PAPPA2 is increased in severe early onset pre-eclampsia and upregulated with hypoxia
Kate Macintire A , Laura Tuohey A , Louie Ye A , Kirsten Palmer A , Michael Gantier B , Stephen Tong A and Tu’uhevaha J. Kaitu’u-Lino A CA Translational Obstetrics Group, The Department of Obstetrics and Gynaecology, Mercy Hospital for Women, University of Melbourne, 163 Studley Road, Heidelberg, Vic. 3084, Australia.
B Monash Institute of Medical Research, Monash University, 27-31 Wright Street, Clayton, Vic. 3168, Australia.
C Corresponding author. Email: t.klino@unimelb.edu.au
Reproduction, Fertility and Development 26(2) 351-357 https://doi.org/10.1071/RD12384
Submitted: 29 November 2012 Accepted: 26 January 2013 Published: 14 March 2013
Abstract
Severe early onset pre-eclampsia is a serious pregnancy complication, believed to arise as a result of persistent placental hypoxia due to impaired placentation. Pregnancy-associated plasma protein A2 (PAPPA2) is very highly expressed in the placenta relative to all other tissues. There is some evidence that PAPPA2 mRNA and protein are increased in association with pre-eclampsia. The aim of the present study was to characterise the mRNA and protein expression, as well as localisation, of PAPPA2 in an independent cohort of severe early onset pre-eclamptic placentas. We also examined whether exposing placental explants to hypoxia (1% oxygen) changed the expression of PAPPA2. Expression of PAPPA2 mRNA and protein was upregulated in severe early onset pre-eclamptic placentas compared with preterm controls and localised to the syncytiotrophoblast. Interestingly, protein localisation was markedly reduced in term placenta. Syncytialisation of BeWo cells did not change PAPPA2 expression. However, hypoxia upregulated PAPPA2 mRNA and protein expression in primary placental explants. Together, our data suggest that PAPPA2 may be upregulated in severe pre-eclampsia and, functionally, this may be mediated via increased placental hypoxia known to occur with this pregnancy disorder.
Additional keywords: placenta, pregnancy.
Introduction
Severe early onset pre-eclampsia (PE) affects 6%–8% of pregnancies and is a leading cause of maternal and perinatal mortality and morbidity (Sibai et al. 2005; Powe et al. 2011). There are currently no treatments available to halt disease progression, and thus clinicians are often forced to deliver infants regardless of gestation.
During the first trimester of pregnancy, placentation occurs in a tightly controlled series of events, specifically designed to allow adequate blood flow to the fetoplacental unit throughout pregnancy. In PE, it is widely believed that trophoblast invasion is shallow and the spiral arteries are not remodelled adequately to provide the high blood volume required by the developing fetoplacental unit (Redman and Sargent 2005). Failure to establish an adequate blood supply results in chronic and prolonged hypoxia, predisposing the fetus to an increased risk of intrauterine growth restriction and stillbirth (Moffett-King 2002; Redman and Sargent 2005). PE is commonly diagnosed when women present with hypertension and proteinuria, clinical hallmarks of mounting end-organ damage resulting from widespread maternal endothelial dysfunction, directly attributable to anti-angiogenic factors that are released from the chronically hypoxic placenta (Redman and Sargent 2005).
Pregnancy-associated plasma protein A2 (PAPPA2) is a homologue of the well known first-trimester serum marker of pathological pregnancies, namely PAPPA, sharing 46% sequence identity (Conover et al. 2011). Importantly, recent reports demonstrate a link between PAPPA2 and PE (Nishizawa et al. 2008; Winn et al. 2009; Paiva et al. 2011). However, the evidence showing raised PAPPA2 protein in severe preterm PE (responsible for most of the morbidity caused by this disease) is limited to 30 cases described to date (Nishizawa et al. 2008; Winn et al. 2009). Thus, this association merits further independent validation.
