The longest-lived spider: mygalomorphs dig deep, and persevere
Leanda Denise Mason A C , Grant Wardell-Johnson A and Barbara York Main BA ARC Centre for Mine Site Restoration, School of Molecular and Life Sciences, Curtin University, GPO Box U1987, Perth, WA 6845, Australia.
B PO Box 668, Nedlands, WA 6909, Australia.
C Corresponding author. Email: leanda.denise.mason@gmail.com
Pacific Conservation Biology 24(2) 203-206 https://doi.org/10.1071/PC18015
Submitted: 31 January 2018 Accepted: 1 April 2018 Published: 19 April 2018
Abstract
We report the longest-lived spider documented to date. A 43-year-old, female Gaius villosus Rainbow, 1914 (Mygalomorphae: Idiopidae) has recently died during a long-term population study. This study was initiated by Barbara York Main at North Bungulla Reserve near Tammin, south-western Australia, in 1974. Annual monitoring of this species of burrowing, sedentary mygalomorph spider yielded not only this record-breaking discovery but also invaluable information for high-priority conservation taxa within a global biodiversity hotspot. We suggest that the life-styles of short-range endemics provide lessons for humanity and sustainable living in old stable landscapes.
Additional keywords: conservation, fragmented landscapes, long-term study, short-range endemism, trapdoor spiders, world-record.
Introduction
All mygalomorph spiders, except some arboreal species (Pérez-Miles and Perafán 2017), live in burrows constructed by dispersing spiderlings, often close to their maternal burrow (Main 1984). Burrow morphology varies between different mygalomorph spider clades but the species are generally sedentary unless disturbed or requirements are not being met (Main 1984). Mygalomorph spiders are considered ‘relictual’, having remained in a similar ecological niche since the mid–late Tertiary, despite diversifying genetically (Rix et al. 2017a). The low dispersal of mygalomorph spiders makes for a high diversity of restricted-range species over evolutionary time scales (Rix et al. 2017a), classifying them as conservation-significant ‘short-range endemics’ (SREs) (Harvey 2002).
SREs are animals found in only a small area (entire distribution within 10 000 km2), due primarily to their low mobility and poor dispersal ability (Harvey 2002). Mygalomorph spiders, as SREs (Rix et al. 2015), represent a largely unrecognised contribution to biodiversity. South-western Australia (SWA), the site of this study, hosts 65 described species of mygalomorph spiders (World Spider Catalog 2018), but also hosts many recorded but unnamed species, as well as many yet to be discovered. Many are restricted to narrow ranges and often require specific microhabitats. Short-range endemism also adds to the overall diversity of regions through high spatial turnover, a situation also well recognised for the plants of SWA (Gibson et al. 2017).
The life-history traits of mygalomorph spiders demonstrate a successful approach for persistence in old, stable landscapes (Mucina and Wardell-Johnson 2011), which are under threat from novel disturbances such as deforestation, fragmentation, exploitation, and introduced biota (Wardell-Johnson et al. 2016). A long-term study established by Barbara York Main in 1974 (Main 1978) enables assessment of the age, longevity and population dynamics of one species of mygalomorph spider – Gaius villosus Rainbow, 1914 (Mygalomorphae: Idiopidae) (Rix et al. 2017b). Here we report the death of an individual from this long-term population study and outline the significance of this event.
Methods
All methodology for this study was derived from the original study conducted by Main (1978).
Study site
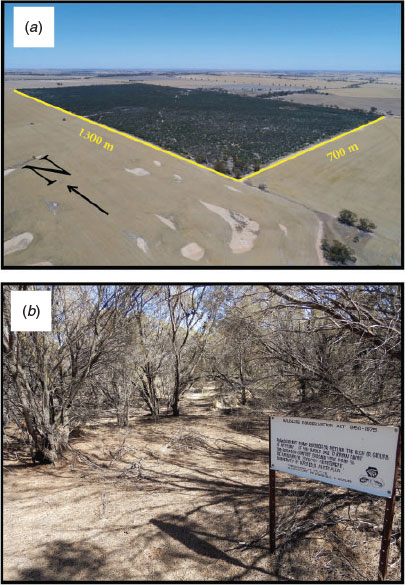
The study site is in the central wheatbelt, SWA. Between 1920 and 1980, this region was subject to substantial clearing for agriculture, and it now retains only 7% native vegetation (Jarvis 1981; Deo 2011). North Bungulla Reserve (area: 104 ha, latitude: −31.525937, longitude: 117.591357) is one of few patches of remnant bushland remaining in the region (Fig. 1a). The reserve comprises mixed mallee, heath and thicket vegetation (Fig. 1b) (Main 1978).
