica, P. A. (2005). Desert tortoise translocation plan for Fort Irwin’s land expansion program at the U.S. Army National Training Center (NTC) at Fort Irwin. U.S. Geological Survey, Las Vegas, NV. Available at: https://tortoise.org/conservation/FtIrwinTranslocationPlan.pdf [accessed 4 December 2017].
Esque, T. C., Nussear, K. E., Drake, K. K., Berry, K. H., Medica, P. A., and Heaton, J. S. (2009). Amendment to Desert Tortoise Translocation Plan for Fort Irwin’s Land Expansion Program at the U.S. Army National Training Center (NTC) & Fort Irwin. U.S. Geological Survey, Las Vegas, NV. Available at: http://www.ww.w.gosolarcalifornia.org/sitingcases/genesis_solar/documents/others/testimony_centr_biological_diversity/exhibits/Exh.%20813.%20Esque%20et%20al.%202009.%20%20Amendment%20to%20DT%20trans.%20Plan.pdf [accessed 4 December 2017].
Esque, T. C., Nussear, K. E., Drake, K. K., Walde, A. D., Berry, K. H., Averill-Murray, R. C., Woodman, A. P., Boarman, W. I., Medica, P. A., Mack, J., and Heaton, J. S. (2010). Effects of subsidized predators, resource variability, and human population density on desert tortoise populations in the Mojave Desert, USA. Endangered Species Research 12, 167–177.
| Effects of subsidized predators, resource variability, and human population density on desert tortoise populations in the Mojave Desert, USA.Crossref | GoogleScholarGoogle Scholar |
Fedriani, J. M., Fuller, T. K., and Sauvajot, R. M. (2001). Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography 24, 325–331.
| Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California.Crossref | GoogleScholarGoogle Scholar |
Ferrel, C. M., Leach, H. R., and Tillotson, D. R. (1953). Food habits of the coyote in California. California Fish and Game 39, 301–341.
Germano, D. J., Rathbun, G. B., and Saslaw, L. R. (2012). Effects of grazing and invasive grasses on desert vertebrates in California. Journal of Wildlife Management 76, 670–682.
| Effects of grazing and invasive grasses on desert vertebrates in California.Crossref | GoogleScholarGoogle Scholar |
Glass, B. P. (1981). ‘Key to the Skulls of North American Mammals.’ (Oklahoma State University: Stillwater, OK.)
Gross, J. E., Stoddart, L. C., and Wagner, F. H. (1974). Demographic analysis of a northern Utah jackrabbit population. Wildlife Monographs 40, 1–68.
Kelly, E. C. (2017). Desert kit fox (Vulpes macrotis arsipus) food habits and competitive interactions with coyotes (Canis latrans) in the Mojave Desert. M.Sc. Thesis, California State University, Bakersfield.
Kelt, D. A. (2011). Comparative ecology of desert small mammals: a selective review of the past 30 years. Journal of Mammalogy 92, 1158–1178.
| Comparative ecology of desert small mammals: a selective review of the past 30 years.Crossref | GoogleScholarGoogle Scholar |
Kristan, W. B., and Boarman, W. I. (2003). Spatial pattern of risk of common raven predation on desert tortoises. Ecology 84, 2432–2443.
| Spatial pattern of risk of common raven predation on desert tortoises.Crossref | GoogleScholarGoogle Scholar |
Legendre, P., and Legendre, L. (1998). ‘Numerical Ecology.’ 2nd edn. (Elsevier: Amsterdam.)
Longshore, K. M., Jaeger, J. R., and Sappington, J. M. (2003). Desert tortoise (Gopherus agassizii) survival at two eastern Mojave Desert sites: death by short-term drought? Journal of Herpetology 37, 169–177.
| Desert tortoise (Gopherus agassizii) survival at two eastern Mojave Desert sites: death by short-term drought?Crossref | GoogleScholarGoogle Scholar |
Lovich, J. E., Yackulic, C. B., Freilich, J., Agha, M., Austin, M., Meyer, K. P., Arundel, T. R., Hansen, J., Vamstad, M. S., and Root, S. A. (2014). Climatic variation and tortoise survival: has a desert species met its match? Biological Conservation 169, 214–224.
| Climatic variation and tortoise survival: has a desert species met its match?Crossref | GoogleScholarGoogle Scholar |
MacCracken, J. G., and Hansen, R. M. (1987). Coyote feeding strategies in southeastern Idaho: optimal foraging by an opportunistic predator. Journal of Wildlife Management 51, 278–285.
| Coyote feeding strategies in southeastern Idaho: optimal foraging by an opportunistic predator.Crossref | GoogleScholarGoogle Scholar |
McClure, M. F., Smith, N. S., and Shaw, W. W. (1995). Diets of coyotes near the boundary of Saguaro National Monument and Tucson, Arizona. The Southwestern Naturalist 40, 101–125.