Furthermore, the major mechanism that may potentially differentially regulate PAPPA2 in placenta remains to be robustly described. Given PE placentas are chronically hypoxic, we hypothesised that PAPPA2 would be upregulated in placental tissues exposed to hypoxia. Although one publication has indeed shown upregulation of PAPPA2 following hypoxic exposure of BeWo cells, a choriocarcinoma-derived cell line (Wagner et al. 2011), it has not been demonstrated in primary placental tissues.
The aim of the present study was to characterise PAPPA2 in an independent cohort of severe early onset PE and gestationally matched preterm control samples at both the mRNA and protein levels. Given that PE is associated with placental hypoxia, we also used functional assays to determine the effects of hypoxia on PAPPA2 mRNA and protein expression in primary placental tissues.
Materials and methods
Tissue collection
Women presenting to two tertiary women’s hospitals in Melbourne, Australia, between 2008 and 2012 (Monash Medical Centre and Mercy Hospital for Women) provided written informed consent for placental tissue collection. Placentas were obtained from preterm pregnancies (PT) not complicated by PE and those complicated by severe early onset PE. Severe pre-eclamptics were diagnosed in accordance with American Congress of Obstetricians and Gynecologists (ACOG) guidelines (ACOG 2002) and included the presence of hypertension >160/110 mmHg on two occasions greater than 6 h apart, proteinuria >5 g day–1, oliguria <500 mL day–1, visual disturbance, pulmonary oedema, right upper quadrant pain, abnormal liver function, thrombocytopenia or fetal growth restriction. In addition, all samples were obtained from cases of early onset preterm PE, defined as requiring delivery <34 weeks gestation. PT control placentas were selected from women presenting with PT rupture of membranes or spontaneous PT labour without evidence of infection (histopathological examination of the placentas), hypertensive disease or maternal comorbidities.
Placental tissue was obtained immediately following delivery. Placental tissue (excluding fetal membranes) was removed and washed briefly in sterile phosphate-buffered saline (PBS). Samples for protein and RNA extraction were frozen within 15 min of delivery and stored at –80°C. A portion of each placenta was also fixed in 10% buffered formalin for histology. Human ethics approval was obtained for this study from both the Southern Health Human Research Ethics Committee and the Mercy Health Human Research Ethics Committee.
Western blot analysis
Placental lysate samples (20 μg) were separated on 10% polyacrylamide gels with wet transfer to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Membranes were blocked before blotting overnight with antibodies targeting PAPPA2 (1 : 500; Sigma, St Louis, MA, USA) and GAPDH (1 : 5000; Cell Signaling Technology, Danvers, MA, USA). Membranes were then visualised using an enhanced chemiluminescence detection system (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and ChemiDoc XRS (BioRad, Hercules, CA, USA). GAPDH was used as a loading control. Relative densitometry was determined using Image Laboratory (BioRad).
Immunohistochemistry
PAPPA2 immunohistochemistry was conducted on placental tissue collected from either PE or PT control pregnancies. Paraffin sections (5 μm) of formalin-fixed tissues were dewaxed in xylene and rehydrated through descending grades of ethanol. Sections were then heated for 20 min on defrost in a 700-W microwave oven, followed by cooling to room temperature for 30 min. Sections were then washed for 10 min in PBS (pH 7.6) and immersed in 3% H2O2 in methanol for 10 min at room temperature before being washed with PBS. The sections were then immersed in Dako blocking buffer (DAKO, Campbelfield, Vic., Australia) for 10 min and then incubated for 1 h at 37°C with rabbit anti-human PAPPA2 (Sigma) at 10 μg mL–1 in 1% bovine serum albumin (BSA)–PBS. For isotype controls, the primary antibody was substituted with mouse IgG. The SuperPicTure kit (Invitrogen, Carlsbad, CA, USA) was used according to the manufacturer’s instructions to reveal the PAPPA2 staining. Sections were lightly counterstained with Harris haematoxylin (Accustain; Sigma Diagnostics, Castle Hill, NSW, Australia), dehydrated and mounted using Di-N-Butyle Phthalate in Xylene (DPX) mounting medium (BDH Laboratory Supplies, Poole, UK).