|
Monitoring
In 1974, a gridded plot 26 m × 40 m (Fig. 1b) was mapped to regularly assess local distribution and demography of a population of G. villosus. Permanent numbered tagged pegs were used to identify individuals in subsequent surveys. Pegs were sited directly behind burrow hinges to prevent foraging being compromised.
As male and female juvenile spiders are morphologically identical, sex cannot be determined before sexual maturity without genetic verification (Hebert et al. 2003). Spiderling emergents were pegged and monitored to determine survivorship and successive recruitment. Adults and associated burrows were monitored at six-monthly intervals or annually to determine age, maturity and reproductive cues.
Males that reach sexual maturity (at ~5 years) seal their burrow and go through a final moult before leaving in search of a female, but perish within the same season. Evidence of a broken burrow lid seal, together with moults confirms that the burrow had hosted a male, rather than being a now-defunct female burrow. Conversely, females always remain in their burrows and, when receptive to mating, will put out a silk ‘doile’, thought to attract males through pheromones. Brooding females are recorded from the presence of a mud-plug, thought to provide extra protection to the spiderlings.
Thriving populations of these spiders include large and mature active burrows inhabited by aging females, as well as smaller burrows inhabited by juveniles of unknown sex. As spiderlings age, they widen their burrows and moult to grow larger each year until reaching sexual maturity. Widening of burrows can leave silk patterns similar to those of tree rings. However, as they don’t widen their burrow once reaching maturity, this is useful only to estimate ages of juveniles between one and five years old. It was therefore imperative to peg burrows to determine the age of mature spiders.
Results
The oldest spider recorded, a Gauis villosus (Fig. 2a), was in the first group of dispersing spiderlings that established a burrow (Fig. 2b) pegged by BYM in the first season of the study in March 1974. It was the 16th spider pegged (Fig. 2c). By 2016, over 150 spiders had been pegged in the 26 × 40 m study site. The first 15 spiders, and spiders numbered well beyond 16, have died in the interim.

|
On 31 October 2016 we found that the lid of the burrow of the oldest spider, #16, had been pierced by a parasitic wasp (Fig. 2c). Having been seen alive in the burrow six months earlier, we therefore report the death of an ancient G. villosus mygalomorph spider matriarch at the age of 43.
On the basis of a diagnostic hole in the burrow lid (Fig. 2c), and her burrow falling into disrepair since the previous recording, we recognise that she was either parasitised or already dead. Thus, it is likely that #16 did not die of old age, but rather was parasitised by a spider wasp (Pompilidae: O’Neill 2001). Once the egg hatches, the spider is consumed from the inside, over the course of several weeks. Detailed data relating to ages, causes of death and life history of the entire population will also be made available.
Discussion
Life-history lessons
To our knowledge, #16 is the oldest documented spider recorded, with the Guinness World Record being a 28-year-old tarantula (Mygalomorphae: Theraphosidae) in captivity, and Tasmanian cave spiders (Araneomorphae: Hickmania troglodytes) thought to live 30–40 years (Mammola et al. 2017).
The findings from the initial years of this long-term study provided invaluable information on spiderling dispersal, sexual maturity, and the proportion of males and females (See Main 1978). Continuance of the study has provided more accurate ages, cause of death, and understanding of the life history of this basal group of spiders (which will be made available elsewhere).