McGee, B. K., Ballard, W. B., Nicholson, K. L., Cypher, B. L., Lemons, P. R., and Kamler, J. F. (2006). Effects of artificial escape dens on swift fox populations in northwest Texas. Wildlife Society Bulletin 34, 821–827.
| Effects of artificial escape dens on swift fox populations in northwest Texas.Crossref | GoogleScholarGoogle Scholar |
McKinney, T., and Smith, T. W. (2007). Diets of sympatric bobcats and coyotes during years of varying rainfall in central Arizona. Western North American Naturalist 67, 8–15.
| Diets of sympatric bobcats and coyotes during years of varying rainfall in central Arizona.Crossref | GoogleScholarGoogle Scholar |
Moore, G. C., and Parker, G. R. (1992). Colonization by the eastern coyote (Canis latrans). In ‘Ecology and Management of the Eastern Coyote’. (Ed. A. H. Boer.) pp. 23–38. (Wildlife Research Unit, University of New Brunswick: Fredericton, NB.)
Moore, J. A., Engeman, R. M., Smith, H. T., and Woolard, J. (2006). Gopherus polyphemus (Gopher tortoise) coyote predation. Herpetological Review 37, 78–79.
Moore, T. D., Spencer, L. E., and Dugnolle, C. E. (1974). Identification of the dorsal guard hairs of some mammals of Wyoming. Wyoming Game and Fish Department Bulletin 14, 1–177.
Nagy, K. A., and Medica, P. A. (1986). Physiological ecology of desert tortoises in southern Nevada. Herpetologica 42, 73–92.
Otten, M. R. M., and Holmstead, G. L. (1996). Effect of seeding burned lands on the abundance of rodents and leporids on Naval Petroleum Reserve No. 1, Kern County, California. The Southwestern Naturalist 41, 129–135.
Page, G. W., Stenzel, L. E., Winkler, D. W., and Swarth, C. W. (1983). Spacing out at Mono Lake: breeding success, nest density, and predation in the snowy plover. The Auk 100, 13–24.
Palmer, R., and Fairall, N. (1988). Caracal and African wild cat diet in the Karoo National Park and the implications thereof for hyrax. South African Journal of Wildlife Research 18, 30–34.
Paltridge, R. (2002). The diets of cats, foxes and dingoes in relation to prey availability in the Tanami Desert, Northern Territory. Wildlife Research 29, 389–403.
| The diets of cats, foxes and dingoes in relation to prey availability in the Tanami Desert, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Peterson, C. C. (1994). Different rates and causes of high mortality in two populations of the threatened desert tortoise Gopherus agassizii. Biological Conservation 70, 101–108.
| Different rates and causes of high mortality in two populations of the threatened desert tortoise Gopherus agassizii.Crossref | GoogleScholarGoogle Scholar |
Pianka, E. R. (1978). ‘Evolutionary Ecology.’ (Harper and Row Publishers: New York.)
Riedle, J. D., Averill-Murray, R. C., and Grandmaison, D. D. (2010). Seasonal variation in survivorship and mortality of desert tortoises in the Sonoran Desert, Arizona. Journal of Herpetology 44, 164–167.
| Seasonal variation in survivorship and mortality of desert tortoises in the Sonoran Desert, Arizona.Crossref | GoogleScholarGoogle Scholar |
Roest, A. I. (1986). ‘A Key-guide to Mammal Skulls and Lower Jaws.’ (Mad River Press: Eureka, CA.)
Rotenberry, J. T., and Wiens, J. A. (1985). Statistical power analysis and community-wide patterns. American Naturalist 125, 164–168.
| Statistical power analysis and community-wide patterns.Crossref | GoogleScholarGoogle Scholar |
Scherer, R. D., and Tracey, J. A. (2011). A power analysis for the use of counts of egg masses to monitor wood frog (Lithobates sylvaticus) populations. Herpetological Conservation and Biology 6, 81–90.
Spencer, E. E., Newsome, T. M., and Dickman, C. R. (2017). Prey selection and dietary flexibility of three species of mammalian predator during an irruption of non-cyclic prey. Royal Society Open Science 4, 170317.
| Prey selection and dietary flexibility of three species of mammalian predator during an irruption of non-cyclic prey.Crossref | GoogleScholarGoogle Scholar |
Steidl, R. J., Hayes, J. P., and Schauber, E. (1997). Statistical power analysis in wildlife research. Journal of Wildlife Management 61, 270–279.
| Statistical power analysis in wildlife research.Crossref | GoogleScholarGoogle Scholar |
Stephens, D. W., and Krebs, J. R. (1986). ‘Foraging Theory.’ (Princeton University Press: Princeton, NJ.)