Reverse transcription–polymerase chain reaction
RNA was extracted from 50–100 mg frozen placental samples by homogenisation, followed by use of the RNeasy mini-kit (Invitrogen) according to the manufacturer’s instructions. The quantity and quality of the RNA were determined using the Nanodrop 2000 (ThermoScientific, Waltham, MA, USA). Only RNA providing a 260/280 ratio of 1.9–2.10 was used in further experiments.
Reverse transcription of RNA was performed using a SuperScript Vilo cDNA Synthesis Kit (Invitrogen) according to the manufacturer’s instructions. Sybr polymerase chain reaction (PCR) analyses were performed using primers obtained from PrimerBank (a public database of primers that have been extensively tested for PCR specificity and efficiency; http://pga.mgh.harvard.edu/primerbank/) with primers synthesised using SigmaGenosys (Sigma), as previously described (Paiva et al. 2011).
Real-time quantitative PCR was performed on the CFX 384 (Bio-Rad, Hercules, CA, USA) using Fast Sybr Green Master Mix (Applied Biosystems) with the following run conditions: for Sybr PCR, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 1 min and 72°C for 30 s, finishing off with 95°C for 15 s, 60°C for 15 s and 95°C for 15 s to obtain a dissociation curve. The specificity of the PCR products obtained using these primers has been confirmed previously (Paiva et al. 2011).
All data were normalised against an appropriate housekeeping gene (GAPDH or YWHAZ) used as an internal control and calibrated against the average cycle threshold (Ct) of the control samples. GAPDH was used as the control for all experiments except in the hypoxia experiments, because it has been suggested previously that GAPDH levels vary with hypoxia (Zhong and Simons 1999). The results are expressed as fold changes relative to control. Samples were run in triplicate. Negative controls lacking template and containing RNA that was not reverse transcribed were included in all runs.
Syncytialisation of BeWo cells
Cells were plated at 50 000 cells per well of a six-well plate and allowed to plate down for 24 h. Cells to be syncytialised were treated with 20 μM forskolin, whereas control cells received an equivalent volume of vehicle control (dimethylsulfoxide; DMSO). Cells were incubated for 48 h before monolayers were processed for RNA or protein extraction.
Hypoxic culture of term placental explants
Fresh placental specimens were dissected into 5–10 mm cubes and allowed to equilibrate at 37°C for 1 h before being transferred to fresh culture medium and cultured under either hypoxic (1% oxygen, 5% CO2) or normal (20% oxygen, 5% CO2) culture conditions for 24 h (n = 6 placentas).
Analysis of the PAPPA2 promoter
Promoter analysis of human PAPPA2 (gene ID 60676) was performed using a 2000-nucleotide region upstream of the PAPPA2 mRNA (NM_020318.2), together with the first exon of PAPPA2, covering a 2248-base region defined by the coordinates chr1 : 176430307–176432554 (hg19 assembly), using MatInspector (Genomatix Software, Munich, Germany). Predictions of hypoxia responsive element (HRE) and nuclear factor (NF)-κB binding sites were confirmed using independent software relying on TRANSFAC (BioBase, Wolfenbuettel, Germany) matrices (Messeguer et al. 2002).
Statistical analysis
Continuous variables were compared using either an unpaired t-test (parametric data) or a Mann–Whitney U-test (non-parametric data). P ≤ 0.05 was considered significant. All statistical analyses were undertaken using GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
Results
Increased PAPPA2 mRNA and protein expression in severe early onset PE
Expression of PAPPA2 mRNA was assessed in a cohort of severe early onset PE (n = 18) and PT control (n = 10) placental samples and was found to be increased in PE placentas (Fig. 1a; P = 0.06). A larger cohort of samples (PE, n = 29; PT, n = 15) was assessed by western blot analysis, which verified a significant (P < 0.01) increase in PAPPA2 protein production with severe early onset PE (Fig. 1b, c).