There is a high level of certainty that #16 lived for 43 years. Neither males nor females re-use the defunct burrow of another spider (Main 1984). Adult spiders do not relocate if their burrow is damaged, but repair their existing burrow. There are three likely reasons for this: (1) the chances of locating a suitable defunct burrow at the time of disturbance to their own burrow is low due to mygalomorphs being relatively blind (Willemart and Lacava 2017); (2) there is a high risk in being exposed or above ground, where they are vulnerable to desiccation or predation (Mason et al. 2013; Canals et al. 2015); and (3) relining the entirety of a burrow with silk and construction of a lid is an exceptionally large energy and time investment (Hils and Hembree 2015). In addition, defunct burrows of adult spiders are too large to accommodate dispersing spiderlings. Further, the burrow of #16 fell into disrepair soon after the lid had been pierced.
Sustainability lessons
A deeper appreciation of the place of biodiversity and sustainability in the ancient landscapes of SWA follows from an understanding of life history (Wardell-Johnson and Horwitz 1996; Main 2001). SRE invertebrates, such as mygalomorph spiders, represent an unquantified contribution to biodiversity. More than 70% of the native vegetation has been removed from SWA (Wardell-Johnson et al. 2015, 2016) and the region was the first Australian global biodiversity hotspot recognised – one of the 25 originally defined by Myers et al. (2000). Global biodiversity hotspots are endemic-rich regions that are also under threat. With so much of the landscape having been cleared, we may never know how many species have already been lost.
Historically, sustainability in the old landscapes of SWA has been vastly overestimated, with influxes of people in the last 180 years who have transformed the environment and pushed much life to the edge of extinction. The European explorers were impressed by the size of trees and apparent productivity (Wardell-Johnson et al. 2015). They would have been better guided by how the Indigenous peoples already dwelling there for tens of millennia were managing, and being managed by, these landscapes (Wardell-Johnson et al. 2016). For them it was about persistence, low-level impact and frugal resource use. These are the same traits that exemplify the character of those now living sustainably in SWA. It is also the very antithesis of contemporary pressures.
SWA is measured in geological time-scales (Myers 1997), by the time discernible in the wearing down of landscapes, and by the time of deep weathering of landscape profiles (Campbell 1997). This is ample time to lose the essential nutrients for growth, especially phosphorus, nitrogen and sulfur. Old landscapes manage the biota, and the people and societies that come and go.
What follows from the challenges presented by old, deeply weathered, nutrient-poor landscapes where carbon is stockpiled, water thirsted for and nutrients extricated? One successful approach results in a long life-time in a small burrow. Unfortunately, their sedentary nature and poor dispersal ability mean that mygalomorph spiders cannot readily break new ground and colonise more broadly. Away from their burrows they are susceptible to desiccation (Mason et al. 2013). In addition, many are confined to small areas and often require specific microhabitats. They are therefore highly vulnerable to disturbance that compromises the quality of their habitat (Harvey et al. 2011; Mason et al. 2016).
Landscapes exemplified by broader SWA may be resilient to disturbances prominent in their evolutionary history such as seasonal instability, fire, and drought (Hopper 2009; Mucina and Wardell-Johnson 2011). However, they are fragile to novel threats. Disturbances such as deforestation, eutrophication, introduced animals, plants and microbes, major substrate disturbance and continued biomass loss transform the landscape into something requiring constant management to be productive (Wardell-Johnson et al. 2016).
As we begin rebuilding with more sustainable technologies and improve the management of known threatening processes (Braby 2018), we can be inspired by an ancient mygalomorph spider and the rich biodiversity she embodied.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
We acknowledge Curtin University’s School of Molecular and Life Sciences and the ARC centre for Mine Site Restoration for funding and for providing an excellent workplace environment. Monica and Rebecca Main have provided ongoing and valued support to their mother – without them both, this finding may never have been documented.
References
Braby, M. F. (2018). Threatened species conservation of invertebrates in Australia: an overview. Austral Entomology , .| Threatened species conservation of invertebrates in Australia: an overview.Crossref | GoogleScholarGoogle Scholar |
Campbell, E. M. (1997). Granite landforms. Journal of the Royal Society of Western Australia 80, 101–112.