Taylor, B. L., and Gerrodette, T. (1993). The uses of statistical power in conservation biology: the vaquita and northern spotted owl. Conservation Biology 7, 489–500.
| The uses of statistical power in conservation biology: the vaquita and northern spotted owl.Crossref | GoogleScholarGoogle Scholar |
Turner, R. M. (1994). Mojave desert scrub. In ‘Biotic Communities, Southwestern United States and Northwestern Mexico’. (Ed. D. E. Brown.) pp. 157–168. (University of Utah Press: Salt Lake City, UT.)
Turner, F. B., Medica, P. A., and Lyons, C. L. (1984). Reproduction and survival of the desert tortoise (Scaptochelys agassizii) in Ivanpah Valley, California. Copeia 1984, 811–820.
| Reproduction and survival of the desert tortoise (Scaptochelys agassizii) in Ivanpah Valley, California.Crossref | GoogleScholarGoogle Scholar |
US Climate Data (2014). Precipitation data for Barstow, CA. Available at: http://www.usclimatedata.com/climate/barstow/california/united-states/usca0069/2014/8 [accessed 4 December 2017].
US Fish and Wildlife Service (1994). Desert tortoise (Mojave population) recovery plan. U.S. Fish and Wildlife Service, Portland, Oregon. Available at: https://www.fws.gov/nevada/desert_tortoise/documents/recovery_plan/1994_dtrp.pdf [accessed 4 December 2017].
US Fish and Wildlife Service (2007). Western snowy plover (Charadrius alexandrinus nivosus) Pacific coast population recovery plan. U.S. Fish and Wildlife Service, Sacramento, California. Available at: https://www.fws.gov/carlsbad/SpeciesStatusList/RP/20070813_RP_WSP.pdf [accessed 4 December 2017].
US Fish and Wildlife Service (2011). Revised recovery plan for the Mojave population of the desert tortoise (Gopherus agassizii). U.S. Fish and Wildlife Service, Sacramento, California. Available at: http://www.fwspubs.org/doi/suppl/10.3996/022015-JFWM-013/suppl_file/022015-jfwm-013.s7.pdf?code=ufws-site [accessed 4 December 2017].
US Fish and Wildlife Service (2016). Range-wide monitoring of the Mojave Desert tortoise (Gopherus agassizii): 2015 and 2016 annual reporting. Report by the Desert Tortoise Recovery Office, U.S. Fish and Wildlife Service, Reno, Nevada. Available at: https://www.fws.gov/nevada/desert_tortoise/documents/reports/2015/201516_rangewide-mojave-desert-tortoise-monitoring.pdf [accessed 4 December 2017].
Voigt, D. R., and Berg, W. E. (1987). Coyote. In ‘Wild Furbearer Management and Conservation in North America’. (Eds M. Novak, J. A. Baker, M. E. Obbard and B. Malloch.) pp. 344–357. (Ontario Ministry of Natural Resources: Toronto, ON.)
Whitford, W. G., and Creusere, F. M. (1977). Seasonal and yearly fluctuations in Chihuahuan Desert lizard communities. Herpetologica 33, 54–65.
Woodbury, A. M., and Hardy, R. (1948). Studies of the desert tortoise, Gopherus agassizii. Ecological Monographs 18, 145–200.
| Studies of the desert tortoise, Gopherus agassizii.Crossref | GoogleScholarGoogle Scholar |
Young, J. A., and Young, C. G. (1992). ‘Seeds of Woody Plants in North America.’ (Dioscorides Press: Portland, OR.)
Zar, J. H. (1984). ‘Biostatistical Analysis.’ 2nd edn. (Prentice-Hall: Englewood Cliffs, NJ.)
Zimmerman, L. C., O’Connor, M. P., Bulova, S. J., Spotila, J. R., Kemp, S. J., and Salice, C. J. (1994). Thermal ecology of desert tortoises in the eastern Mojave Desert: seasonal patterns of operative and body temperatures, and microhabitat utilization. Herpetological Monograph 8, 45–59.
| Thermal ecology of desert tortoises in the eastern Mojave Desert: seasonal patterns of operative and body temperatures, and microhabitat utilization.Crossref | GoogleScholarGoogle Scholar |