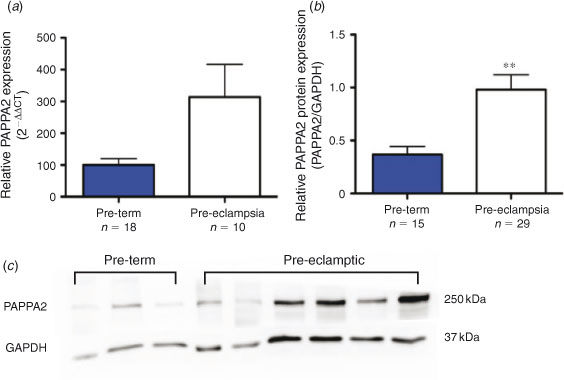
|
Localisation of PAPPA2 to the syncytiotrophoblast of PE and PT placentas does not change with syncytialisation
Immunohistochemistry was used to investigate the localisation of PAPPA2 in placental tissue (Fig. 2). Strong syncytiotrophoblast staining was observed in both the PT (Fig. 2a) and PE (Fig. 2b) cohort. Interestingly, little to no staining was observed in term placental sections (Fig. 2c, d). Negative controls, in which the primary antibody was substituted with an IgG control, were clean (Fig. 2e). Given PAPPA2 was localised to the syncytiotrophoblast, we assessed PAPPA2 mRNA levels in BeWo cells that were induced to syncytialise, but observed no significant changes in PAPPA2 expression (Fig. 2f).
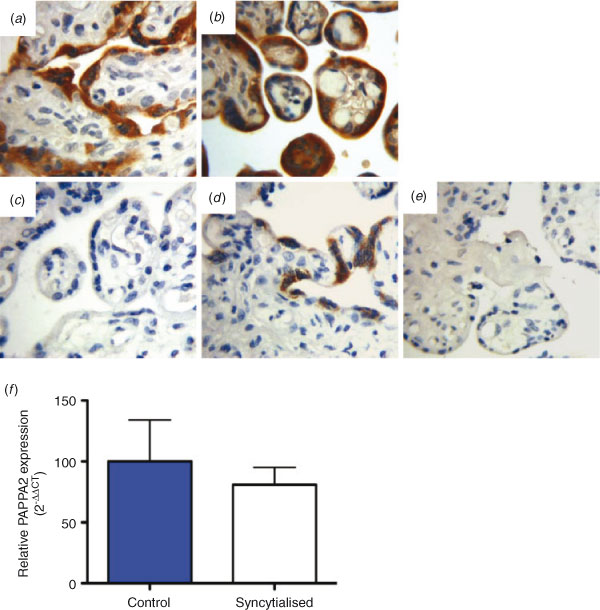
|
Increased PAPPA2 mRNA and protein expression in primary term placental explants exposed to hypoxia
In silico analysis identified the presence of an HRE and two NF-κB target sites within the PAPPA2 promoter region (Fig. 3a). To examine whether placental PAPPA2 expression changed with exposure to hypoxia, we exposed primary term placental explants to hypoxia (1% oxygen) for 24 h. There was a significant (P < 0.001) increase in PAPPA2 mRNA expression (Fig. 3b) following exposure to hypoxia. There was also a tendency for increased protein expression in the same samples on densitometric analysis, but the differences did not reach statistical significance (Fig. 3c, d).