Canals, M., Veloso, C., and Solís, R. (2015). Adaptation of the spiders to the environment: the case of some Chilean species. Frontiers in Physiology 6, 220.
| Adaptation of the spiders to the environment: the case of some Chilean species.Crossref | GoogleScholarGoogle Scholar |
Deo, R. C. (2011). Links between native forest and climate in Australia. Weather 66, 64–69.
| Links between native forest and climate in Australia.Crossref | GoogleScholarGoogle Scholar |
Gibson, N., Prober, S., Meissner, R., and van Leeuwen, S. (2017). Implications of high species turnover on the south-western Australian sandplains. PLOS ONE 12, e0172977.
| Implications of high species turnover on the south-western Australian sandplains.Crossref | GoogleScholarGoogle Scholar |
Harvey, M. S. (2002). Short-range endemism in the Australian fauna: some examples from non-marine environments. Invertebrate Systematics 16, 555–570.
| Short-range endemism in the Australian fauna: some examples from non-marine environments.Crossref | GoogleScholarGoogle Scholar |
Harvey, M. S., Rix, M. G., Framenau, V. W., Hamilton, Z. R., Johnson, M. S., Teale, R. J., Humphreys, G., and Humphreys, W. F. (2011). Protecting the innocent: studying short-range endemic taxa enhances conservation outcomes. Invertebrate Systematics 25, 1–10.
| Protecting the innocent: studying short-range endemic taxa enhances conservation outcomes.Crossref | GoogleScholarGoogle Scholar |
Hebert, P. D., Cywinska, A., and Ball, S. L. (2003). Biological identifications through DNA barcodes. Proceedings of the Royal Society of London. Series B, Biological Sciences 270, 313–321.
| Biological identifications through DNA barcodes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktVWiu7g%3D&md5=9cc74ca9094086122f3e2559c4bb37caCAS |
Hils, J. M., and Hembree, D. I. (2015). Neoichnology of the burrowing spiders Gorgyrella inermis (Mygalomorphae: Idiopidae) and Hogna lenta (Araneomorphae: Lycosidae). Palaeontologia Electronica 18, 1–62.
Hopper, S. D. (2009). OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant and Soil 322, 49–86.
| OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVWisrnN&md5=dc610cba3f620d664d2cd8c35b2d7120CAS |
Jarvis, N. T. (1981). Western Australia: An Atlas of Human Endeavour, 1829–1979. Education and Lands and Surveys Departments of Western Australia, Perth.
Main, B. Y. (1978). Biology of the arid-adapted Australian trapdoor spider Anidiops villosus (rainbow). Bulletin of the British Arachnological Society 4, 161–175.
Main, B. Y. (1984). ‘Spiders.’ (William Collins Pty Ltd: Sydney.)
Main, B. Y. (2001). Historical ecology, responses to current ecological changes and conservation of Australian spiders. Journal of Insect Conservation 5, 9–25.
| Historical ecology, responses to current ecological changes and conservation of Australian spiders.Crossref | GoogleScholarGoogle Scholar |
Mammola, S., Michalik, P., Hebets, E. A., and Isaia, M. (2017). Record breaking achievements by spiders and the scientists who study them. PeerJ 5, e3972.
| Record breaking achievements by spiders and the scientists who study them.Crossref | GoogleScholarGoogle Scholar |
Mason, L., Tomlinson, S., Withers, P., and Main, B. (2013). Thermal and hygric physiology of Australian burrowing mygalomorph spiders (Aganippe spp.). Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 183, 71–82.
| Thermal and hygric physiology of Australian burrowing mygalomorph spiders (Aganippe spp.).Crossref | GoogleScholarGoogle Scholar |
Mason, L. D., Wardell-Johnson, G., and Main, B. Y. (2016). Quality not quantity: conserving species of low mobility and dispersal capacity in south-western Australian urban remnants. Pacific Conservation Biology 22, 37–47.
| Quality not quantity: conserving species of low mobility and dispersal capacity in south-western Australian urban remnants.Crossref | GoogleScholarGoogle Scholar |
Mucina, L., and Wardell-Johnson, G. (2011). Landscape age and soil fertility, climate stability, and fire regime predictability: beyond the OCBIL framework. Plant and Soil 341, 1–23.