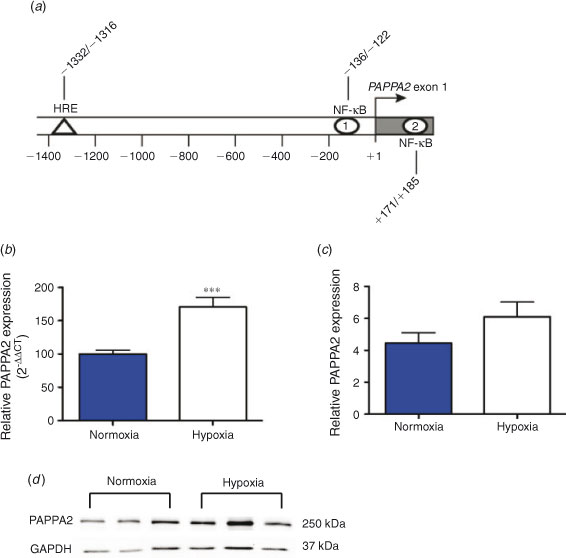
|
Discussion
In the present study we demonstrated a significant increase in PAPPA2 mRNA and protein expression in an independent cohort of severe early onset PE placentas. We demonstrated protein localisation to the syncytiotrophoblast layer in PE and PT placentas but, surprisingly, observed a marked decrease and/or absent staining in term placenta. We also demonstrated no change in PAPPA2 expression with syncytialisation, but a significant increase in PAPPA2 mRNA expression in term placental explants exposed to hypoxia.
Several previous studies have published data indicating that PAPPA2 is increased in PE (Buimer et al. 2008; Sitras et al. 2009; Várkonyi et al. 2010; Wagner and Christians 2010). In addition, PAPPA2 has also been localised to the syncytiotrophoblast previously (Nishizawa et al. 2008; Wagner and Christians 2010) in both PE and PT placentas.
Severe preterm PE represents the most serious spectrum of this disease and is responsible for most of the serious morbidity and mortality. The collective cohort size in all reports demonstrating PAPPA2 protein is increased in severe preterm PE has thus far been limited to only 30 (Nishizawa et al. 2008; Winn et al. 2009). Therefore, we thought it was important to provide independent confirmation of this finding. Thus, in the present study, we demonstrated increased PAPPA2 in a further large cohort of 29 severe PE placentas. In light of our findings, we now believe there is collectively very strong evidence showing PAPPA2 protein is increased in severe preterm PE.
Interestingly, we observed little to no PAPPA2 staining within term placentas. Nishizawa et al. (2008) previously assessed PAPPA2 immunohistochemistry in term control (but not preterm) and severe early onset PE placentas and also suggested a decrease in PAPPA2 in control samples compared with PE. However, the present study appears to be the first to comprehensively compare PAPPA2 immunohistochemistry between PT, PE and term samples and to observe a lack of staining in term samples. Although variable, placental PAPPA2 mRNA levels have also been reported to decline with gestation (Winn et al. 2009) and PAPPA2 has been shown to be a cleavage protease for insulin-like growth factor (IGF) binding protein 5 (Wang et al. 2009), suggesting that it contributes to IGF bioavailability. Thus, the decline in placental PAPPA2 protein observed at term in our immunohistochemical samples is possibly a result of the decreased placental growth occurring during this phase of pregnancy.
It is now well accepted that the pre-eclamptic placenta is exposed to chronic hypoxia throughout gestation as a consequence of the failure to establish an adequate blood supply during implantation (Redman and Sargent 2005; Sibai et al. 2005). Recent studies have also suggested that reactive oxygen species triggered by hypoxia produce oxysterols that may contribute to the pathogenesis of PE (Valbuena-Diez et al. 2012) and that oxysterols inhibit differentiation and fusion of primary trophoblasts (Aye et al. 2011) For this reason, we examined PAPPA2 mRNA and protein expression in term placental explants exposed to hypoxia for 24 h. Previous data indicate a 47-fold increase in PAPPA2 mRNA when BeWo cells are exposed to hypoxia (2% oxygen; Wagner et al. 2011). However, primary tissue has not been examined previously. Given that we identified a putative HRE within the PAPPA2 promoter, it was not surprising to observe a highly significant increase in PAPPA2 mRNA levels when placental explants were exposed to hypoxia. Although the increase in protein levels from these same samples did not reach statistical significance, there was the same trend for increased levels with hypoxia. It is possible that the lack of statistically significant increases was related to the timing of the experiment (only 24 h exposure), and that protein levels would have been more definitively elevated if given further time in culture. Interestingly, we also identified two NF-κB binding sites within the promoter region, which may also contribute to the decrease in PAPPA2 observed with hypoxia. Certainly there is good evidence to suggest a strong overlap between hypoxia and NF-κB activity, whereby hypoxia induces NF-κB activation and NF-κB regulates hypoxia-inducible factor-1 expression (Rius et al. 2008; Culver et al. 2010). Thus, the presence of NF-κB binding sites in PAPPA2 may also contribute to the effect observed.
Currently soluble FMS-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng), anti-angiogenic factors produced by the failing placenta in PE, are considered the best biomarkers of PE, with levels of both rising before the clinical hallmarks of PE are apparent (Levine et al. 2004; Venkatesha et al. 2006). Interestingly, PAPPA, which is used clinically to screen for the presence of Downs syndrome, has been reported to be decreased in all trimesters of patients with PE (for a review, see Grill et al. 2009). Given the present data confirming that PAPPA2 is increased in PE and previous data indicating that it is significantly increased in the blood of PE patients (Paiva et al. 2011), it is possible that PAPPA2 may also represent a potential biomarker for this disease. It is interesting that PAPPA2 appears to trend in the opposite direction of PAPPA, suggesting that despite their homology, they may be regulated very differently and probably perform very different cellular functions.
In conclusion, the findings in the present study confirm and extend previous evidence to indicate that, in severe PE, there is a marked increase in PAPPA2. We also demonstrate that the upregulation of PAPPA2 in PE may be attributable to placental hypoxic exposure in utero.
References
American Congress of Obstetricians and Gynecologists (ACOG) (2002). Diagnosis and management of pre-eclampsia and eclampsia. ACOG Practice Bulletin No. 33. Obstet. Gynecol. 99, 159–167.| Diagnosis and management of pre-eclampsia and eclampsia. ACOG Practice Bulletin No. 33.Crossref | GoogleScholarGoogle Scholar | 16175681PubMed |
Aye, I. L., Waddell, B. J., Mark, P. J., and Keelan, J. A. (2011). Oxysterols inhibit differentiation and fusion of term primary trophoblasts by activating liver X receptors. Placenta 32, 183–191.
| Oxysterols inhibit differentiation and fusion of term primary trophoblasts by activating liver X receptors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhvFWnurs%3D&md5=9a5b1fe56b99084daab279473e7c4e97CAS | 21208656PubMed |
Buimer, M., Keijser, R., Jebbink, J. M., Wehkamp, D., van Kampen, A. H., Boer, K., van der Post, J. A., and Ris-Stalpers, C. (2008). Seven placental transcripts characterize HELLP-syndrome. Placenta 29, 444–453.
| Seven placental transcripts characterize HELLP-syndrome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvVSgu7o%3D&md5=16c82be55d1789e5dec1961cc549742fCAS | 18374411PubMed |
Conover, C. A., Boldt, H. B., Bale, L. K., Clifton, K. B., Grell, J. A., Mader, J. R., Mason, E. J., and Powell, D. R. (2011). Pregnancy-associated plasma protein-A2 (PAPP-A2): tissue expression and biological consequences of gene knockout in mice. Endocrinology 152, 2837–2844.
| Pregnancy-associated plasma protein-A2 (PAPP-A2): tissue expression and biological consequences of gene knockout in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXosFait7Y%3D&md5=25c4e11b8126d4f8079dc4a291fc72c0CAS | 21586553PubMed |
Culver, C., Sundqvist, A., Mudie, S., Melvin, A., Xirodimas, D., and Rocha, S. (2010). Mechanism of hypoxia-induced NF-kappaB. Mol. Cell. Biol. 30, 4901–4921.