| Landscape age and soil fertility, climate stability, and fire regime predictability: beyond the OCBIL framework.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjt1Wjur8%3D&md5=5ccf3df28447a20258e18e61a630b230CAS |
Myers, J. S. (1997). Geology of granite. Journal of the Royal Society of Western Australia 80, 87–100.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhs1Olsr4%3D&md5=faeb3f1a9fb6a1a6e0b62f4d864c151aCAS |
O’Neill, K. M. (2001). ‘Solitary Wasps: Behavior and Natural History.’ (Cornell University Press: Cornell, NY.)
Pérez-Miles, F., and Perafán, C. (2017). Behavior and biology of Mygalomorphae. In ‘Behaviour and Ecology of Spiders: Contributions from the Neotropical Region’. (Eds C. Viera and M. O. Gonzaga.) pp. 29–54. (Springer: Cham.)
Rix, M. G., Edwards, D. L., Byrne, M., Harvey, M. S., Joseph, L., and Roberts, J. D. (2015). Biogeography and speciation of terrestrial fauna in the south‐western Australian biodiversity hotspot. Biological Reviews of the Cambridge Philosophical Society 90, 762–793.
| Biogeography and speciation of terrestrial fauna in the south‐western Australian biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Rix, M. G., Cooper, S. J., Meusemann, K., Klopfstein, S., Harrison, S. E., Harvey, M. S., and Austin, A. D. (2017a). Post-Eocene climate change across continental Australia and the diversification of Australasian spiny trapdoor spiders (Idiopidae: Arbanitinae). Molecular Phylogenetics and Evolution 109, 302–320.
| Post-Eocene climate change across continental Australia and the diversification of Australasian spiny trapdoor spiders (Idiopidae: Arbanitinae).Crossref | GoogleScholarGoogle Scholar |
Rix, M. G., Raven, R. J., Main, B. Y., Harrison, S. E., Austin, A. D., Cooper, S. J. B., and Harvey, M. S. (2017b). The Australasian spiny trapdoor spiders of the family Idiopidae (Mygalomorphae: Arbanitinae): a relimitation and revision at the generic level. Invertebrate Systematics 31, 566–634.
| The Australasian spiny trapdoor spiders of the family Idiopidae (Mygalomorphae: Arbanitinae): a relimitation and revision at the generic level.Crossref | GoogleScholarGoogle Scholar |
Wardell-Johnson, G., and Horwitz, P. (1996). Conserving biodiversity and the recognition of heterogeneity in ancient landscapes: a case study from south-western Australia. Forest Ecology and Management 85, 219–238.
| Conserving biodiversity and the recognition of heterogeneity in ancient landscapes: a case study from south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Wardell-Johnson, G., Calver, M., Burrows, N., and Virgilio, G. D. (2015). Integrating rehabilitation, restoration and conservation for a sustainable jarrah forest future during climate disruption. Pacific Conservation Biology 21, 175–185.
| Integrating rehabilitation, restoration and conservation for a sustainable jarrah forest future during climate disruption.Crossref | GoogleScholarGoogle Scholar |
Wardell‐Johnson, G., Wardell‐Johnson, A., Bradby, K., Robinson, T., Bateman, P. W., Williams, K., Keesing, A., Braun, K., Beckerling, J., and Burbridge, M. (2016). Application of a Gondwanan perspective to restore ecological integrity in the south‐western Australian global biodiversity hotspot. Restoration Ecology 24, 805–815.
| Application of a Gondwanan perspective to restore ecological integrity in the south‐western Australian global biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Willemart, R. H., and Lacava, M. (2017). Foraging strategies of cursorial and ambush spiders. In ‘Behaviour and Ecology of Spiders: Contributions from the Neotropical Region’. (Eds C. Viera and M. O. Gonzaga.) pp. 227–245. (Springer: Cham.)
World Spider Catalog (2018). World Spider Catalog. Natural History Museum Bern. Available at: http://wsc.nmbe.ch, version 19.0, accessed 23 March 2018.