| Mechanism of hypoxia-induced NF-kappaB.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlyqtL3K&md5=ac12763f15b36dbed2f274e1d56c330dCAS | 20696840PubMed |
Grill, S., Rusterholz, C., Zanetti-Dallenbach, R., Tercanli, S., Holzgreve, W., Hahn, S., and Lapaire, O. (2009). Potential markers of preeclampsia: a review. Reprod. Biol. Endocrinol. 7, 70.
| Potential markers of preeclampsia: a review.Crossref | GoogleScholarGoogle Scholar | 19602262PubMed |
Levine, R. J., Maynard, S. E., Qian, C., Lim, K.-H., England, L. J., Yu, K. F., Schisterman, E. F., Thadhani, R., Sachs, B. P., Epstein, F. H., Sibai, B. M., Sukhatme, V. P., and Karumanchi, S. A. (2004). Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683.
| Circulating angiogenic factors and the risk of preeclampsia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFCrt7w%3D&md5=6dcae1f04ac71fb6378848af726dd9f4CAS | 14764923PubMed |
Messeguer, X., Escudero, R., Farre, D., Nunez, O., Martinez, J., and Alba, M. M. (2002). PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 18, 333–334.
| PROMO: detection of known transcription regulatory elements using species-tailored searches.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitFentr4%3D&md5=ab41923198fa56c639d69f2c666671d4CAS | 11847087PubMed |
Moffett-King, A. (2002). Natural killer cells and pregnancy. Nat. Rev. Immunol. 2, 656–663.
| Natural killer cells and pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xmslantrk%3D&md5=f4e28499622a145ee4e53ac94d38e438CAS | 12209134PubMed |
Nishizawa, H., Pryor-Koishi, K., Suzuki, M., Kato, T., Kogo, H., Sekiya, T., Kurahashi, H., and Udagawa, Y. (2008). Increased levels of pregnancy-associated plasma protein-A2 in the serum of pre-eclamptic patients. Mol. Hum. Reprod. 14, 595–602.
| Increased levels of pregnancy-associated plasma protein-A2 in the serum of pre-eclamptic patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltV2jtg%3D%3D&md5=154d96a0b620ea6ced9ba6bb3e64bf1bCAS | 18805800PubMed |
Paiva, P., Whitehead, C., Saglam, B., Palmer, K., and Tong, S. (2011). Measurement of mRNA transcripts of very high placental expression in maternal blood as biomarkers of preeclampsia. J. Clin. Endocrinol. Metab. 96, E1807–E1815.
| Measurement of mRNA transcripts of very high placental expression in maternal blood as biomarkers of preeclampsia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsV2nurzM&md5=44bffeb5784b28cd16ebf99212c0fa3dCAS | 21865357PubMed |
Powe, C. E., Levine, R. J., and Karumanchi, S. A. (2011). Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123, 2856–2869.
| Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease.Crossref | GoogleScholarGoogle Scholar | 21690502PubMed |
Redman, C. W., and Sargent, I. L. (2005). Latest advances in understanding preeclampsia. Science 308, 1592–1594.
| Latest advances in understanding preeclampsia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltFemsbo%3D&md5=b965c3a743fbd27a262d35c464623e89CAS | 15947178PubMed |
Rius, J., Guma, M., Schachtrup, C., Akassoglou, K., Zinkernagel, A. S., Nizet, V., Johnson, R. S., Haddad, G. G., and Karin, M. (2008). NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453, 807–811.
| NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmvVGmsL8%3D&md5=07c9ca3f98ab72bc0f828284a6f0f745CAS | 18432192PubMed |
Sibai, B., Dekker, G., and Kupferminc, M. (2005). Pre-eclampsia. Lancet 365, 785–799.
| 15733721PubMed |
Sitras, V., Paulssen, R. H., Gronaas, H., Leirvik, J., Hanssen, T. A., Vartun, A., and Acharya, G. (2009). Differential placental gene expression in severe preeclampsia. Placenta 30, 424–433.
| Differential placental gene expression in severe preeclampsia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFelsrk%3D&md5=756c5513d9e0a1fc45d747c850a0245bCAS | 19249095PubMed |
Valbuena-Diez, A. C., Blanco, F. J., Oujo, B., Langa, C., Gonzalez-Nunez, M., Llano, E., Pendas, A. M., Diaz, M., Castrillo, A., Lopez-Novoa, J. M., and Bernabeu, C. (2012). Oxysterol-induced soluble endoglin release and its involvement in hypertension. Circulation 126, 2612–2624.
| Oxysterol-induced soluble endoglin release and its involvement in hypertension.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslGjs73J&md5=fb142dd3dfc340533c17708612b4d608CAS | 23110859PubMed |
Várkonyi, T., Nagy, B., Fule, T., Tarca, A. L., Karaszi, K., Schonleber, J., Hupuczi, P., Mihalik, N., Kovalszky, I., Rigo, J., Meiri, H., Papp, Z., Romero, R., and Than, N. G. (2011). Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar. Placenta 32, S21–S29.
| Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar.Crossref | GoogleScholarGoogle Scholar | 20541258PubMed |
Venkatesha, S., Toporsian, M., Lam, C., Hanai, J., Mammoto, T., Kim, Y. M., Bdolah, Y., Lim, K. H., Yuan, H. T., Libermann, T. A., Stillman, I. E., Roberts, D., D’Amore, P. A., Epstein, F. H., Sellke, F. W., Romero, R., Sukhatme, V. P., Letarte, M., and Karumanchi, S. A. (2006). Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 12, 642–649.
| Soluble endoglin contributes to the pathogenesis of preeclampsia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltlOjtrs%3D&md5=f1fb1933b900fa42c7e78ba1eddb74d1CAS | 16751767PubMed |
Wagner, P. K., and Christians, J. K. (2010). Altered placental expression of PAPPA2 does not affect birth weight in mice. Reprod. Biol. Endocrinol. 8, 90.
| Altered placental expression of PAPPA2 does not affect birth weight in mice.Crossref | GoogleScholarGoogle Scholar | 20642865PubMed |
Wagner, P. K., Otomo, A., and Christians, J. K. (2011). Regulation of pregnancy-associated plasma protein A2 (PAPPA2) in a human placental trophoblast cell line (BeWo). Reprod. Biol. Endocrinol. 9, 48.
| Regulation of pregnancy-associated plasma protein A2 (PAPPA2) in a human placental trophoblast cell line (BeWo).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltlGrs78%3D&md5=b19616b42d078652712b8593d74f4738CAS | 21496272PubMed |
Wang, J., Qiu, Q., Haider, M., Bell, M., Gruslin, A., and Christians, J. K. (2009). Expression of pregnancy-associated plasma protein A2 during pregnancy in human and mouse. J. Endocrinol. 202, 337–345.
| Expression of pregnancy-associated plasma protein A2 during pregnancy in human and mouse.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFahtLnM&md5=76f0d011ecefcf98c9d9c3f3e0ea1db9CAS | 19474058PubMed |
Winn, V. D., Gormley, M., Paquet, A. C., Kjaer-Sorensen, K., Kramer, A., Rumer, K. K., Haimov-Kochman, R., Yeh, R. F., Overgaard, M. T., Varki, A., Oxvig, C., and Fisher, S. J. (2009). Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology 150, 452–462.
| Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmt1ejtw%3D%3D&md5=5270be1f96f5ee1f79d075f57186ffc6CAS | 18818296PubMed |
Zhong, H., and Simons, J. W. (1999). Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem. Biophys. Res. Commun. 259, 523–526.
| Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjs1Gntbk%3D&md5=839e78290ac1ec3b8cb4ed408e69710fCAS | 10364451PubMed |


